Abstract
There is much promise in the use of immunotherapy for the treatment of cancer. Approaches such as those using antibodies or adoptive cell transfer can mediate complete tumor regression in a proportion of patients. However, the tumor microenvironment can inhibit immune responses leading to ineffective or suboptimal responses of tumors to immunotherapy in the majority of cases. As our knowledge of the tumor microenvironment increases, many strategies are emerging for changing the immunosuppressive nature of the tumor toward a microenvironment able to support immunity. These strategies aim to enhance the ability of immunotherapies to initiate effective immune responses able to destroy tumors. In this article, we review approaches that use immunomodulators specifically to modify the tumor microenvironment, and their use in combination with other immune-based strategies for cancer therapy.
Introduction
Immunotherapy holds much promise for the treatment of cancer. A wide variety of approaches have been implemented in order to stimulate a range of immune activities including innate and adaptive components. Strategies include the use of immunomodulatory antibodies, vaccines and adoptive cell transfer. Notable clinical successes include the use of the immune check-point inhibitor, ipilimumabCitation1 for melanoma, and rituximab targeting CD20 for lymphoma.Citation2 Adoptive immunotherapy, involving transfer of ex vivo activated autologous T cells, is also showing promise for the treatment of melanoma.Citation3
However, most immunotherapeutic approaches on their own are of limited value against the majority of malignancies. Reasons for this limited success include immune regulation mediated by cancer cells and leukocyte populations through a variety of cell-expressed and secreted molecules. In many cases, immune regulation occurs locally within the tumor, leading to an ineffectual or suppressed antitumor response.
Tumors are not just a mass of proliferating genetically abnormal cells, but they are now well defined as a heterogeneous and structurally complex tissue. Malignant tumor cells can recruit a variety of cell types, including fibroblasts, immune inflammatory cells, and endothelial cells, through production and secretion of stimulatory growth factors and cytokines.Citation4 This assortment of cells and molecules together comprises the tumor microenvironment.
Antitumor immunity within the tumor microenvironment can be suppressed by a variety of tumor infiltrating leukocytes, including regulatory T cells (Treg), myeloid-derived suppressor cells (MDSC) and alternatively activated (type 2) macrophages (M2).Citation5-Citation7 Mechanisms employed by these cell types to suppress effective immunity include secretion of cytokines such as IL-10 and TGF-β, and expression of inhibitory receptors such as CTLA-4 and PD-L1. Secretion of amino acid-depleting enzymes including arginase and IDO by these cell types in the microenvironment can also negatively impact on tumor immunity.
In addition to these effects mediated by infiltrating cells, tumor cells themselves can actively inhibit immunity through a number of mechanisms. Malignant cells can block T cell function through secretion of soluble forms of ligands for effector molecules, as reported for shed ligands of NKG2D; MICA and MICB.Citation8 Additionally, cytokines released by tumor cells, such as VEGF and TGF-β can inhibit T cell recognition and destruction of malignant cells.Citation9 IL-10 as well, can skew T cell responses toward a type 2 immune response that is less effective against tumor cells.Citation10 Other secreted factors such as galectins can also impede T cell activity and survival.Citation11
Many of these regulatory mechanisms can occur concurrently within the tumor microenvironment resulting in multiple redundant levels of immune suppression, which reduces the effectiveness of immunotherapy. Not surprisingly then, the tumor microenvironment can impede immunotherapy, and approaches to specifically reduce immune suppression within the tumor microenvironment are gaining momentum as a companion to additional immunotherapy. This review focuses on immune-based strategies to change the microenvironment to enable the effectiveness of immunotherapy, with discussion largely restricted to studies that demonstrate changes to the tumor microenvironment and synergy between that and additional immunotherapy.
