Abstract
Some of the anti-angiogenic agents currently used to treat solid malignancies have effects on tumor endothelial cells as well as on immune cells. We have recently demonstrated that targeting the vascular endothelial growth factor A (VEGFA)/VEGF receptor 2 (VEGFR2) signaling pathway reduces the proportion of regulatory T cells (Treg) in a mouse model of colorectal cancer (CRC) and in metastatic CRC patients as it inhibits tumor-induced Treg proliferation.
Neoangiogenesis is the pathophysiological process whereby tumors stimulate the formation of novel blood vessels to support their growth and metastatic dissemination. The production of pro-angiogenic factors including vascular endothelial growth factor (VEGF) by malignant cells robustly contributes to neoangiogenesis. In line with this notion, targeting pro-angiogenic factors or their receptors by means of specific anti-angiogenic agents has significantly improved the clinical course of several types of cancer in the last two decades. Two main classes of anti-angiogenic agents have been developed so far: small tyrosine kinase inhibitors (TKIs), which often target multiple pro-angiogenic signal receptors at the same time, and monoclonal antibodies, which generally operate by directly neutralizing pro-angiogenic factors. Thus, sunitinib—a TKI that inhibits vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), KIT and fms-related tyrosine kinase 3 (FLT3)—is nowadays employed for the treatment of metastatic renal cancer, while bevacizumab, an anti-VEGFA antibody, is currently used in metastatic colorectal carcinoma (CRC) patients.
Sunitinib, like other relatively unselective TKIs,Citation1 affects not only malignant and endothelial cells but also immune cells. Indeed, it has been shown that sunitinib decreases the number of regulatory T cells (Tregs) in a mouse model of renal cancerCitation2,Citation3 as well as in metastatic renal cancer patients.Citation4,Citation5 Tregs accumulate in tumor-bearing mice and in the peripheral blood of cancer patients and negatively regulate antitumor T-cell responses. Moreover, although this remains a matter of debate, the accumulation of Tregs is generally associated with poor clinical outcomes.Citation6 In a relevant mouse model of CRC (based on murine CT26 cells), we confirmed that sunitinib can inhibit the accumulation of Tregs, both in the spleen and within neoplastic lesions.Citation7 Next, since sunitinib is a multi-kinase inhibitor that targets VEGFR, PDGFR, KIT and FLT3, we analyzed the impact of blocking the VEGFA/VEGFR signaling pathway in this model. The blockade of VEGFA with a specific antibody resulted in a decrease in Tregs similar to that achieved with sunitinib. In addition, the administration of masitinib (a TKI that targets KIT and PDGFR, but not VEGFR) had no effects on the accumulation of Tregs in CRC-bearing mice. These findings suggest that blocking the VEGFA/VEGFR signaling pathway is sufficient to inhibit Treg accumulation in a mouse model of CRC. In line with these preclinical data, we observed that the combination of bevacizumab with standard chemotherapeutic agents, but not chemotherapy alone, decreased the proportion of circulating Tregs in metastatic CRC patients.
Although we found that blocking VEGFA/VEGFR-transduced signals counteracts the induction of Tregs by malignant cells, the underlying mechanisms remained unclear. The accumulation of Tregs in the course of tumor progression is essentially attributed to the conversion of conventional T cells into Tregs or to the proliferation of pre-existing Tregs. It has previously been shown that sunitinib can prevent the conversion of CD4+FOXP3− T cells into CD4+FOXP3+ T lymphocytes in vitro and in vivo by inhibiting the phosphorylation of signal transducer and activator of transcription 3 (STAT3) in melanoma-bearing mice.Citation8 VEGFA also inhibits the maturation of dendritic cells and induce the expansion of myeloid-derived suppressor cells (MDSCs). Immature dendritic cells as well as MDSCs can preferentially induce the development of Tregs.Citation9 Since the administration of sunitinib had previously been shown to decrease the amount of MDSCs and as bevacizumab was known to restore dendritic cell maturation, we reasoned that both these mechanisms could be involved in the modulation of Treg abundance by VEGFA/VEGFR-targeting therapies. However, the direct effect of VEGFA on Tregs had never been studied before.
Since VEGFA is a mitogenic factor for endothelial cells and some types of cancer cells, we investigated the impact of VEGFA on Treg proliferation. Circulating VEGFA levels are increased in both tumor-bearing mice and metastatic CRC patients as compared with tumor-free animals and healthy volunteers, respectively. We observed that the proliferation of Tregs is accelerated in tumor-bearing mice and in metastatic CRC patients, a phenomenon that—in both settings—could be limited by the administration of anti-VEGFA antibodies.Citation7 Of note, this effect was restricted to CD44hi memory Tregs, suggesting that VEGFA-targeting agents could reduce the cycling of a specific subset of tumor antigen-specific Tregs. In vitro, VEGFA stimulated the proliferation of Tregs purified from tumor-bearing but not from tumor-free mice. This observation could be attributed to the restricted expression of VEGFR1 and VEGFR2 on Tregs obtained from mice bearing neoplastic lesions but not from tumor-naïve animals. Of note, only anti-VEGFR2 antibodies turned out to decrease the proliferation of Tregs exposed to VEGFA in vitro and their accumulation in tumor-bearing mice. Taken together, our results highlight the ability of tumor-derived VEGFA to stimulate the proliferation of VEGFR2+ Tregs, and that this phenomenon is efficiently inhibited by VEGFA/VEGFR-targeted therapies in preclinical models of CRC as well as in CRC patients.
