Abstract
Natural killer (NK) cells hold great promise for adoptive cancer immunotherapy. The antitumor activity of NK cells can be enhanced by the transgene driven expression of chimeric antigen receptors that facilitate the selective recognition and killing of malignant cells. Recent data from our laboratory suggest that NK cells may similarly be “armed” against neoplastic cells by the expression of cancer-specific granzyme B-containing fusion proteins that are released as soluble factors upon NK-cell activation.
Keywords: :
Natural killer (NK) cells play an important role in the defense against viral infection and the elimination of neoplastic cells. The natural cytotoxic function of NK cells can be rapidly activated upon appropriate stimulation, and is regulated by a complex balance of signals originating from germline-encoded activating and inhibitory receptors. Following the recognition of target cells and activation, lytic granules within NK cells are polarized toward the immunological synapse, where they fuse with the plasma membrane and release their contents into the so-called synaptic cleft, i.e., the small space between effector and target cells ().Citation1 The importance of NK cells for cancer immunosurveillance has been extensively documented in mouse models.Citation2 A correlation between a reduced activity of circulating NK cells and an increased risk of cancer has been demonstrated also in humans.Citation3 Of note, in contrast to B and T lymphocytes, NK cells are intrinsically programmed to detect modifications in cellular metabolism or gene expression that are concurrent with oncogenesis.Citation4 Hence, different strategies are being developed to employ NK cells for anticancer immunotherapy. These include the activation of autologous NK cells, the adoptive transfer of allogeneic NK cells, and the pharmacological or genetic modulation of NK-cell functions.Citation5
Figure 1. Enhancement of NK-cell antitumor activity by the expression of tumor-specific receptors and cytotoxic fusion proteins. (A) The natural cytotoxicity of natural killer (NK) cells against tumor cells is controlled by signals from stimulatory receptors, such as natural cytotoxicity receptors (NCRs) and NKG2D, and inhibitory receptors, such as inhibitory killer-cell immunoglobulin-like receptors (KIRs). (B) The expression of a chimeric antigen receptor (CAR) specific for tumor-associated cell surface antigens efficiently redirects NK cells to malignant cells, and facilitates their cytolytic activity independently from the activation of endogenous stimulatory receptors. (C) Alternatively, NK cells can be modified to express targeted cytotoxic chimeras such as the pro-apoptotic serine protease granzyme B (GrB) fused to a tumor cell-specific ligand or antibody fragment. Such molecules are stored together with endogenous granzymes and perforin in cytotoxic granules. Upon the activation of endogenous NK-cell receptors, tumor-targeted GrB is released together with perforin and hence can cooperate with natural cytotoxicity mechanisms in the killing of target cells. Nonetheless, insufficient signals via endogenous NK-cell stimulatory receptors can prevent the release of retargeted GrB variants. (D) This issue may be overcome by the co-expression of tumor-targeted GrB derivatives together with a CAR that ensures NK-cell activation even by otherwise resistant cancer cells. In this scenario, CARs and targeted GrB may be directed to the same or distinct tumor-associated cell surface antigens.
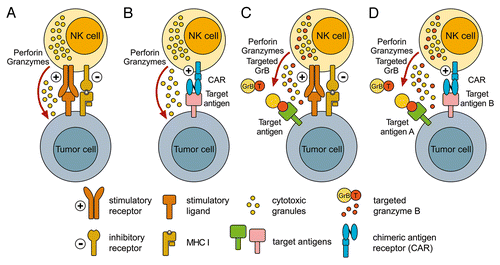
Like T cells, NK cells can be genetically modified to express chimeric antigen receptors (CARs), allowing them to selectively recognize tumor-associated cell surface antigens and hence increasing their specificity and antineoplastic activity.Citation6,Citation7 Typically, a CAR consists of an extracellular single-chain antibody fragment (scFv, for the recognition for tumor-associated antigens) that is linked via a transmembrane domain to an intracellular signaling moiety such as a CD3 ζ chain or a CD3 ζ chain coupled to a co-stimulatory protein domain. The engagement of CARs expressed by NK cells triggers the antigen-specific lysis of target cells, hence bypassing the need for the activation of endogenous cytotoxicity receptors (). This may be of particular advantage in the case of solid neoplasms, as malignant cells from this type of cancer display a varying degree of resistance to the lytic activity of unmodified NK cells,Citation8 but can be readily killed in an antigen-dependent manner by NK cells expressing suitable CARs.Citation6,Citation7
As a novel approach to provide NK cells with target-specific cytotoxic functions and augment their antitumor activity, we have recently tested the ectopic expression of a chimeric granzyme B (GrB)-containing fusion protein by NK cells.Citation9 GrB is a pro-apoptotic serine protease that plays a crucial role in NK-cell mediated cytotoxicity. GrB is synthesized as an inactive precursor protein (pre-pro-GrB) bearing an N-terminal signal peptide that directs the protein into secretory granules and an activation dipeptide. The removal of this activation peptide by the cysteine protease cathepsin C generates the enzymatically active form of GrB, which is stored together with other granzymes and perforin in the dense core of lytic granules. Following the recognition of target cells and activation, NK cells release mature GrB specifically in the immunological synapse, from where it enters target cells in cooperation with perforin, rapidly inducing their death.