Check Point Inhibitors
Immune inhibitory receptors can be expressed on, or secreted, by tumor cells and stromal components and constitute an important part of the tumor microenvironment. A variety of molecules, often referred to as immune checkpoints, including PD-1 and TIM3 can mediate immune inhibition through their respective inhibitory ligands, PD-L1 and galectin 9, expressed by tumor cells.Citation12 CTLA-4, which can be expressed by antigen presenting cells, is an inhibitory competitor for CD80 and CD86 costimulation of T cells through CD28, which can effectively inhibit T cell activation and expansion. Blockade of CTLA-4 interactions can itself enable endogenous immunity against tumors, and promising results have been observed in clinical and preclinical settings.Citation1,Citation13,Citation14 However, targeting immune checkpoints to reduce an immunosuppressive microenvironment within tumors in combination with other immunotherapies can result in dramatically improved antitumor responses.
Programmed Cell Death-1 (PD-1) is expressed on activated T cells, B cells and myeloid cells, and can induce inhibition and apoptosis of T cells following ligation by programmed death ligands-1 or -2, the former of which can be expressed on tumor cells. The PD-1 pathway performs a crucial role in the normal regulation of immunity, but the utilization of this pathway by tumors can inhibit immune control of malignancy. Agents in use for blocking the PD-1 pathway include neutralizing antibodies and soluble PD-1 ligands ().
Figure 1. Immune modulators of the tumor microenvironment that enhance cancer immunotherapy. Different therapies are depicted as described in the text. ATRA, all-trans retinoic acid; IDO, Indoleamine 2, 3-dioxygenase; M1 or M2, M1 or M2 macrophage; MDSC, Myeloid-derived suppressor cell; PD-1, programmed cell death protein 1; TLR, Toll-like receptor
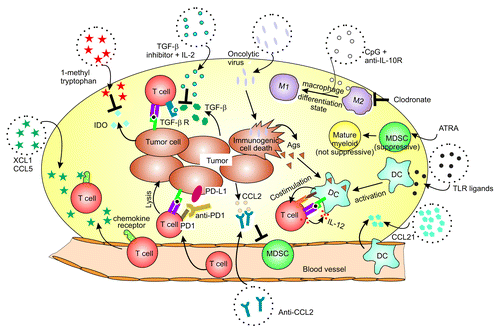
Immune therapies used in combination with PD-1 include adoptive transfer of tumor-reactive T cells, where enhanced tumor localization of T cells was observed together with increased inhibition of tumor growth.Citation15 While PD-1 blockade can augment passive transfer of immunity, it can also be used to enhance endogenous antitumor immune responses as seen in studies that combine it with anti-CD137 or tumor cell vaccines expressing Flt3L or GM-CSF, which could prolong survival of miceCitation16 or even eradicate tumors in some cases.Citation17,Citation18
Therapies targeting PD-1 or CTLA-4 are relatively well advanced, but manipulating the tumor microenvironment through inhibiting other checkpoints is also demonstrating potential for enhancing other immunotherapeutics. Preliminary studies suggest blocking TIM3 can enhance cancer vaccine efficacy, at least in a prophylactic setting.Citation19
Additional improvements can be achieved by blocking multiple checkpoints, as was observed in the study by Curran et al.,Citation18 when anti-CTLA-4 was added to the anti-PD-1, anti-PD-L1 plus Flt3L vaccine treatment regimen. Similarly, when both PD-1 and CTLA-4 inhibitory pathways were blocked, a better outcome was observed using IL-15 to treat intravenously injected CT26 metastatic colon cancer in mice.Citation20 Combining blockade of TIM3 and other checkpoints can also have enhanced anti-tumor effects.Citation21 Indeed, there is evidence to suggest that further benefit can be achieved using more complex combinations. For example, optimal effects against melanoma in mice were demonstrated when anti-PD-L1 was combined with adoptive transfer of tumor-specific CD8+ T cells, DC-peptide vaccine, IL-2 and irradiation.Citation22
Some recent clinical trials suggest that not all components of combination therapies are necessary. A Phase III clinical trial using check-point blockade (Ipilimumab) and/or a gp100 vaccine resulted in increased median overall survival in patients receiving the combined therapy, but this was not greater than those receiving Ipilimumab alone.Citation1
Immune inhibition can also be mediated by adenosine generated within the tumor microenvironment by the action of CD73. Blockade of this immunoregulatory pathway can lead to increased activity of adoptively transferred T cells against CD73-expressing tumors.Citation23,Citation24 In addition to tumor cells, CD73 can be expressed on endothelium, and inhibition of CD73 can increase T cell adhesion to endothelial cells and localization to tumors.Citation25
Targeting Regulatory Cells
Many different cells with immunosuppressive potential can infiltrate the tumor environment, including M2 macrophages, Treg cells, and MDSCs. Suppressive mechanisms employed by these cells involve secretion of cytokines (e.g., IL-10 and TGFβ), secretion of enzymes (e.g., arginase, NOS and IDO), and expression of inhibitory receptors (e.g., CTLA-4 and PD-L1). Depleting or modifying these regulatory cells and targeting each of the mechanisms they use within the tumor microenvironment can reverse immunosuppression (). In combination with other immunotherapies, it can lead to enhanced tumor regression.