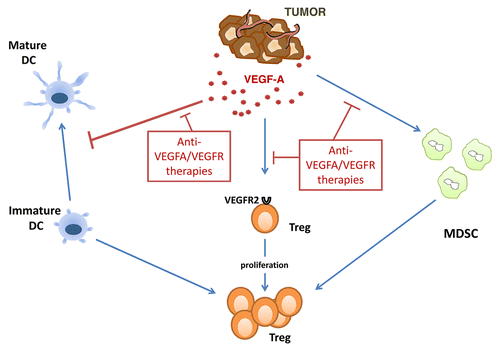
Thus, VEGFA plays a key role in the establishment of an immunosuppressive tumor microenvironment, as it inhibits the maturation of dendritic cells, induces the expansion of MDSCs and stimulates the proliferation of Tregs (). The administration of recombinant VEGFA to tumor-free mice decreases the absolute and relative abundance of T cells and inhibits their functions.Citation10 VEGFA also controls the infiltration of neoplastic lesions by T lymphocytes by modulating the expression of intercellular adhesion molecule 1 (ICAM1) and ICAM2 on endothelial cells.Citation9 Thus, VEGFA can contribute to prevent the development of efficient antitumor immune responses by promoting local and systemic immunosuppression. VEGFA/VEGFR-targeting therapies may therefore revert such an immunosuppressive state () and, in addition to their effects on the tumor stroma, positively modulate anticancer immunity. These agents offer some advantages since (1) they can simultaneously inhibit several immunosuppressive pathways; (2) they may restore the physiological proportion of Tregs and minimize the occurrence of autoimmune side effects, which are often associated with complete Treg depletion; and (3) they do not deplete conventional T cells. The safety and antineoplastic activity of VEGFA/VEGFR-targeting agents combined with immunotherapy should now be evaluated in clinical settings.
Figure 1. Impact of VEGFA/VEGFR-targeted therapeutics on tumor-induced immunosuppression. Tumor-derived vascular endothelial growth factor A (VEGFA) can block the maturation of dendritic cells (DCs) and induce the expansion of myeloid-derived suppressor cells. Immature DCs as well as MDSCs favor the generation of regulatory T cells (Tregs). VEGFA can also induce the proliferation of VEGF receptor 2 (VEGFR2)+ Tregs. VEGFA/VEGFR-targeted therapies can exert antineoplastic effects by interfering with all these mechanisms.
Dislosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Borg C, Terme M, Taïeb J, Ménard C, Flament C, Robert C, et al. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J Clin Invest 2004; 114:379 - 88; PMID: 15286804
- Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res 2009; 69:2514 - 22; http://dx.doi.org/10.1158/0008-5472.CAN-08-4709; PMID: 19276342
- Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res 2009; 15:2148 - 57; http://dx.doi.org/10.1158/1078-0432.CCR-08-1332; PMID: 19276286
- Finke JH, Rini B, Ireland J, Rayman P, Richmond A, Golshayan A, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res 2008; 14:6674 - 82; http://dx.doi.org/10.1158/1078-0432.CCR-07-5212; PMID: 18927310
- Adotevi O, Pere H, Ravel P, Haicheur N, Badoual C, Merillon N, et al. A decrease of regulatory T cells correlates with overall survival after sunitinib-based antiangiogenic therapy in metastatic renal cancer patients. J Immunother 2010; 33:991 - 8; http://dx.doi.org/10.1097/CJI.0b013e3181f4c208; PMID: 20948437
- Tanchot C, Terme M, Pere H, Tran T, Benhamouda N, Strioga M, et al. Tumor-Infiltrating Regulatory T Cells: Phenotype, Role, Mechanism of Expansion In Situ and Clinical Significance. Cancer Microenviron 2012; http://dx.doi.org/10.1007/s12307-012-0122-y; PMID: 23104434
- Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res 2013; 73:539 - 49; http://dx.doi.org/10.1158/0008-5472.CAN-12-2325; PMID: 23108136
- Kujawski M, Zhang C, Herrmann A, Reckamp K, Scuto A, Jensen M, et al. Targeting STAT3 in adoptively transferred T cells promotes their in vivo expansion and antitumor effects. Cancer Res 2010; 70:9599 - 610; http://dx.doi.org/10.1158/0008-5472.CAN-10-1293; PMID: 21118964
- Terme M, Colussi O, Marcheteau E, Tanchot C, Tartour E, Taieb J. Modulation of immunity by antiangiogenic molecules in cancer. Clin Dev Immunol 2012; 2012:492920; http://dx.doi.org/10.1155/2012/492920; PMID: 23320019
- Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 1998; 92:4150 - 66; PMID: 9834220