Abstract
The ability of Listeria monocytogenes-based anticancer vaccines to induce tumor regression depends on the responsiveness of malignant cells to interferon γ (IFNγ). Inhibition of IFNγ limits the recruitment of T cells to the tumors of vaccinated mice. We hypothesized that vaccination with immunotherapeutic L. monocytogenes induces the IFNγ-dependent production of chemokines that regulate the migration of tumor-infiltrating T cells. To gain further insights into this issue, we examined the chemokine responses of a transplantable, human papillomavirus (HPV)-immortalized murine tumor model (TC-1) following the administration of a L. monocytogenes-based immunotherapeutic agent that expresses E7 from HPV-16. Here, we report that the administration of L. monocytogenes-based anticancer vaccines increases the secretion of chemokine (C-X-C motif) ligand 9 (CXCL9), and CXCL10 by tumors, hence favoring the recruitment of T cells bearing the cognate chemokine (C-X-C motif) receptor 3 (CXCR3). Furthermore, the expression of CXCL9, but not CXCL10, in TC-1 tumors was significantly reduced upon anti-IFNγ antibody treatment. CXCL9 was highly expressed by TC-1 cells following the administration of IFNγ and tumor necrosis factor α (TNFα), in vitro. Moreover, the inhibition of CXCL9 in TC-1 cells reduced the proportion of CD8+ T cells infiltrating tumors in vaccinated mice, while increasing that of CD4+ T cells, thus altering T-cell subset distribution. We conclude that the administration of L. monocytogenes-based anticancer vaccines regulates TH1 chemokine responses and that malignant cells are an important source of these chemokines.
Introduction
Effective bacterial anticancer immunotherapies stimulate the proliferation of tumor-associated antigen specific T cells that are capable of overcoming peripheral tolerance. While these cells are indispensable for tumor regression, they are not necessarily sufficient for the therapeutic efficacy of bacterial vaccines.Citation1 Efforts to establish immune correlates of vaccine efficacy have focused on the function and distribution of lymphocytes within tumors. Using TC-1 cells that express type 16 human papillomavirus (HPV-16) E7, Listeria monocytogenes-based vaccines were shown to induce the secretion of pro-inflammatory cytokines, including interleukin (IL)-12 and tumor necrosis factor α (TNFα),Citation2 rely on interferon γ (IFNγ)Citation3,Citation4 and induce tumor regressionCitation4. Such responses correlate with the induction of TH1-promoting, mature dendritic cellsCitation2, the presence of CD4+ and CD8+ T cells and the production of IFNγ.Citation4 Loss of IFNγ sensitivity by malignant cells results in defects in the migration and infiltration of T cells into tumors and renders treatment ineffective.Citation3 Based on these studies, it was proposed that TC-1 derived, IFNγ-driven chemokines play a critical role in vaccine-induced T-cell infiltration into the tumor and effective TH1 responses.Citation3
As discussed in a recent review, chemokine (C-X-C motif) receptor 3 (CXCR3) is typically absent on naïve T cells while it is upregulated during the course of TH1 responses on effector T cells.Citation5 CXCR3 ligands induce tumor regression by inhibiting angiogenesis.Citation6-Citation7 and by orchestrating TH1 responses. Several studies have demonstrated a role for chemokine (C-X-C motif) ligand 9 (CXCL9)Citation8-Citation11 and CXCL10Citation6,Citation10,Citation12,Citation13 in models of tumor suppression. Indeed, therapeutic strategies that induce antitumor chemokine activity have been examinedCitation12 Groom and Luster have recently reviewed the functions of CXCR3 ligands, noting that while whole-organ studies of chemokine expression during inflammatory episodes have frequently been performed, most often these studies did not focus on the identity of the cells responsible for chemokine secretion within the inflammatory environment.Citation14 Recently, Muthuswamy et al. have addressed the roles of fibroblasts and other tumor-infiltrating inflammatory cells in the production of chemokines that recruit effector T cells to malignant lesions.Citation15 Earlier studies conducted in our laboratory indicated that transformed cells initiating oncogenesis also play a significant role in regulating T-cell migration, putatively through IFNγ-dependent chemokine responses.Citation3
Using the transplantable HPV-immortalized murine lung cancer, TC-1, and a Listeria monocytogenes based immunotherapeutic, Lm-LLO-E7, that expresses a hly-E7 fusion gene that is transcribed and secreted as a truncated form of LLO (first 442 residues) fused to E7,Citation4 we investigated the contribution of chemokines secreted by malignant cells to the intratumoral distribution of T cells upon vaccination, finding that Listeria-vaccines can upregulate TC-1 derived CXCR3 ligands that are known to facilitate tumor regression. We also identified and further characterized T cells that migrate to and infiltrate tumors in vaccinated mice. Herein, we show that Lm-LLO-E7 upregulates the production of CXCL9 and CXCL10 by tumor cells, and induces tumor antigen-specific T cells bearing CXCR3, their cognate receptor. CXCL9 expression by TC-1 cells was stimulated by pro-inflammatory cytokines and was selectively inhibited by anti-IFNγ treatment. Finally, we show that CXCL9 derived from TC-1 cells regulates the distribution of CD4+ and CD8+ T cells within the tumor microenvironment.
We conclude that the administration of L. monocytogenes-based anticancer vaccines not only induces antitumor effector T cells but can also remodel the tumor microenvironment from an immunosuppressive to an immunostimulatory state through IFNγ-dependent, chemokine-mediated mechanisms.