Blocking differentiation or recruitment
MDSC differentiation can be blocked using cyclooxygenase (COX) inhibitors, which prevent the production of prostaglandin.Citation26 In combination with tumor lysate-pulsed DCs, a COX inhibitor could decrease the immunosuppressive function of myeloid cells and improve the survival of tumor bearing mice.Citation27 All-trans retinoic acids (ATRA) have also been shown to reduce the presence of immature MDSC by converting them to non-immunosuppressive mature myeloid cells, thereby prolonging the anti-tumor effect of cancer vaccines.Citation28 Complete rejection of tumors was also achieved when CpG therapy was combined with an antibody blocking CCL1, neutralizing de novo conversion of Treg.Citation29
In addition to abrogating the function of regulatory cells by blocking their differentiation, accumulation of regulatory cells in the tumor microenvironment can be reduced by targeting chemokine pathways. CCL2 for instance, is an important chemo-attractant for myeloid suppressor cells and its neutralization could augment the antitumor activity of vaccineCitation30 or adoptive CTL transfer.Citation31 Treg recruitment, through CCL17 and CCL22, could also be inhibited using a small molecule antagonist of CCR4, which led to improved responses to vaccine.Citation32
Blocking immunosuppressive enzymes
The suppressive activity of myeloid cells has been associated with the catabolism of the amino acids arginine and tryptophan. Arginase (ARG) can deplete arginine, and indoleamine 2,3-dioxygenase (IDO) can degrade tryptophan present in the tumor micro-environment.Citation33,Citation34 Recently, ARG-expressing M2 have been targeted using N-hydroxy-L-Arg (NOHA) and survival of sarcoma tumor bearing mice have been increased when combined with αOX40 therapy.Citation35 Both ARG and NOS are blocked simultaneously by nitroaspirin or sildenafil (Viagra®). Combined with a tumor vaccine or adoptive T cell therapy, these molecules could reduce function of MDSC, increase number of tumor infiltrating specific CTLCitation36 and improve the survival of tumor-bearing mice.Citation37
Several immunotherapies including the use of vaccines,Citation38-Citation40 and cytokinesCitation41,Citation42 have been improved when used in combination with the IDO inhibitor, 1-methyl-tryptophan. Knockdown of IDO by small-interfering RNA has also demonstrated the benefit of IDO inhibition combined with immunotherapy. Efficacy has been demonstrated when it was directly loaded in DCs used as a vaccineCitation43 or used in combination with a DNA vaccine encoding the tumor-associated antigen HER-2.Citation44
Regulatory cell depletion
Multiple regulatory mechanisms within the tumor microenvironment can be targeted simultaneously by depletion of subsets of regulatory leukocytes, which has been demonstrated to enhance immunotherapy in mouse models. Clodronate encapsulated in liposomes is a reagent for the depletion of macrophages in vivo. This reagent can deplete M2 macrophages and increase the efficacy of therapies including anti-angiogenic therapy using anti-VEGF or α-CD137 and CpG combination immunotherapy.Citation45,Citation46
Monoclonal antibodies specific for Gr-1 could deplete MDSC, and combined with adoptive cell therapy,Citation47 or OVA protein based vaccineCitation48 or anti-VEGF antibody therapyCitation49 resulted in an enhancement of immunotherapy and regression of established tumors. Welford et al. showed that depleting Tie2-expressing proangiogenic macrophages from mammary tumors, through a suicide gene based strategy, improve the effect of a vascular disrupting agent.Citation50
