Abstract
Natural killer (NK) cells are integral components of the antitumor immune response. The downregulation of ligands for NK-cell stimulatory receptors represents a strategy whereby glioblastoma cells can evade NK-cell attacks. Histone deacetylase inhibitors can stimulate the (re)expression of these ligands, driving cytotoxic responses against glioblastoma cells that efficiently inhibit tumor growth.
Natural killer (NK) cells are lymphocytes of the innate immune system that play a critical role in the immunosurveillance of several tumors, including glioblastoma multiforme (GBM). Effective antitumor immune responses depend on the interaction between the activating receptor NKG2D, which is expressed on NK, CD8+ and γδ T cells, and its ligands (NKG2DLs) on the surface of target cells, including MHC Class I-related chain A and B MICA/B as well as multiple UL16-binding proteins (ULBP1–4). NKG2DL are upregulated by cells, including malignant cells, in response to stress. Through NKG2D, NK cells prevent the growth of malignant cells expressing NKG2DLs, and the blockade of NKG2D impairs the NK cell-mediated lysis of target cells. Both the downregulation of NKG2DLs or their matrix metalloproteinase (MMP)-dependent shedding, resulting in the release of soluble NKG2DL fragments, represent strategies whereby GBM cells evade NKG2D-mediated immunosurveillance.Citation1 In line with this notion, the induction or ectopic overexpression of MICA in glioma cells enhances NK and T cell-mediated antitumor responses in vitro and delays GBM growth in vivo.Citation2
Several physiological and pathological cellular processes are governed by epigenetic events such as histone acetylation and deacetylation. Histone acetylation is mediated by histone acetyltransferases (HATs) and generally allows for active gene transcription. Conversely, histone deacetylation is catalyzed by histone deacetylases (HDACs), and favors gene repression. Histone acetylation is a reversible, dynamic and highly regulated process that plays a crucial role in the regulation of gene expression (). In addition, a growing number of non-histone proteins has been shown to undergo reversible acetylation by HATs and HDACs. Alterations in this dynamic equilibrium, such as those caused by the aberrant expression or functional activation of HATs and HDACs, can disturb cell homeostasis and result in pathological states. Deletions or inactivating mutations in multiple genes coding for HATs as well as an increased activity of HDACs have indeed been associated with oncogenesis and tumor progression, as they alter the transcription of genes that regulate key functions such as proliferation, cell cycle progression and apoptosis.Citation3,Citation4 More interestingly, the transcription of many immunomodulatory genes such as those encoding MHC Class I molecules, proteins of the antigen-processing machinery (APM) like transporter associated with antigen processing 1 and 2 (TAP1/2), proteins associated with the proteasome like large multifunctional protease 2 (LMP2) and tapasin as well as multiple NKG2DLs appears to be regulated by histone acetylation/deacetylation. Numerous studies have demonstrated that a variety of HDAC inhibitors (HDACis) like valproic acid, sodium butyrate, vorinostat, romidepsinor and trichostatin A (TSA) induces the expression of NKG2DLs on tumor cells, facilitating their recognition and destruction by cytotoxic lymphocytes.Citation5,Citation6 In addition, HDACis downregulate the expression of MMP9, thus inhibiting the release of MICA and MICB from the surface of tumor cells.Citation7 Finally, it has been shown that HDACis enhance the NK cell-mediated lysis of tumor cells and reduce tumor growth in vivo as they promote the expression of MICA or ULBP2.Citation5
Figure 1. Antitumor activity of HDAC inhibitors. Left: The inhibition of histone deacetylases (HDACs) causes both transcriptional and non-transcriptional effects, leading to profound alterations in cell homeostasis. Middle: The re-acetylation of histones upon HDAC inhibition stimulates gene transcription. Right: As a result of HDAC inhibition, NKG2D ligands (NKG2DLs) such as MHC Class I-related chain A and B (MICA/B) or UL16-binding proteins (ULBPs) are upregulated, rendering glioblastoma multiforme (GBM) susceptible to recognition and lysis by natural killer (NK) cells.
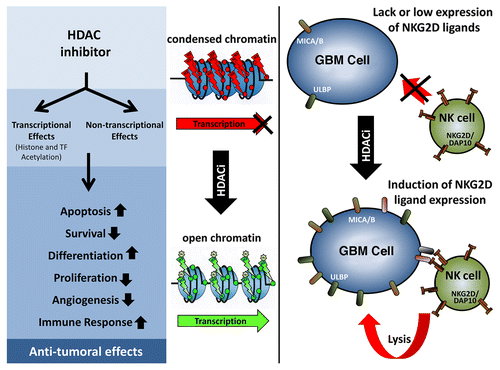
We have recently investigated the immunomodulatory effects of TSA on GBM cells in vitro as well as its therapeutic activity in vivo, in a GBM xenograft model.Citation8 We were able to demonstrate that, besides its acute cytotoxicity, TSA synergized with death receptor ligands in the killing of GBM cells, putatively as it inhibits the expression of anti-apoptotic factors such as cellular CASP8 and FADD-like apoptosis regulator (CFLAR) or X-linked inhibitor of apoptosis (XIAP). More interestingly, TSA influenced several processes that are involved in antitumor immune responses. Thus, whereas no changes in the expression levels of APM components were detectable upon TSA administration, GBM cells responding to TSA released high-mobility group box 1 (HMGB1), an endogenous Toll-like receptor 4 (TLR4) ligand that promotes cytotoxic T-cell mediated antitumor immune responses. Furthermore, TSA led to the upregulation of the NKG2DLs MICA und ULBP2, at both the mRNA and surface protein level, resulting in the recognition and efficient lysis of GBM cells by lymphokine activate killer and CD56+ NK cells ().Citation8 Such an enhanced cytotoxic response was at least partially dependent on NKG2DL expression by glioma cells, as it was significantly reduced when NK cells were pre-treated with a NKG2D-neutralizing antibody. In a mouse model of GBM, TSA delayed tumor growth independently from the induction of cancer cell death, an effect that was strictly dependent on the presence of functional NK cells. These findings provide proof-of-principle evidence in support of a therapeutically relevant immunostimulatory activity of HDACis against GBM.