Results
Lm-LLO-E7 upregulates TH1-associated chemokines in TC-1 tumors
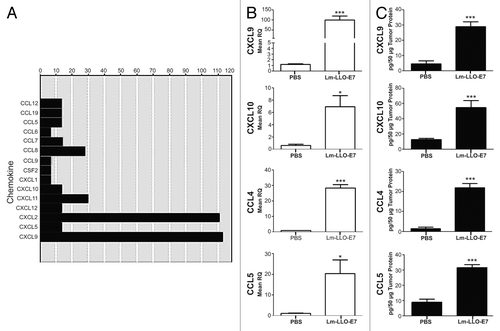
Listerial vaccines have previously been shown to stimulate the production of IFNγ as well as TNFα,Citation2–Citation3 two cytokines that in turn synergize to promote the secretion of TH1 chemokines.Citation14 Thus, we hypothesized that the vaccination of mice bearing TC-1 tumors with Lm-LLO-E7 upregulates the cytokine-dependent production of TH1 chemokines by malignant cells. To gain further insights into this issue, we performed qPCR chemokine arrays and individual primer assays as well as ELISAs on TC-1 tumors harvested from Dulbecco’s PBS (DPBS)- and Lm-LLO-E7-treated mice. To this aim, TC-1 tumor-bearing mice were treated twice with DPBS or Lm-LLO-E7 and tumors were harvested 5 d after the second vaccination, as previously described.Citation16 Chemokine arrays revealed that Lm-LLO-E7 upregulates chemokines that attract myeloid and lymphoid cells, notably CXCL2 (also known as macrophage inflammatory protein 2, MIP2) and CXCL9 respectively (). Among other TH1 chemokines, CXCL10, CXCL11, CCL4 and CCL5 were also moderately increased in TC-1 tumors after vaccination with Lm-LLO-E7 (). We corroborated these observations using individual primer assays (). Of note, elevations in the abundance of transcripts reflected increases in the protein levels of multiple TH1 chemokines, notably CXCL9, CXCL10, chemokine (C-C motif) ligand 4 (CCL4) and CCL5 (). Given the expected lack of CXCL11 expression in C57BL/6 mice,Citation17 CXCL11 was not further considered in our study.
Figure 1. Vaccination with Lm-LLO-E7 induces the expression of chemokines by TC-1 tumors. (A) cDNA from three mice that were treated twice with either Dulbecco’s PBS (DPBS) or Lm-LLO-E7 bacteria were pooled and applied to chemokine PCR arrays. Data are reported as fold changes in the expression of the indicated mRNA species in TC-1 tumors isolated from Lm-LLO-E7-treated mice relative to DPBS-treated animals. Data are from a single experiment conducted once. (B and C) Conventional quantitative RT-PCR assays were conducted on tumor RNA samples (n = 3) (B) and tumor lysates were analyzed using chemokine ELISAs in order to confirm the presence of vaccine-induced TH1 chemokines (CXCL9 and CXCL10, n = 8, pooled from 2 experiments; CCL4 and CCL5, n = 3) (C). Mean RQ (B) or protein concentration values (C) ± SEM are reported (*P≤ 0.05, ** P ≤ 0.01, ***P < 0.001). Conventional quantitative RT-PCR primer assays and ELISAs were conducted twice with similar results.
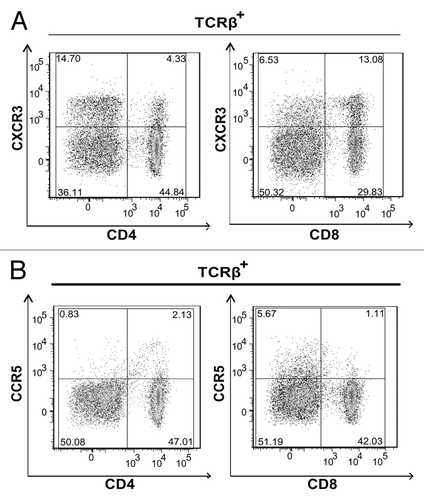
Next, we determined which cognate chemokine receptors were expressed by CD4+ and CD8+ T cells present in the lymph nodes of tumor bearing, vaccinated mice. CXCR3, but not chemokine (C-C motif) receptor 5 (CCR5), was the predominant receptor expressed on CD4+ and CD8+ T cells (). A greater proportion of CD8+ T cells expressed CXCR3 when compared to CD4+ T cells (). Moreover, the majority of E7-specific CD8+ T cells maintained similar chemokine receptor distribution profiles (Fig. S1A). Taken together, these data suggest that CXCR3, as opposed to CCR5, ligands play a dominant role in regulating CD8+ T-cell migration in the tumor microenvironment.
Figure 2. CD4+ and CD8+ T cells express CXCR3. (A and B) Lymph node (LN) cells were isolated from 3–5 tumor bearing mice that were vaccinated with live Lm-LLO-E7 bacteria, pooled and labeled with a panel of antibodies to identify multiple T-subsets. Labeled cells were analyzed by flow cytometry for the expression of CXCR3 and CCR5. Representative density plots are shown. CXCR3+ (A) and CCR5+ (B) T cells were identified among live, single TCRβ+ cells.
CXCL9, but not CXCL10, CCL4 or CCL5, production by malignant cells relies on intact IFNγ signaling induced by Lm-LLO-E7 vaccination
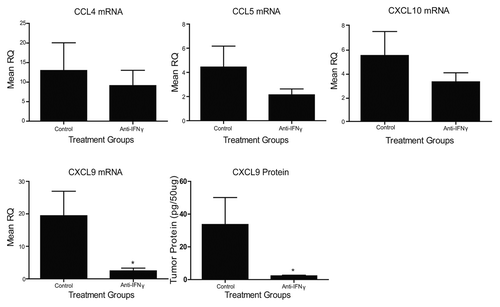
The administration of anti-IFNγ antibodies has previously been shown to inhibit CD4+ and CD8+ T-cell infiltration into TC-1 tumors upon vaccination,Citation3 perhaps owing to the absence of IFNγ-inducible chemokines. Given the profound increases in TH1 chemokines that we observed following the administration of Lm-LLO-E7 to TC-1 tumor-bearing mice, we asked which TH1 chemokines in the tumor microenvironment relies on systemic IFNγ signaling. To address this question, we treated tumor-bearing mice with anti-IFNγ antibodies or control IgG, as previously described.Citation3 Both groups of mice were then vaccinated with Lm-LLO-E7 and TC-1 tumors were harvested 7 d later. Anti-IFNγ treatment had no effect on the expression of CCL4, CCL5 or CXCL10 transcripts (), suggesting that these chemokines–at least in the TC-1 tumor microenvironment–are regulated in an IFNγ-independent manner. Conversely, anti-IFNγ antibody treatments significantly decreased levels of both CXCL9 mRNA and protein (). Thus, IFNγ is critical for the Lm-LLO-E7-mediated induction of CXCL9, but not other TH1 chemokines, consistent with observations made in other experimental models.Citation14
Figure 3. Vaccine-induced chemokine expression is affected by anti-interferon γ antibody administration. TC-1 tumor-bearing mice (n = 3-5 mice per group) were treated with anti-interferon γ (IFNγ) or IgG control antibodies and vaccinated with Lm-LLO-E7 bacteria. CCL4, CCL5, CXCL9 and CXCL10 expression levels were quantified by quantitative RT-PCR or ELISA, as indicated. Mean RQ or protein concentration values ± SEM are reported (*p ≤ 0.05). These experiments were conducted twice with similar results.