While M2 macrophages and MDSC can be found in large numbers in tumors and their immunomodulatory activity is often exerted locally within the tumor microenvironment, it is less clear where the immunoregulatory activity of Treg is performed since it can occur in lymphoid tissue and/or the tumor itself. Nevertheless, there are several approaches where depletion of Treg in tumors has enhanced immunotherapies. Several investigators have shown that depletion of Treg using anti-CD25 antibody can enhance the efficacy of a variety of immunotherapies.Citation51-Citation53 However, the potential benefit of Treg depletion through anti-CD25 antibody can be lost by the concurrent elimination of activated effector lymphocytes. In the DEREG mouse model, Foxp3+ Tregs express a diphtheria toxin receptor and so can be selectively eliminated with diphtheria toxin. The use of this model has already shown that elimination of Treg in tumors leads to tumor infiltration with CD8+ T cells and enhances survival of mice when combined with various types of vaccination.Citation54,Citation55
Re-programming immunosuppressive cells
An alternate, and more translationally feasible, approach to use instead of depleting regulatory cells is to re-program them to circumvent immunosuppression. Macrophages possess a certain degree of plasticity with regard to phenotype, and it is possible to manipulate tumor-associated immunosuppressive M2 macrophages to become immunosupportive M1-like. A range of strategies including CpG oligodeoxynucleotides combined with anti-IL-10 receptor and adenoviral delivery of CCL6 chemokineCitation56 or with anti-CD40 antibody and chemotherapyCitation57 or with anti-CD40 and anti-disialogangliosideCitation58 are able synergize in the induction of anti-tumor effects and to be associated with repolarization of tumor-associated macrophages. Immunotherapy using agonist anti-CD40 antibody combined with chemotherapy has shown remarkable effects in both mice and patients with pancreatic carcinoma by redirecting infiltrating macrophages to anti-tumor potential.Citation59
Treg have been recently observed to possess an unexpected degree of phenotypic plasticity and could lose their suppressor phenotype and become reprogrammed into T helper-like cells. Combining IDO inhibitor and an anti-tumor vaccine caused upregulation of IL-6 in plasmacytoid DCs and in situ conversion of a majority of Tregs to a Th17 phenotype, with marked enhancement of CD8+ T cell activation and antitumor efficacy.Citation60,Citation61 Furthermore, gemcitabine, in combination with a recombinant adenovirus expressing the tumor-associated antigen Her-2 and an anti-GITR antibody, was demonstrated to revert in vivo Treg immunosuppressive activity, achieving therapeutic effectiveness against pre-existing tumors.Citation62
Modifying the Chemokine Profile of the Tumor Microenvironment
The cellular composition of tumors is influenced by the chemokine profile of the microenvironment. Individual types of leukocytes are attracted in response to specific chemokines. Manipulation of the chemokine makeup can be used to swing the balance of cell types, and their associated molecules, from immunosuppressive to immunopotentiating with anti-tumor activity. This section of the review highlights the variety of chemokines which are being exploited to manipulate and alter the microenvironment, and which are then used in conjunction with one or more other immunotherapies to improve localization of effector lymphocytes to tumors ().
CXCL10 and XCL1, which attract CD8+ T cells, NK cells and monocytes, are chemokines used in a number of studies. The intratumoral injection of adenovirus- or plasmid-encoded XCL1 has been combined with a variety of immunotherapies including adoptive transfer of effector T cells,Citation63,Citation64 delivery of cytokines such as IL-12Citation65,Citation66 or IL-18,Citation67 and DC vaccine.Citation68 Together they caused considerable tumor regression or eradicated all tumors, which could include non-injected distant tumors, with a role for CD4+ and CD8+ T cells together with NK cells identified.
