Abstract
Myeloid-derived suppressor cells (MDSC) potently repress antitumor immunity. The amount of MDSC in the blood of melanoma patients declines in response to vemurafenib, an inhibitor of oncogenic BRAF signaling that abrogates the ability of malignant cells to induce MDSC. This suggests that vemurafenib may be used in combination with various immunotherapeutic agents for the induction of long-lasting tumor regression.
Melanoma cells express an array of specific antigens that are recognized of cytotoxic CD8+ T cells, allowing for the selective eradication of malignant cells.Citation1 Despite such an elevated intrinsic immunogenicity, melanoma develop, progress and eventually spread to distant organs, even when antitumor responses are boosted by immunotherapy. Nowadays, it has been clearly established that the activity of T cells is hampered within the tumor microenvironment. Different leukocytes infiltrate malignant lesions and exert immunosuppressive effects, myeloid-derived suppressor cells (MDSC) playing a major role in this setting.Citation2 We have recently analyzed the peripheral blood of melanoma patients for the presence of these highly immunosuppressive cells and identified two distinct MDSC subsets: the previously known subset of monocytic MDSC (moMDSC)Citation3 as well as a new granulocytic MDSC subset (grMDSC). MoMDSC and grMDSC share several surface markers including CD45, CD33 and CD11b but can be distinguished and isolated from each other thanks to the subset-specific expression of CD14 (on moMDSC) and CD66b (on grMDSC). Both these MDSC subsets were found to suppress autologous T-cell proliferation independently of each other, thus constituting fully functional human MDSC.Citation4 Investigating the presence of MDSC in the peripheral blood of melanoma patients in the course of disease progression, we observed that, in comparison to healthy donors and patients with localized disease, patients with metastatic melanoma exhibited a higher frequency of circulating moMDSC and grMDSC. Interestingly, Stage IV melanoma patients with no evidence of disease at the time of blood draw exhibited MDSC frequencies similar to those of healthy donors.Citation4 These findings suggested us that both moMDSC and grMDSC are directly linked to the presence of metastatic melanoma lesions.
Prompted by this observation, our attention was attracted by recent clinical studies reporting the regression of melanoma metastases in response to small inhibitors of mutant BRAFV600E like vemurafenib and dabrafenib, so-called selective BRAF inhibitors (BRAFi).Citation5 Such therapeutic agents can indeed induce impressive clinical responses by blocking oncogenic signaling pathways within malignant cells. However, in the majority of patients, the effects of selective BRAFi are temporary and tumors become resistant.Citation5 The fact that selective BRAFi induce strong, though transient, reductions in the metastatic tumor burden led us to investigate the impact of these agents on MDSC. The analysis of MDSC frequencies in the peripheral blood of patients under vemurafenib therapy revealed that moMDSC and grMDSC decline over time in individual achieving clinical responses. We have not determined whether vemurafenib affects not only circulating MDSC but also the tumor-infiltrating myeloid cells. Tumor samples from patients under treatment have not yet been analyzed in this respect. However, we set up a cell culture model that allowed us to mimic the effect of vemurafenib on MDSC in the melanoma microenvironment. In this model, peripheral blood mononuclear cells (as a source of CD14+ monocytic cells) were exposed to conditioned medium (CM) from BRAF-mutant melanoma cells that had been treated or not with vemurafenib. Interestingly, the CM from untreated melanoma cells induced MDSC with a monocytic phenotype, exerting robust immunosuppressive effects on T cells. In contrast, the CM from vemurafenib-treated melanoma cells did not. Of note, the ability of the CM from untreated tumor cells to induce moMDSC was not impaired by the addition of vemurafenib, indicating that this BRAFi exerts indirect immunomodulatory effects by acting on tumor cells.Citation4 In this context it should be noted that the constitutive activation of BRAF not only promotes the proliferation of melanoma cells but also stimulates them to secrete immunosuppressive cytokines.Citation6 Thus, vemurafenib appears to exert immunomodulatory effects by inhibiting the ability of BRAF-mutant melanoma cells to secrete factors that induce moMDSC in vitro, such as interleukin-6 (IL-6) ().Citation7
Figure 1. Vemurafenib abrogates the immunosuppressive effects of MDSC in melanoma patients. Vemurafenib inhibits mutant BRAFV600E signaling in melanoma cells, not only limiting their proliferation and survival, but also interfering with the secretion of soluble factors that are responsible for the recruitment, induction and differentiation of myeloid-derived suppressor cells (MDSC). Vemurafenib appears to have no direct effects on MDSC induction, though a potential modulation of MDSC function by vemurafenib has not been studied yet.