IFNγ upregulates TC-1 cell-derived chemokines
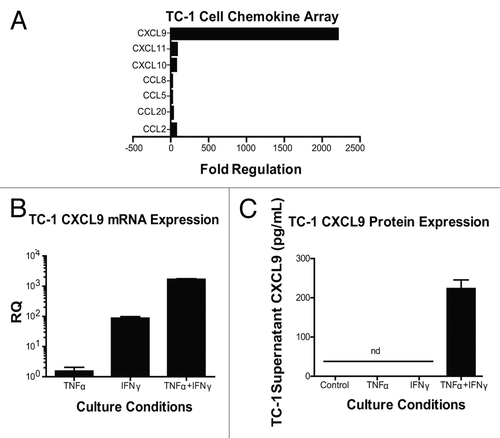
Chemokines that are found in the tumor microenvironment are likely derived from immune cells as well as non-immune cells. Previous studies have demonstrated that IFNγ signaling within implanted TC-1 cells is important for T-cell infiltration into TC-1 tumors and required for the efficacy of listerial vaccines.Citation3 We thus asked whether or not TC-1 tumor cells themselves could respond to IFNγ by upregulating and secreting IFNγ-dependent T cell chemoattractants. To gain further insights into this issue, we stimulated TC-1 tumor cells with IFNγ alone or in combination with TNFα. We included TNFα in these assays as (1) it is also produced in response to vaccination,Citation2 and (2) it is known to regulate IFNγ signalingCitation18,Citation19 Stimulation of TC-1 cells with both IFNγ and TNFα upregulated a multitude of chemokines, especially CXCL9 (). The production of CXCL9 by TC-1 tumor cells appeared to be primarily regulated by IFNγ, since IFNγ alone induced a 100-fold increase in the abundance of CXCL9 transcripts whereas TNFα alone had negligible effects (). However, the administration of both cytokines amplified the response by another 10-fold over that seen with IFNγ only (). TNFα also synergized with IFNγ at inducing detectable levels of CXCL9 protein (). These data suggest that TC-1 cells are capable of producing TH1 chemokines, especially CXCL9. TC-1 cells are not unique in their capacity to produce CXCL9 in response to pro-inflammatory cytokines, as several malignant cell lines stimulated with IFNγ plus TNFα did so as well (Fig. S2). These observations suggest that our findings regarding TC-1 tumors may be broadly applicable to other tumor models.
Figure 4. Interferon γ induces the expression of chemokines by - cells. (A–C) Samples from TC-1 cells that were maintained under control conditions or cultured with tumor necrosis factor α (TNFα) and interferon γ (IFNγ), were collected and then processed for chemokine PCR arrays (A), CXCL9-specific quantitative RT-PCR assays (B) or CXCL9-specific ELISAs (C). In (A and B) data are reported as fold changes in mRNA expression relative to cells maintained in control conditions. Mean RQ or protein concentration values ± SEM (of 2 independent experiments) are reported in (B and C) respectively. nd, not detected.
TC-1 cell derived CXCL9 alters the distribution of various T-cell subsets within tumors.
Given that the administration of a listerial vaccine to TC-1 tumor-bearing mice induces the production of CXCL9 in the tumor microenvironment in an IFNγ-dependent manner and that TC-1 cells produce CXCL9 in response to IFNγ, we investigated the contribution of TC-1 cell-derived CXCL9 to the intratumoral distribution of T cells in vivo. To address this aim, we inhibited the ability of TC-1 cells to express CXCL9 by means of a short-hairpin RNA (shRNA). We also generated TC-1 cells containing a control plasmid. We implanted TC-1 cells bearing the control vector or CXCL9-shRNA in basement membrane exctract (BME) in order to form tumor plugs (TC-1-BME). We then analyzed the distribution of T cell subsets that bear the CXCL9 receptor CXCR3 seven days after a single dose of Lm-LLO-E7, in keeping with a previously established protocol.Citation3 Surprisingly, the proportions of total CXCR3+ T lymphocytes infiltrating TC-1-BME plugs, which were generated with cells expressing a control plasmid or a CXCL9-silencing plasmid, were similar (). However, a deficiency in CXCL9 expression led to a significant decrease in the proportion of CXCR3+CD8+ T cells along with a concomitant increase in the proportion of CXCR3+CD4+ T cells (), thus resulting in a significant increase in the CD4+:CD8+ T-cell ratio (). Thus, we conclude that CXCL9 produced by implanted TC-1 cells regulates the migration of CXCR3-bearing CD8+ T cells in the tumor microenvironment.