Other chemokines used to attract T cells into tumors include CCL5. An oncolytic vaccinia virus encoding CCL5 was given in combination with tumor lysate-pulsed dendritic cells to achieve greater tumor inhibition.Citation69 CCL2 has also been used to recruit T cells to tumor. Two herpes simplex virus vaccines were used in one study, one encoding the chemokine CCL2 and the other encoding IL-12. Intratumoral injection of these together resulted in significant tumor infiltration by CD8+ T cells and enhanced inhibition of neuroblastoma.Citation70
Recruitment of DCs and monocytes to tumors can also enhance immunotherapy. CCL21 and CCL16 can attract DCs and macrophages in addition to T cells. CCL21 injected intratumorally in combination with injection of CpG-oligonucleotideCitation71 led to tumor inhibition associated with infiltration of CD4+ T cells and DCs. CCL16 delivered by adenoviral vector to the tumor has been used in combination with CpG and αIL-10 monoclonal antibody resulting in 60% of tumors rejecting both primary 4T1 tumors and distant metastases.Citation56 Macrophages shifted from an M2 to M1 phenotype intratumorally and DCs upregulated costimulatory molecules, secreting cytokines to stimulate T cell proliferation and activation.
Thus, chemokines can be a potent way of changing the cellular composition of tumors, although efforts to date have largely focused on attracting T cells. Future approaches may employ chemokines better suited to recruiting other leukocytes with anti-tumor potential. Indeed, harnessing response capabilities normally reserved for pathogens through Toll-like receptors (TLRs) can be a potent way of attracting a variety of leukocytes and triggering changes to the microenvironment.
Danger Signals (TLR)
TLR agonists can trigger broad inflammatory responses that elicit rapid innate immunity and promote the activation of the adaptive immune reaction.Citation72 Two of the most commonly used TLR agonists are Polyinosinic:Polycytidylic Acid [Poly(I:C)] and Cytosine-phosphorothioate-guanine [CpG] (agonists for TLR3 and TLR9 respectively). Intratumoral injection of CpG aloneCitation73 or in combination with PolyI:CCitation74 enhanced the anti-tumor efficacy of adoptive transfer of tumor specific T cells. Stimulation of endogenous tumor immunity can also benefit from TLR agonist delivery. This was demonstrated using anti-CD137 together with local tumor injection of CpG that led to increased expression of genes associated with antigen presentation together with an increased frequency of tumor-infiltrating T cells, resulting in total regression of the majority of established tumors.Citation46 Enhanced antigen presentation was also thought to play a role in optimal antitumor effects observed when using intratumoral injection of CpG and poly(I:C) in combination with intratumoral delivery of CD40 ligand.Citation75
Oncolytic viruses are highly immunogenic pathogens able to stimulate TLR, and because they infect or replicate predominantly in tumor cells, much of their activity is localized to tumor. They can induce inflammatory mediators attracting myeloid cells and lymphocytes.Citation76 In addition, they can lyse a proportion of tumor cells, thereby releasing immunogenic antigens. In one study, oncolytic vaccinia virus was injected into subcutaneous tumors and combined with intraperitoneal delivery of anti-CD137. This combination therapy resulted in increased tumor infiltration by NK cells, neutrophils, and CD8+ effector T cells and enhanced inhibition of tumor growth and survival of mice.Citation77
There are many other strains of oncolytic virus under development that may induce novel and desirable changes to the microenvironments of tumors in various locations. For example, oncolytic myxoma virus when injected intratumorally and combined with adoptively transferred tumor-specific T cells was demonstrated to lead to enhanced survival of mice with syngeneic B16-SIY melanoma brain tumors.Citation78
In addition, studies have shown that loading antigen-specific T cells with viruses such as the vaccinia virus or vesicular stomatitis virus, have led to more efficient delivery of virus to tumor, resulting in increased T cell localization and activation, associated with effective tumor regression, and significantly increased survival.Citation79,Citation80 The ability of retroviral particles to adhere to the surface of T cells was utilized enabling viruses encoding IL-12 or Herpes simplex thymidine kinase (HSVtk) to “hitchhike” on antigen-specific T cells, which were delivered by adoptive transfer. Between 5–14% of the injected dose localized to the tumor, curing 60–90% of mice carrying B16-OVA tumors.Citation81 Further elegant strategies like these may lead to more specific and effective delivery of both TLR agonists and other immune modulators to tumors.