For expression in NK cells, we generated a tumor-specific GrB-based chimera by fusing the epidermal growth factor receptor (EGFR)-specific ligand transforming growth factor α (TGFα) to the C-terminus of human pre-pro-GrB. Unlike bacterial or yeast expression systems previously employed for the generation of recombinant GrB fusion proteins,Citation10 NK cells possess the entire molecular machinery that is required for the processing, packaging, and triggered release of endogenous GrB, which may also be employed by an ectopically expressed, re-targeted GrB derivative. Following lentiviral vector-based gene transfer, NK cells readily expressed the GrB-TGFα fusion protein in amounts comparable to endogenous wild-type GrB.Citation9 Moreover, the activation of genetically modified NK cells by target cells led to the release of correctly processed and enzymatically active GrB-TGFα together with endogenous granzymes and perforin. This facilitated the cooperation between tumor-specific GrB-TGFα and natural cytotoxic effectors of NK cells, resulting in enhanced antitumor activity against NK cell-sensitive targets (). The GrB-TGFα chimera enriched from the supernatant of artificially activated NK cells displayed EGFR-specific binding as well as the ability to kill EGFR-expressing neoplastic cells in the presence of an endosomolytic activity. Nevertheless, EGFR-expressing malignant cells originating from solid tumors failed to activate natural cytotoxicity receptors, thereby avoiding lysis by parental as well as by GrB-TGFα-expressing NK cells.Citation9
To overcome this issue, we are currently investigating the co-expression of tumor-targeted GrB variants with CARs that ensure NK-cell activation by otherwise resistant target cells, resulting in NK-cell degranulation and efficient release of cytotoxic molecules (). In this setting, CARs and GrB may be targeted to the same or to distinct tumor-associated cell surface antigens. In the latter case, surface-expressed CARs would mediate the tumor-specific targeting and activation of NK cells, while a GrB variant directed to a different tumor-associated antigen could potentiate their antitumor activity via a second hit on the same target cell and/or by killing bystander neoplastic cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol 2008; 8:713 - 25; http://dx.doi.org/10.1038/nri2381; PMID: 19172692
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011; 29:235 - 71; http://dx.doi.org/10.1146/annurev-immunol-031210-101324; PMID: 21219185
- Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet 2000; 356:1795 - 9; http://dx.doi.org/10.1016/S0140-6736(00)03231-1; PMID: 11117911
- Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol 2012; 12:239 - 52; http://dx.doi.org/10.1038/nri3174; PMID: 22437937
- Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol 2008; 9:486 - 94; http://dx.doi.org/10.1038/ni1580; PMID: 18425105
- Uherek C, Tonn T, Uherek B, Becker S, Schnierle B, Klingemann H-G, et al. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood 2002; 100:1265 - 73; PMID: 12149207
- Sahm C, Schönfeld K, Wels WS. Expression of IL-15 in NK cells results in rapid enrichment and selective cytotoxicity of gene-modified effectors that carry a tumor-specific antigen receptor. Cancer Immunol Immunother 2012; 61:1451 - 61; http://dx.doi.org/10.1007/s00262-012-1212-x; PMID: 22310931
- Stojanovic A, Cerwenka A. Natural killer cells and solid tumors. J Innate Immun 2011; 3:355 - 64; http://dx.doi.org/10.1159/000325465; PMID: 21502747
- Oberoi P, Jabulowsky RA, Bähr-Mahmud H, Wels WS. EGFR-targeted granzyme B expressed in NK cells enhances natural cytotoxicity and mediates specific killing of tumor cells. PLoS One 2013; 8:e61267; http://dx.doi.org/10.1371/journal.pone.0061267; PMID: 23573299
- Oberoi P, Jabulowsky RA, Wels WS. Selective induction of cancer cell death by targeted granzyme B. Antibodies 2013; 2:130 - 51; http://dx.doi.org/10.3390/antib2010130