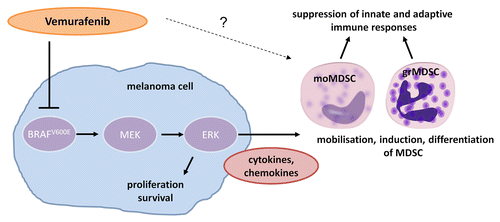
In summary, our findings suggest that the immunosuppressive activity of MDSCs is decreased in melanoma patients achieving clinical responses upon the administration of vemurafenib. Thus, by neutralizing MDSC-dependent immunosuppressive networks, selective BRAFi might allow antigen-specific CD8+ T cells to efficiently target autologous tumor cells. In line with this notion, a study of melanoma biopsies from patients under BRAFi therapy has revealed that the number of CD8+ T cells infiltrating malignant lesions increased in response to vemurafenib, correlating with a reduction in tumor size.Citation8 Additional studies have suggested that the recognition of melanoma cells by T cells is enhanced by vemurafenib owing to an increased availability of specific CD8+ T-cell antigens.Citation9 Thus, it seems reasonable to combine vemurafenib with immunotherapeutic interventions. Vemurafenib has indeed a direct effect on tumor burden, promotes the antigenicity of malignant cells as well as the infiltration of neoplastic lesions by T cells, and limits MDSC-dependent immunosuppressive networks. Unfortunately, a Phase I clinical trial testing vemurafenib in combination with ipilimumab, an anti-CTLA4 antibody that operates as an immune checkpoint inhibitor, had to be discontinued due to severe liver toxicity.Citation10 Thus, although translational data suggest that specific immunochemotherapeutic approaches may be beneficial to cancer patients, only appropriately designed clinical trials can assess the actual safety, tolerability and efficacy of such novel (and sometimes rather intense) treatment schemes.
| Abbreviations: | ||
| BRAFi | = | BRAF inhibitor |
| CM | = | conditioned medium |
| grMDSC | = | granulocytic MDSC |
| MDSC | = | myeloid-derived suppressor cells |
| moMDSC | = | monocytic MDSC |
Disclosure of Potential Conflicts of Interest
BS received an honoraria from Roche
References
- Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol 2006; 24:175 - 208; http://dx.doi.org/10.1146/annurev.immunol.24.021605.090733; PMID: 16551247
- Umansky V, Sevko A. Melanoma-induced immunosuppression and its neutralization. Semin Cancer Biol 2012; 22:319 - 26; http://dx.doi.org/10.1016/j.semcancer.2012.02.003; PMID: 22349515
- Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol 2007; 25:2546 - 53; http://dx.doi.org/10.1200/JCO.2006.08.5829; PMID: 17577033
- Schilling B, Sucker A, Griewank K, Zhao F, Weide B, Görgens A, et al. Vemurafenib reverses immunosuppression by myeloid derived suppressor cells. Int J Cancer 2013; In press http://dx.doi.org/10.1002/ijc.28168; PMID: 23526263
- Johnson DB, Sosman JA. Update on the targeted therapy of melanoma. Curr Treat Options Oncol 2013; 14:280 - 92; http://dx.doi.org/10.1007/s11864-013-0226-8; PMID: 23420410
- Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med 2006; 203:1651 - 6; http://dx.doi.org/10.1084/jem.20051848; PMID: 16801397
- Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol 2010; 185:2273 - 84; http://dx.doi.org/10.4049/jimmunol.1000901; PMID: 20644162
- Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res 2012; 18:1386 - 94; http://dx.doi.org/10.1158/1078-0432.CCR-11-2479; PMID: 22156613
- Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res 2010; 70:5213 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-10-0118; PMID: 20551059
- Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med 2013; 368:1365 - 6; http://dx.doi.org/10.1056/NEJMc1302338; PMID: 23550685