Thus oncolytic viruses can trigger immunogenic cell death and inflammation that can lead to an enhanced immune response against cancer. Immunogenic cell death can also be mediated by triggering death receptors on tumor cells. Targeting one such receptor, using anti-DR5, on a variety of mouse tumors can synergize with immunotherapies that augment antigen presentation (CD40) and T cell costimulation (CD137/4–1BB) to eradicate established tumors.Citation82 Recent investigations also demonstrate that targeting DR5 can have profound effects on the tumor microenvironment by disrupting tumor vasculature, although this specific feature has not been investigated in combination with additional immunotherapy.Citation83
Manipulating Cytokines in the Tumor Microenvironment
The cytokine content of the microenvironment can influence the balance of immunosuppressive and immunosupportive factors within tumors. Many types of immunotherapy can benefit from co-administration of cytokines, but delivery is often systemic making it difficult to distinguish between contributions from microenvironment modification and systemic immune modification. However, some studies have directed cytokines specifically to tumors and demonstrated changes to the microenvironment and increased efficacy of additional immunotherapeutic agents. For example, T cell-mediated production of IL-12 within tumors has been demonstrated to reprogram immunosuppressive leukocytes to enable tumor destruction by adoptively transferred T cells.Citation84,Citation85 Localized delivery of IFN-α to tumors has also been demonstrated to enhance immunotherapies using a DC vaccine or agonist anti-CD137 antibody.Citation86,Citation87
TGF-β secretion within tumors can suppress the antitumor activity of leukocytes in the tumor microenvironment. Thus various inhibitors have been used in cancer therapy to block TGF-β and lift immunosuppression, while simultaneously delivering other immunotherapies to eradicate the tumor.Citation88 For example, TGF-β was inhibited by intratumoral injection of two inhibitory peptides which were combined with simultaneous i.t. injections of poly(I:C) and α-CD40 antibody.Citation89 The TGF-β inhibited was mainly that produced by Tregs rather than tumor cells, leading to 70% rejection of tumors in mice. Another innovative method of TGF-β inhibition involved nanoscale liposomal polymeric gels releasing a TGF-β inhibitor that, when combined with IL-2, significantly delayed B16 tumor growth in mice.Citation90 There were significantly increased numbers of intratumoral NK cells (required for maximal tumor regression) and intratumoral infiltration of activated CD8+ T cells, in parallel with demonstration of localized therapy and drug to the tumor.
Concluding Remarks
Thus, there are a variety of strategies that can be used to modify the tumor microenvironment to render it less immunosuppressive and enable additional immunotherapies (). The majority of the above studies were preclinical in mice, which allowed interpretation of mechanistic contributions to therapy, but these types of combination therapies are gaining momentum in the clinic.Citation91,Citation92
Table1. Examples of strategies for manipulating the tumor microenvironment to enable immunotherapy
Checkpoint blockade has been used in combination with other immunomodulators, and benefit to patients demonstrated.Citation93 However, most clinical studies using combinations of immunotherapeutic agents have been early stage trials and mechanistic insight into the relative roles of immunomodulation and microenvironment modulation has not been possible. Nevertheless, there is much excitement in the use of combination therapy with remarkable response rates reported in a recent clinical study by combining anti-CTLA-4 and anti-PD1.Citation94 More complex combinations are possible,Citation95 although careful evaluation of potential toxicities prior to commencing clinical trial is crucial to the safety of these studies.