Figure 5. Knockdown of CXCL9 in TC-1 cells alters T-cell subset distribution in the tumor plugs of vaccinated mice. (A–C) TC-1 cells bearing a control plasmid or a shRNA plasmid for the downregulation of CXCL9 were co-injected with basement membrane extract (BME) and allowed to form plugs for 7 d. On day 7, mice were vaccinated with Lm-LLO-E7 bacteria and, 7 d later, tumor plugs were removed and processed for the cytofluorometric measurement of the percentages of CXCR3+TCRβ+ as well as CD8+CXCR3+TCRβ+ and CD4+CXCR3+TCRβ+ T lymphocytes (among live single cells). The percentage of CXCR3+TCRβ+ cells is reported in panel (A) whereas representative density plots of the relative abundance of CD8+CXCR3+TCRβ+ and CD4+CXCR3+TCRβ+ T lymphocytes is depicted in (B) In (C) quantitative data on the percentages of CD8+CXCR3+TCRβ+ and CD4+CXCR3+TCRβ+ T cells as well as on their ratio are reported. In (A and C) columns represent mean values ± SEM of pooled data (n = 7) from two independent experiments (2–5 mice per group) (*P ≤ 0.05, **P ≤ 0.01, ***P < 0.001).
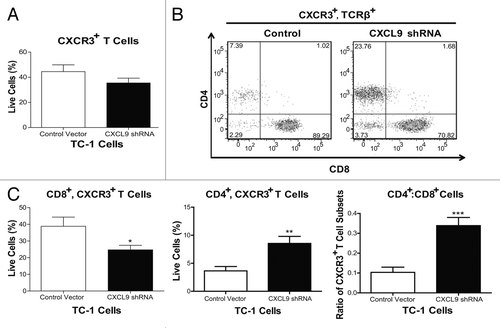
Lm-LLO-E7 elicits E7-specific CXCR3+CD8+ T cells as well as L. monocytogenes antigen-specific CD4+ T cells
The efficacy of listerial vaccines has previously been shown to rely on the presence of tumor-associated antigen-specific CD8+ T cells.Citation4 Given that CXCR3 is upregulated on activated effector T cells during TH1 responses,Citation5 we hypothesized that vaccine-induced E7-specific CD8+ T cells would express CXCR3. Of note, although only a minority of total CD8+ T cells expressed CXCR3, the majority of E7-specific CD8+ T cells expressed CXCR3 upon vaccination (Fig. S1A, bottom left panel). Such E7-specific CD8+ T cells did not express CCR5 (Fig. S1A, top left panel), lending further support to the limited role of tumor-derived CCR5 ligands in the elevation of intratumoral E7-specific CD8+ T cells upon vaccination. The knock down of CXCL9 in TC-1 cells resulted in > 30% decrease in the proportion of CXCR3+CD8+ T cells infiltrating TC-1-BME plugs. This reduction was a statistically significant, albeit minor, decrease in the proportion of CXCR3+CD8+ T cells; however, given the percentages of tumor antigen specific cells that typically develop following treatment, such reductions may exert a profound impact on tumor regression. Our data also point to a concomitant increase in the proportion of CD4+ T cells (). Additional analyses subdivided the CXCR3+CD4+ T-cell population into FoxP3+ and FoxP3- subsets (Fig. S1A, bottom right panel) that are likely to respond to CXCR3 gradients established by tumors in vaccinated animals. CCR5 was also expressed to lesser extents by a minor percentage of both FoxP3+ and FoxP3- CD4+ T cells (Fig. S1A, top right panel). Subsequent studies of effector CD4+ T cells showed them to be largely LLO- (and not E7-) specific as LLO-sensitive CD4+ T cells secreted IFNγ upon re-stimulation with a MHC class II-restricted LLO-derived peptide (Fig. S1B). Additional studies are underway to characterize the impact of the chemokine milieu on effector cell populations as well as the physiological relevance of LLO-specific CD4+ T cells.
Discussion
L. monocytogenes-based anticancer vaccines promote the generation of tumor-specific cytotoxic T lymphocytes that are necessary for their therapeutic activity.Citation4 Moreover, both the infiltration of tumors by T cells and the efficacy of listerial vaccines are dependent on the production of IFNγ as well as on the sensitivity of malignant cells to its effects, at least in the TC-1 tumor model.Citation3 The accumulation of lymphocytes in inflamed tissues and tumors is governed by temporally and spatially regulated CXCR3-ligand responsesCitation14 that may be generated by transformed cells. CXCR3 is upregulated during the course of TH1 responses on effector T cells following activation,Citation5 and CXCR3 ligands are known to contribute to T-cell migration in models of renal cancer,Citation20 melanoma, Citation21 and colorectal cancer.Citation22 In the current study, we investigated IFNγ-dependent chemokine responses that are induced by Lm-LLO-E7. We hypothesized that such a listerial vaccine elicits the IFNγ-dependent production of T-cell chemoattractants required for the optimal infiltration of tumors by CD8+ T cells. We have shown that the same vaccination protocol used in previous studies to induce tumor regressionCitation3,Citation4 also alters the proportion of intratumoral T-cell subsets and induces the secretion of chemoattractants for effector T cells. The ability of TC-1 cells to secrete CXCL9, but not other chemokines elicited by Lm-LLO-E7, appeared to be regulated by IFNγ as it was 1) highly upregulated by pro-inflammatory cytokines, in vitro, 2) upregulated within tumors in response to vaccination and 3) significantly inhibited in vaccinated mice treated with anti-IFNγ antibodies.
CXCL9 inhibits tumor growth by inducing vascular damageCitation7 and by recruiting effector T cells to tumors.Citation10 In a recent study, CXCL9-deficient cancer cells were shown to grow more aggressively as compared with their CXCL9-sufficient counterparts, and the reintroduction of CXCL9 into these cells led to T-cell dependent tumor rejection,Citation8 suggesting that CXCL9 is critical for antitumor T-cell functions, at least in some tumor models. We hypothesized that TC-1 cell-derived CXCL9 is necessary for the optimal infiltration of tumors by CD8+ T lymphocytes. In previous studies, the inhibition of IFNγ signaling in TC-1 cells has been shown to reduce the amount of intratumoral T cells.Citation3 Our results suggest that the effects of inhibiting a single chemokine, namely CXCL9, are more subtle than completely blocking T-cell infiltration. In our study, a CXCL9 deficiency in TC-1 cells manifested as an increase in the overall CD4+:CD8+ T-cell ratio, reflecting a combined decrease in CXCR3+CD8+ T cells and increase in CXCR3+CD4+ T cells. The expression of CXCR3 by these cells would suggest an activated state, allowing us to propose that transformed cell-derived CXCL9 is important for the migration of activated CD8+ T cells to the tumor. Additional studies are underway to determine the antigen specificity of these cells at later time points. Observations from the current study suggest that these cells also recognize E7.
