Abstract
For a long time, the transcription factor interferon-regulatory factor 8 (IRF8) has been recognized as a masterpiece for the development of myeloid cells, and its role as a central regulator of immune responses has now been clarified. IRF8 is also critical for tumor progression, suggesting its fundamental relevance in multiple aspects of cancer immunosurveillance.
Tumor progression and metastatic invasion are 2 interconnected phenomena characterized by a plethora of relatively well characterized, sequential events. The immune system can respond to the presence of cancer by actively infiltrating neoplastic lesions and interacting with cancer cells and other components of the tumor microenvironment.Citation1 The profile of immune cell populations and soluble immunomodulatory factors (i.e., cytokines, chemokines) found within neoplastic lesions is sometimes referred to as “immunoenvironment” and is thought to critically dictate tumor progression and metastatic spread, although the mechanisms underpinning the crosstalk between immune system and cancer are still poorly defined.
Interferon-regulatory factor 8 (IRF8) shares with all the other members of the IRF protein family the ability to regulate type I and II interferon-dependent signaling pathways. IRF8 specifically directs the developmental program of the myeloid cell lineage. Mice lacking the murine counterpart of IRF8 are devoid of plasmacytoid dendritic cells (pDCs) and display a selective decrease in the number and functionality of CD8α+ DCs and macrophages along with abnormally elevated frequencies of granulocytes.Citation2,Citation3 Of note, Irf8−/− mice are characterized by an unusual recurrence of a hematologic malignancy that closely resembles chronic myelogenous leukemia (CML),Citation2 which in humans is associated with the expression of the oncogenic fusion protein BCR-ABL.Citation4 IRF8 also plays a role in the biology of solid tumors, a setting in which IRF8 has been ascribed with oncosuppressive functions.Citation5
The mechanisms by which intratumoral IRF8 expression is regulated in vivo and how this affects the immunoenvironment have long remained elusive. By using Irf8−/− mice transplanted with highly metastatic B16.F10 melanoma cells, we recently highlighted a dual role for IRF8 in the regulation of tumor progression and metastasis. On one hand, the lack of IRF8 in the host results in failing anticancer immunosurveillance, allowing B16 melanoma cells to rapidly grow and form lung metastases.Citation6 These findings could be attributed to the immunological defects displayed by Irf8−/− mice. In fact, the analysis of melanoma lesions developing in Irf8−/− hosts revealed profound immunoenvironmental alterations as compared with similar tumors growing in wild-type (WT) mice. Indeed, whereas the latter were highly infiltrated by conventional DCs, pDCs, CD4+, and CD8+ T lymphocytes, the former were rich of immunosuppressive cells populations, in particular myeloid-derived suppressor cells (which have recently been shown to downregulate IRF8 as a mechanism to avoid apoptosis).Citation7 Accordingly, gene expression analysis of melanoma developing in Irf8−/− mice evidenced a chemokine/chemokine receptor profile compatible with an immunosuppressive microenvironment that supports tumor growth and metastasis. Conversely, melanoma growing in WT animals displayed a more immunogenic chemokine expression pattern, supporting the recruitment of DCs and T cells ().
Figure 1. Dual role of IRF8 in cancer immunosurveillance. In comparison to immunocompetent (wild-type) hosts, interferon-regulatory factor 8 (IRF8)-deficient mice receiving B16.F10 melanoma cells exhibit accelerated tumor growth and an increased propensity to form lung metastasis. For the most part, this reflects the establishment of a highly immunosuppressive tumor microenvironment characterized by the expression of cytokines, chemokines, and pro-angiogenic factors that support tumor growth and metastasis. All these events are closely correlated with the epigenetic silencing of Irf8 in melanoma cells.
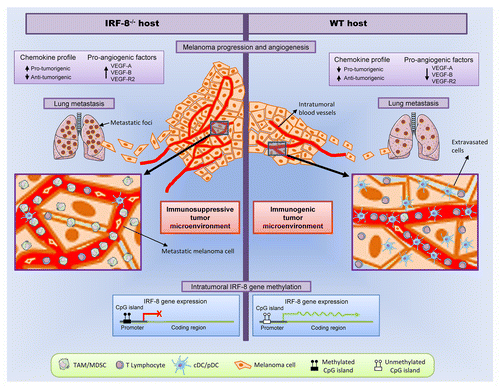
On the other hand, the immunological defects of Irf8−/− mice were paralleled by an early loss of IRF8 expression by transplanted B16 melanoma cells. In contrast, melanoma lesions developing in WT mice displayed detectable IRF8 expression, although the levels progressively declined along with tumor progression. These results suggest that the immunoenvironment is capable of shaping the phenotype of cancer cells in terms of gene expression, notably concerning the expression of oncosuppressive factors such as IRF8. Co-culture experiments in transwell chambers using splenocytes (as a representative population of bulk immune cells) and melanoma cells revealed that the expression levels of IRF8 in the latter can be directly modulated by soluble factors released by immune cells. Hence, while the immunosuppressive microenvironment provided by Irf8−/− mice was unable to sustain intratumoral IRF8 expression, the immunoenvironment of WT hosts released the soluble mediators that are required to this aim. Additional co-culture experiments performed in a microfluidic environment (using nanofabricated silicon-based devices) confirmed the existence of an active crosstalk between immune cells and malignant cells modulated by IRF8.Citation8 Thus, IRF8 is capable to selectively induce and sustain the production of soluble factors that play a pivotal role in the balance between immunosurveillance and immune escape. Interleukin (IL)-27 and IL-6 were identified as central factors that modulate IRF8 expression, although other mediators may also be involved in this process. IL-27 is an attractive candidate for the development of anticancer immunotherapeutic interventions, as it mediates antineoplastic effects against melanoma cells through a mechanism that involves the upregulation of IRF1 and IRF8.Citation9
The administration of the demethylating agent 5-aza-2′-deoxycytidine (decitabine) to melanoma-bearing Irf8−/− mice induced a transient reversion of the cancer immunoenvironment toward a state characterized by DC and T-cell infiltration as well as by a specific chemokine/chemokine receptor pattern that mediated antineoplastic effects. Of note, while the Irf8 promoter in melanoma lesions developing in untreated Irf8−/− mice was highly methylated, the administration of decitabine resulted in its specific demethylation, restoring intratumoral IRF8 expression. These findings confirm the epigenetic control of Irf8 gene and demonstrate a close correlation between intratumoral IRF8 expression levels and the immune contexture of melanoma ().