HDACis have pleiotropic effects on malignant cells. They inhibit proliferation, sensitize cells to death receptor ligand- or radiation-induced apoptosis, mitigate migration, modulate angiogenesis and induce the expression of oncosuppressor genes as well as that of a variety of immunostimulatory genes. Because of these qualities, HDACis are considered as exciting anticancer agents. Several epigenetic modulators have already been approved by FDA and EMEA for cancer therapy and appear to be well tolerated by patients. Up to date, about a dozen HDACis have been tested in clinical trials for the treatment of different types of cancer, including GBM, either as standalone therapeutic interventions or in combination with other anticancer agents.Citation6,Citation9 It has become increasingly clearer that—besides their intrinsic effects on tumor cells—HDACis limit tumor progression by regulating immune responses. HDACis can provide immunomodulatory properties by enhancing the antigenicity of tumor cells (via the upregulation of MHC Class I and II molecules or MICA/B), by regulating the production of several cytokines such as tumor necrosis factor α (TNFα), interleukin-1 (IL-1) and interferon γ (IFNγ), as well as by inhibiting the immunosuppressive functions of regulatory T cells (Tregs).Citation10 In summary, accumulating evidence provides a strong rationale in support of clinical studies to further evaluate the safety and therapeutic profile of HDACis in combination with anticancer immunotherapy.
| Abbreviations: | ||
| APM | = | antigen presenting machinery |
| CFLAR | = | CASP8 and FADD-like apoptosis regulator |
| GBM | = | glioblastoma multiforme, HDAC, histone deacetylase |
| HAT | = | histone acetyltransferase |
| HDACi | = | HDAC inhibitor |
| HMGB1 | = | high mobility group box 1 |
| IL-1 | = | interleukin-1 |
| IFNγ | = | interferon γ |
| NK | = | natural killer |
| MIC | = | MHC Class I-related chain |
| MMP | = | matrix metalloproteinase |
| NKG2DL | = | NKG2D ligand |
| TAP | = | transported associated with antigen processing |
| TLR4 | = | Toll-like receptor 4 |
| TNFα | = | tumor necrosis factor α |
| Treg | = | regulatory T cell |
| TSA | = | trichostatin A |
| ULBP | = | UL16-binding protein |
| XIAP | = | X-linked inhibitor of apoptosis |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Eisele G, Wischhusen J, Mittelbronn M, Meyermann R, Waldhauer I, Steinle A, et al. TGF-beta and metalloproteinases differentially suppress NKG2D ligand surface expression on malignant glioma cells. Brain 2006; 129:2416 - 25; http://dx.doi.org/10.1093/brain/awl205; PMID: 16891318
- Friese MA, Platten M, Lutz SZ, Naumann U, Aulwurm S, Bischof F, et al. MICA/NKG2D-mediated immunogene therapy of experimental gliomas. Cancer Res 2003; 63:8996 - 9006; PMID: 14695218
- Hagelkruys A, Sawicka A, Rennmayr M, Seiser C. The biology of HDAC in cancer: the nuclear and epigenetic components. Handb Exp Pharmacol 2011; 206:13 - 37; http://dx.doi.org/10.1007/978-3-642-21631-2_2; PMID: 21879444
- Ropero S, Esteller M. The role of histone deacetylases (HDACs) in human cancer. Mol Oncol 2007; 1:19 - 25; http://dx.doi.org/10.1016/j.molonc.2007.01.001; PMID: 19383284
- Armeanu S, Bitzer M, Lauer UM, Venturelli S, Pathil A, Krusch M, et al. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res 2005; 65:6321 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-04-4252; PMID: 16024634
- Kim HJ, Bae SC. Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anti-cancer drugs. Am J Transl Res 2011; 3:166 - 79; PMID: 21416059
- Yamanegi K, Yamane J, Kobayashi K, Ohyama H, Nakasho K, Yamada N, et al. Downregulation of matrix metalloproteinase-9 mRNA by valproic acid plays a role in inhibiting the shedding of MHC class I-related molecules A and B on the surface of human osteosarcoma cells. Oncol Rep 2012; 28:1585 - 90; PMID: 22923031
- Höring E, Podlech O, Silkenstedt B, Rota IA, Adamopoulou E, Naumann U. The histone deacetylase inhibitor trichostatin a promotes apoptosis and antitumor immunity in glioblastoma cells. Anticancer Res 2013; 33:1351 - 60; PMID: 23564772
- Galanis E, Jaeckle KA, Maurer MJ, Reid JM, Ames MM, Hardwick JS, et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J Clin Oncol 2009; 27:2052 - 8; http://dx.doi.org/10.1200/JCO.2008.19.0694; PMID: 19307505
- Shen L, Ciesielski M, Ramakrishnan S, Miles KM, Ellis L, Sotomayor P, et al. Class I histone deacetylase inhibitor entinostat suppresses regulatory T cells and enhances immunotherapies in renal and prostate cancer models. PLoS One 2012; 7:e30815; http://dx.doi.org/10.1371/journal.pone.0030815; PMID: 22303460