The alterations in the intratumoral distribution of T-cell subsets observed in our studies presumably originate from an imbalance in the otherwise finely orchestrated chemokine response that normally arises during immunotherapy. Our listerial vaccine also induced CXCL10 as well as CCR5 ligands, two groups of chemokines that, together with CXCL9, may work in concert to position distinct T-cell subsets within tumors, hence facilitating tumor regression. These chemokines are produced by a variety of cells that compose tumors,Citation15 which may explain the limited—albeit significant—impact on CD8+ T-cell infiltration observed in our studies. Inhibition of TC-1-cell derived CXCL9 significantly altered the composition of CXCR3+ T cells in BME plugs (). However, these effects were not complete, indicating that either another source of CXCL9 in the tumor or intact CXCL10 responses may offset, at least in part, deficits associated with the silencing of CXCL9 in implanted cells. Many chemokines synergize, cooperate or antagonize each other in a non-redundant, context dependent manner Citation14,Citation23 and a deficiency or an imbalance in this system may alter the recruitment of T cells to tumors. In our model, altered chemokine expression patterns resulting from the silencing of CXCL9 in implanted cells led to an increase in the CD4+:CD8+ T-cell ratio in TC-1 tumor plugs upon vaccination. These alterations may cause reductions in antigen specific CTL as well as proportional increases in regulatory and conventional CD4+ T cells. Additional studies are underway to further characterize the impact of the chemokine balance on effector cell populations.
The ability of tumor-initiating cells to respond to inflammatory cues may dictate therapeutic outcome and should be carefully considered during the design of novel therapeutic regimens. Furthermore, effective cancer immunotherapy should not only elicit antitumor T cells but should also drive critical cytokine and chemokine responses. Strategies involving chemokine-inducing chemotherapeutic approaches have been shown to synergize with immunotherapy at inducing tumor regressionCitation24. L. monocytogenes-based immunotherapeutic vaccines are under clinical development for the treatment of cancer.Citation25 The present work, coupled with earlier studies,Citation1-Citation4highlight multiple benefits stemming from the use of multifunctional listerial anticancer vaccines. These agents promote robust antitumor cellular responses as they elicit 1) beneficial pro-inflammatory responses, 2) antigen-specific T lymphoctyes and 3) chemokine gradients that these cells require to migrate. Such chemokine responses should add useful immune correlates of vaccine efficacy in several tumor types and across a variety of tissues.
Materials and Methods
Tumor cell lines
TC-1 cells (ATCC, CRL-2785), which have been derived from a murine lung cell,Citation26 were cultured as previously described.Citation16 CXCL9 knock down TC-1 cells were prepared by means of a specific shRNA according to the manufacturer’s protocol (SABiosciences, KM02973P). Briefly, TC-1 cells were transfected with a negative control or a CXCL9-specific shRNA-coding plasmid and initially selected using 4 µg/mL puromycin. Following selection, plasmid-bearing cells were maintained in 1.5 µg/mL puromycin prior to implantation. Inhibition of CXCL9 expression at the mRNA level (~70%) was confirmed by qPCR analyses, as described below.
Murine colorectal carcinoma CT26 cellsCitation27 and renal cell carcinoma Renca cellsCitation28 are derived from BALB/c mice. CT26mugR cell line is a variant of the CT26 cell line that was rendered insensitive to IFNγ.Citation29 The FVB/N syngeneic NT-2 mammary tumor cell line was developed as previously described.Citation30 4T1 cells are derived from a mouse mammary tumor virus (MMTV)-induced mammary carcinoma.Citation31–Citation32 All cell lines were maintained in standard culture conditions.
Animal experiments
Two-hundred fifty thousand TC-1 cells were implanted s.c. in the flank of 6–8-wk-old, female C57BL/6 mice (Jackson Laboratories, 000664). Mice were injected i.p. with 1 × 108 colony forming units (CFU) of live Lm-LLO-E7 or DPBS when tumors reached a size of 3–4 mm. Tumor growth was quantified as previously described.Citation4 Mice were treated with a single dose or 2 equivalent doses of Lm-LLO-E7 at 1 week intervals. When appropriate, TC-1 tumor-bearing mice were vaccinated with Lm-LLO-E7 combined with the administration of 1.2 mg anti-IFNγ XMG1.2 antibodies (BioXcell, BE0055) or control antibodies (anti-β-galactosidase or anti-HRPN; BioXcell, BE0088) on days −1, 0, +1 and +4 (relative to vaccination). Five-to-seven days following the first or second immunization, animals were sacrificed and lymph nodes (axillary and brachial), tumors and/or spleens were harvested and processed, or frozen at −80 °C for further evaluation.
Three million control or CXCL9 knock down TC-1 cells were co-implanted with 400 µL BME (Cultrex PathClear® HC20+, Trevigen, 3444–005–02) s.c. in the flanks of 6–8-week old, female C57BL/6 mice, a strategy that facilitates the analyses of tumor infiltrates.Citation33 TC-1 cells were allowed to form tumor plugs with BME (TC-1-BME) for 1 week. Mice were then immunized with 1 × 108 Lm-LLO-E7 or treated with DPBS once i.p. TC-1 BME plugs were removed and processed for cytofluorometric analyses one week later, as described below. All mouse experiments were performed according to the regulations of the Institutional Animal Care and Use Committee of the University of Pennsylvania.