Globally, the results of our studies identify IRF8 as a crucial modulator of melanoma progression operating at the interface between malignant cells and the immune infiltrate. We propose that immune effector cells actively infiltrating neoplastic lesions sustain the expression of IRF8 by melanoma cells, and, in turn, basal intratumoral levels of IRF8 are indispensable for maintaining an appropriate immunological microenvironment, in part through the modulation of soluble antitumor factors, thus favoring disease control. We envisage that this mechanism of mutual interplay between malignant and immune cells may also apply to other oncosuppressive factors, as recently proposed for IRF1 and NK cells in a murine model of lung metastasis.Citation10 In this respect, oncosuppressive proteins like IRF8 may be re-viewed as clinically relevant biomarkers for the early treatment of melanoma and other solid tumors with approaches that combine targeted antineoplastic agents and immunotherapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Schiavoni G, Gabriele L, Mattei F. The tumor microenvironment: a pitch for multiple players. Front Oncol 2013; 3:90; http://dx.doi.org/10.3389/fonc.2013.00090; PMID: 23616948
- Holtschke T, Löhler J, Kanno Y, Fehr T, Giese N, Rosenbauer F, et al. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell 1996; 87:307 - 17; http://dx.doi.org/10.1016/S0092-8674(00)81348-3; PMID: 8861914
- Schiavoni G, Mattei F, Sestili P, Borghi P, Venditti M, Morse HC 3rd, et al. ICSBP is essential for the development of mouse type I interferon-producing cells and for the generation and activation of CD8alpha(+) dendritic cells. J Exp Med 2002; 196:1415 - 25; http://dx.doi.org/10.1084/jem.20021263; PMID: 12461077
- Tamura T, Kong HJ, Tunyaplin C, Tsujimura H, Calame K, Ozato K. ICSBP/IRF-8 inhibits mitogenic activity of p210 Bcr/Abl in differentiating myeloid progenitor cells. Blood 2003; 102:4547 - 54; http://dx.doi.org/10.1182/blood-2003-01-0291; PMID: 12933588
- Abrams SI. A multi-functional role of interferon regulatory factor-8 in solid tumor and myeloid cell biology. Immunol Res 2010; 46:59 - 71; http://dx.doi.org/10.1007/s12026-009-8125-6; PMID: 19756408
- Mattei F, Schiavoni G, Sestili P, Spadaro F, Fragale A, Sistigu A, et al. IRF-8 controls melanoma progression by regulating the cross talk between cancer and immune cells within the tumor microenvironment. Neoplasia 2012; 14:1223 - 35; PMID: 23308054
- Hu X, Bardhan K, Paschall AV, Yang D, Waller JL, Park MA, et al. Deregulation of Apoptotic Factors Bcl-xL and Bax Confers Apoptotic Resistance to Myeloid- Derived Suppressive Cells and Contributes to their Persistence in Tumors. J Biol Chem 2013; In press http://dx.doi.org/10.1074/jbc.M112.434530; PMID: 23677993
- Businaro L, De Ninno A, Schiavoni G, Lucarini V, Ciasca G, Gerardino A, et al. Cross talk between cancer and immune cells: exploring complex dynamics in a microfluidic environment. Lab Chip 2013; 13:229 - 39; http://dx.doi.org/10.1039/c2lc40887b; PMID: 23108434
- Yoshimoto T, Morishima N, Mizoguchi I, Shimizu M, Nagai H, Oniki S, et al. Antiproliferative activity of IL-27 on melanoma. J Immunol 2008; 180:6527 - 35; PMID: 18453571
- Ksienzyk A, Neumann B, Nandakumar R, Finsterbusch K, Grashoff M, Zawatzky R, et al. IRF-1 expression is essential for natural killer cells to suppress metastasis. Cancer Res 2011; 71:6410 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-11-1565; PMID: 21900395