RNA isolation, chemokine mRNA expression and quantitative PCR
The expression of chemokine-coding mRNA by the aforementioned cell lines maintained in control conditions or stimulated with 10 ng/mL IFNγ and/or 10 ng/mL TNFα was measured by quantitative RT-PCR. To this end, total RNA was isolated from cryopulverized TC-1 tumors or cultured tumor cells using a commercial kit (Qiagen Inc., 74134), according to the manufacturer’s protocol, and converted into cDNA using reverse transcription kits (SABiosciences, C-03 or ABI Applied Biosystems, 4375575). cDNA was amplified using gene specific primers (SABiosciences, CCL4: PPM02948F-200, CCL5: PPM02960E-200, CXCL9: PPM02973A-200, CXCL10: PPM02978D-200) or applied to chemokine PCR arrays following manufacturer’s instructions (SABiosciences, PAMM-022C). Quantitative PCR (ABI Applied Biosystems, StepOne Plus) was conducted using SYBR Green Master Mix (SABiosciences, PA-012 or ABI systems, 4309155). Data were analyzed using the ABI StepOne software (ABI Applied Biosystems) or SABiosciences online analysis programs (SABiosciences). Individual primer assays and PCR arrays were analyzed using the comparative CT method and individual primer assays were normalized based on HSP90AB1 expression levels (SABiosciences, HSP90AB1: PPM04803E-200).
ELISA
TC-1 tumors were frozen, cryopulverized and resuspended in tissue lysis bufferCitation34 in the presence of protease inhibitors (Roche Diagnostics, 11 836 170 001). Tumor lysates were freeze-thawed twice, disrupted with a tissue homogenizer and centrifuged to collect supernatants. Protein content was determined and normalized using the Bio-Rad Protein Assay Reagent according to the manufacturer’s protocol (Bio-Rad, 500–0006). Supernatants were collected from TC-1 cells stimulated with IFNγ and/or TNFα, centrifuged to remove cells and debris and stored at −80 °C until analysis. Tumor lysates were equilibrated for total protein content using bovine serum albumin (BSA) and 50 µg per sample were used in sandwich ELISAs for chemokine detection (overnight incubation, 4 °C). Equal volumes of TC-1 supernatants from experimental and control conditions were similarly incubated at 4°C, overnight. All samples were analyzed for the presence of chemokines according to the manufacturer’s protocol (R&D Systems: CCL4/MIP-1β: MMB00, CCL5/RANTES: MMR00, CXCL9/MIG: MCX900, CXCL10/IP-10: MCX100). Background optical density (OD) values recorded at 570 nm were subtracted from OD values recorded at 450 nm and adjusted for protein concentration based on standard curves.
Cell isolation and cytofluorometric analyses
The lymph nodes, spleens, tumors and TC-1-BME plugs were dissected from DPBS- or Lm-LLO-E7 treated mice. Spleens and lymph nodes were mechanically dissociated in cold FACS buffer (1× DPBS supplemented with 0.5% heat inactivated fetal bovine serum). TC-1-BME tumor plugs were ground and filtered through a 100 µm mesh filter. Red blood cells were removed by lysis in ACK buffer (Lonza, 10–548E). tumors were dissected and subjected to enzymatic digestion in cRPMI medium containing 2 mg/mL collagenase P (Roche Diagnostics, 11 249 002 001), 1 mg/mL DNase I (Roche Diagnostics, 10 104 159 001) and 50 μg/mL protease inhibitors (Sigma, T9253) for 30 min at 37°C. Upon digestion, tumor samples were ground and filtered through a 100 µm mesh filter, the enzymatic buffer was removed by centrifugation, and red blood cells were lysed in ACK buffer. Lymph node cells and tumor cells were incubated with an Fc-blocking reagent (CD16/CD32 Fcγ II/III, clone 93) (eBiosciences, 16–0161–82) followed by biotin-conjugated or fluorochrome-conjugated primary antibodies. Cells were labeled with antibodies specific for TCRβ, clone H57–597 (eBiosciences, 47–5961); CD8a, clone 53–6.7 (eBiosciences, 48–0081); CD8b, clone eBioH35–17.2 (eBiosciences, 53–0083); CD4, clone GK1.5, (eBiosciences, 53–0041–82); FoxP3, cloneFJK-16s (eBiosciences, 12–5773); CCR5, clone CTC5 (R&D Systems, FAB1802F); CXCR3, clone CXCR3–173 (Biolegend, 126512) and/or MHC class I E7-specific tetramers (NIH Tetramer Core Facility, Atlanta, GA or Beckman Coulter, Inc., T03012) in Fc block. Dead cells were identified using the Aqua Blue Dead Cell Exclusion Dye (Invitrogen, L34957). Cells were fixed with fixation/permeabilization buffer (eBiosciences, 00–5521–00) and washed with FACS buffer or permeabilization buffer (eBiosciences, 00–8333). Cells analyzed for FoxP3 were fixed and permeabilized overnight according to the manufacturer’s protocol, and only thereafter incubated with anti-FoxP3 antibodies. Cells were washed prior to cytofluorometric acquisition. Regulatory T cells (Tregs) were defined as TCRβ+CD4+FoxP3+ cells. Conventional T cells (Tconvs) were defined as TCRβ+CD4+FoxP3- cells. E7-CD8+ cells were defined as TCRβ+ CD8b+ E7 tetramer-binding cells. Acquisition was performed using a flowcytometer and FACSDiva software (LSRII BD Biosciences). Cytofluorometric data was processed and analyzed using the FLOWJO software (Tree Star Inc., Ashland, OR).
IFNγ-specific ELISPOT assays
Splenocytes from DPBS- or Lm-LLO-E7-treated mice were isolated as described above. One hundred thousand CD4+ cells, isolated using a CD4+ isolation kit (Miltenyi Biotec, 130–095–248), were incubated with antigen-presenting cells in the presence of 50 U/mL recombinant human IL-2 (R&D Systems, 202-IL-010) and Listerial MHC II epitope LLO190–201 (NEKYAQAYPNVS)Citation35or a MHC class II-restricted E7 epitope (DRAHYNI)Citation36 (GenWay Biotech, Inc.) at 37 °C in 7% CO2. On day 4, cells were collected and added to an ELISpot plate coated with anti-IFNγ capture antibody (Mabtech, Inc., mAb AN18, 3321–3-1000) for 4 h at 37°C in 7% CO2. Cells were then removed and plates were processed according to the manufacturer’s protocol, using a specific detection antibody (Mabtech, Inc., mAb R4–6A2-Biotin, 3321–6-1000). The plates were developed with ExtrAvidin-Alkaline Phosphatase (Sigma-Aldrich, E2636-.2ML, 120M4789) using nitro-blue tetrazolium chloride/5-bromo-4-chloro-3′-indoly phosphate phosphate toluidine salt (NBT/BCIP) as substrate (Sigma-Aldrich, 72091). The colorimetric reaction was terminated with 1M NaH2PO4. Spots were enumerated on a CTL-ImmunoSpot Reader (Cellular Technology Limited).
Statistical analyses
Data obtained from control groups and treatment groups were subjected to t tests with significant differences reported as follows: *P ≤ 0.05, **P ≤ 0.01, ***P < 0.001. Differences among multiple groups were statistically analyzed using ANOVA and means were compared using the Bonferroni post-hoc test only when ANOVA P was < 0.05 (GraphPad Prism v.4.0, GraphPad Software Inc.).
Acknowledgments
We wish to acknowledge Dr Zhen-Kun Pan for providing technical assistance. This work was supported by NIH RO1 CA69632 (YP), NIH T32AI060516–03 (PG), NIH K12GM081259 (PG) and NIH T32CA009140 (PG).
| Abbreviations: | ||
| BSA | = | bovine serum albumin |
| IFNγ | = | interferon γ |
| shRNA | = | short hairpin RNA |
| TNFα | = | tumor necrosis factor α |
Additional material
Download Zip (250 KB)Disclosure of Potential Conflicts of Interest
YP wishes to disclose that she has a financial interest in Advaxis Inc., a publicly-traded immunotherapy company that has licensed or has an option to license all patents from the University of Pennsylvania that concern the use of Listeria monocytogenes or listerial products as immunotherapies. All other authors do not have any conflict of interest.
References
- Hussain SF, Paterson Y. What is needed for effective antitumor immunotherapy? Lessons learned using Listeria monocytogenes as a live vector for HPV-associated tumors. [doi] Cancer Immunol Immunother 2005; 54:577 - 86; http://dx.doi.org/10.1007/s00262-004-0600-2; PMID: 15650885
- Peng X, Hussain SF, Paterson Y. The ability of two Listeria monocytogenes vaccines targeting human papillomavirus-16 E7 to induce an antitumor response correlates with myeloid dendritic cell function. J Immunol 2004; 172:6030 - 8; PMID: 15128786
- Dominiecki ME, Beatty GL, Pan ZK, Neeson P, Paterson Y. Tumor sensitivity to IFN-gamma is required for successful antigen-specific immunotherapy of a transplantable mouse tumor model for HPV-transformed tumors. [doi] Cancer Immunol Immunother 2005; 54:477 - 88; http://dx.doi.org/10.1007/s00262-004-0610-0; PMID: 15750832
- Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol 2001; 167:6471 - 9; PMID: 11714814
- Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res 2011; 317:620 - 31; http://dx.doi.org/10.1016/j.yexcr.2010.12.017; PMID: 21376175
- Sgadari C, Angiolillo AL, Cherney BW, Pike SE, Farber JM, Koniaris LG, et al. Interferon-inducible protein-10 identified as a mediator of tumor necrosis in vivo. Proc Natl Acad Sci USA 1996; 93:13791 - 6; http://dx.doi.org/10.1073/pnas.93.24.13791; PMID: 8943014
- Sgadari C, Farber JM, Angiolillo AL, Liao F, Teruya-Feldstein J, Burd PR, et al. Mig, the monokine induced by interferon-gamma, promotes tumor necrosis in vivo. Blood 1997; 89:2635 - 43; PMID: 9108380
- Gorbachev AV, Kobayashi H, Kudo D, Tannenbaum CS, Finke JH, Shu S, et al. CXC chemokine ligand 9/monokine induced by IFN-gamma production by tumor cells is critical for T cell-mediated suppression of cutaneous tumors. J Immunol 2007; 178:2278 - 86; PMID: 17277133
- Ruehlmann JM, Xiang R, Niethammer AG, Ba Y, Pertl U, Dolman CS, et al. MIG (CXCL9) chemokine gene therapy combines with antibody-cytokine fusion protein to suppress growth and dissemination of murine colon carcinoma. Cancer Res 2001; 61:8498 - 503; PMID: 11731434
- Tannenbaum CS, Tubbs R, Armstrong D, Finke JH, Bukowski RM, Hamilton TA. The CXC chemokines IP-10 and Mig are necessary for IL-12-mediated regression of the mouse RENCA tumor. J Immunol 1998; 161:927 - 32; PMID: 9670971
- Dorsey R, Kundu N, Yang Q, Tannenbaum CS, Sun H, Hamilton TA, et al. Immunotherapy with interleukin-10 depends on the CXC chemokines inducible protein-10 and monokine induced by IFN-gamma. Cancer Res 2002; 62:2606 - 10; PMID: 11980656
- Narvaiza I, Mazzolini G, Barajas M, Duarte M, Zaratiegui M, Qian C, et al. Intratumoral coinjection of two adenoviruses, one encoding the chemokine IFN-gamma-inducible protein-10 and another encoding IL-12, results in marked antitumoral synergy. J Immunol 2000; 164:3112 - 22; PMID: 10706701
- Pertl U, Luster AD, Varki NM, Homann D, Gaedicke G, Reisfeld RA, et al. IFN-gamma-inducible protein-10 is essential for the generation of a protective tumor-specific CD8 T cell response induced by single-chain IL-12 gene therapy. J Immunol 2001; 166:6944 - 51; PMID: 11359856
- Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol 2011; 89:207 - 15; http://dx.doi.org/10.1038/icb.2010.158; PMID: 21221121
- Muthuswamy R, Berk E, Junecko BF, Zeh HJ, Zureikat AH, Normolle D, et al. NF-κB hyperactivation in tumor tissues allows tumor-selective reprogramming of the chemokine microenvironment to enhance the recruitment of cytolytic T effector cells. Cancer Res 2012;; 72:3735 - 43; http://dx.doi.org/10.1158/0008-5472.CAN-11-4136; PMID: 22593190
- Guirnalda P, Paterson Y. Vaccination with immunotherapeutic Listeria monocytogenes induces IL-17+ γδ T cells in a murine model for HPV associated cancer. OncoImmunology 2012; 1:822 - 828; http://dx.doi.org/10.4161/onci.20491
- Carr DJ, Wuest T, Ash J. An increase in herpes simplex virus type 1 in the anterior segment of the eye is linked to a deficiency in NK cell infiltration in mice deficient in CXCR3. [doi] J Interferon Cytokine Res 2008; 28:245 - 51; http://dx.doi.org/10.1089/jir.2007.0110; PMID: 18439102
- Raitano AB, Scuderi P, Korc M. Upregulation of interferon-gamma binding by tumor necrosis factor and lymphotoxin: disparate potencies of the cytokines and modulation of their effects by phorbol ester. J Interferon Res 1991; 11:61 - 7; http://dx.doi.org/10.1089/jir.1991.11.61; PMID: 1903142
- Raitano AB, Korc M. Tumor necrosis factor up-regulates gamma-interferon binding in a human carcinoma cell line. J Biol Chem 1990; 265:10466 - 72; PMID: 2141333
- Kondo T, Nakazawa H, Ito F, Hashimoto Y, Osaka Y, Futatsuyama K, et al. Favorable prognosis of renal cell carcinoma with increased expression of chemokines associated with a Th1-type immune response. Cancer Sci 2006; 97:780 - 6; http://dx.doi.org/10.1111/j.1349-7006.2006.00231.x; PMID: 16863511
- Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res 2009; 69:3077 - 85; http://dx.doi.org/10.1158/0008-5472.CAN-08-2281; PMID: 19293190
- Mlecnik B, Tosolini M, Charoentong P, Kirilovsky A, Bindea G, Berger A, et al. Biomolecular network reconstruction identifies T-cell homing factors associated with survival in colorectal cancer. Gastroenterology 2010; 138:1429 - 40; http://dx.doi.org/10.1053/j.gastro.2009.10.057; PMID: 19909745
- Hong M, Puaux AL, Huang C, Loumagne L, Tow C, Mackay C, Kato M, Prevost-Blondel A, Avril MF, Nardin A, Abastado JP. Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T-cell infiltration and tumor control. Cancer Res:2011; 71:22 6997 - 7009
- Huang H, Liu Y, Xiang J. Synergistic effect of adoptive T-cell therapy and intratumoral interferon gamma-inducible protein-10 transgene expression in treatment of established tumors. Cell Immunol 2002; 217:12 - 22; http://dx.doi.org/10.1016/S0008-8749(02)00508-7; PMID: 12425997
- Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a Phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine 2009; 27:3975 - 83; http://dx.doi.org/10.1016/j.vaccine.2009.04.041; PMID: 19389451
- Lin KY, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res 1996; 56:21 - 6; PMID: 8548765
- Fearon ER, Itaya T, Hunt B, Vogelstein B, Frost P. Induction in a murine tumor of immunogenic tumor variants by transfection with a foreign gene. Cancer Res 1988; 48:2975 - 80; PMID: 3259156
- Murphy GP, Hrushesky WJ. A murine renal cell carcinoma. J Natl Cancer Inst 1973; 50:1013 - 25; PMID: 4703766
- Beatty GL, Paterson Y. IFN-gamma can promote tumor evasion of the immune system in vivo by down-regulating cellular levels of an endogenous tumor antigen. J Immunol 2000; 165:5502 - 8; PMID: 11067903
- Reilly RT, Gottlieb MB, Ercolini AM, Machiels JP, Kane CE, Okoye FI, et al. HER-2/neu is a tumor rejection target in tolerized HER-2/neu transgenic mice. Cancer Res 2000; 60:3569 - 76; PMID: 10910070
- Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res 1992; 52:1399 - 405; PMID: 1540948
- Pulaski BA, Ostrand-Rosenberg S. Mouse 4T1 breast tumor model. Curr Protoc Immunol 2001; Chapter 20:Unit 20 22. http://dx.doi.org/10.1002/0471142735.im2002s39
- Kowalczyk DW, Wlazlo AP, Blaszczyk-Thurin M, Xiang ZQ, Giles-Davis W, Ertl HC. A method that allows easy characterization of tumor-infiltrating lymphocytes. J Immunol Methods 2001; 253:163 - 75; http://dx.doi.org/10.1016/S0022-1759(01)00356-8; PMID: 11384678
- Darnell JC, Albert ML, Darnell RB. Cdr2, a target antigen of naturally occuring human tumor immunity, is widely expressed in gynecological tumors. Cancer Res 2000; 60:2136 - 9; PMID: 10786675
- Krawczyk CM, Shen H, Pearce EJ. Memory CD4 T cells enhance primary CD8 T-cell responses. Infect Immun 2007; 75:3556 - 60; http://dx.doi.org/10.1128/IAI.00086-07; PMID: 17438031
- Tindle RW, Fernando GJ, Sterling JC, Frazer IH. A “public” T-helper epitope of the E7 transforming protein of human papillomavirus 16 provides cognate help for several E7 B-cell epitopes from cervical cancer-associated human papillomavirus genotypes. Proc Natl Acad Sci U S A 1991; 88:5887 - 91; http://dx.doi.org/10.1073/pnas.88.13.5887; PMID: 1712110