In summary, immunotherapy of cancer can induce anti-tumor responses, although these responses are not often complete. The immunoregulatory nature of the tumor microenvironment can inhibit fully effective immune responses against cancer, and modulation of the microenvironment can enhance the efficacy of immunotherapy to achieve eradication of tumors.
References
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711 - 23; http://dx.doi.org/10.1056/NEJMoa1003466; PMID: 20525992
- Salles G, Seymour JF, Offner F, López-Guillermo A, Belada D, Xerri L, Feugier P, Bouabdallah R, Catalano JV, Brice P, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet 2011; 377:42 - 51; http://dx.doi.org/10.1016/S0140-6736(10)62175-7; PMID: 21176949
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 2008; 26:5233 - 9; http://dx.doi.org/10.1200/JCO.2008.16.5449; PMID: 18809613
- Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature 2001; 411:375 - 9; http://dx.doi.org/10.1038/35077241; PMID: 11357145
- Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev 2008; 222:162 - 79; http://dx.doi.org/10.1111/j.1600-065X.2008.00602.x; PMID: 18364001
- Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 2009; 30:636 - 45; http://dx.doi.org/10.1016/j.immuni.2009.04.010; PMID: 19464986
- Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol 2008; 18:349 - 55; http://dx.doi.org/10.1016/j.semcancer.2008.03.004; PMID: 18467122
- Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 2002; 419:734 - 8; http://dx.doi.org/10.1038/nature01112; PMID: 12384702
- Smyth MJ, Cretney E, Kershaw MH, Hayakawa Y. Cytokines in cancer immunity and immunotherapy. Immunol Rev 2004; 202:275 - 93; http://dx.doi.org/10.1111/j.0105-2896.2004.00199.x; PMID: 15546400
- Aruga A, Aruga E, Tanigawa K, Bishop DK, Sondak VK, Chang AE. Type 1 versus type 2 cytokine release by Vbeta T cell subpopulations determines in vivo antitumor reactivity: IL-10 mediates a suppressive role. J Immunol 1997; 159:664 - 73; PMID: 9218581
- Rubinstein N, Alvarez M, Zwirner NW, Toscano MA, Ilarregui JM, Bravo A, Mordoh J, Fainboim L, Podhajcer OL, Rabinovich GA. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell 2004; 5:241 - 51; http://dx.doi.org/10.1016/S1535-6108(04)00024-8; PMID: 15050916
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8:793 - 800; http://dx.doi.org/10.1038/nm0902-1039c; PMID: 12091876
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28:3167 - 75; http://dx.doi.org/10.1200/JCO.2009.26.7609; PMID: 20516446
- Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A 2003; 100:8372 - 7; http://dx.doi.org/10.1073/pnas.1533209100; PMID: 12826605
- Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, Yagita H, Overwijk WW, Lizée G, Radvanyi L, et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-γ inducible chemokines. Cancer Res 2012; 72:5209 - 18; http://dx.doi.org/10.1158/0008-5472.CAN-12-1187; PMID: 22915761
- Li B, VanRoey M, Wang C, Chen TH, Korman A, Jooss K. Anti-programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor--secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumors. Clin Cancer Res 2009; 15:1623 - 34; http://dx.doi.org/10.1158/1078-0432.CCR-08-1825; PMID: 19208793
- Verbrugge I, Hagekyriakou J, Sharp LL, Galli M, West A, McLaughlin NM, Duret H, Yagita H, Johnstone RW, Smyth MJ, et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res 2012; 72:3163 - 74; http://dx.doi.org/10.1158/0008-5472.CAN-12-0210; PMID: 22570253
- Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A 2010; 107:4275 - 80; http://dx.doi.org/10.1073/pnas.0915174107; PMID: 20160101
- Lee MJ, Woo MY, Heo YM, Kim JS, Kwon MH, Kim K, Park S. The inhibition of the T-cell immunoglobulin and mucin domain 3 (Tim3) pathway enhances the efficacy of tumor vaccine. Biochem Biophys Res Commun 2010; 402:88 - 93; http://dx.doi.org/10.1016/j.bbrc.2010.09.121; PMID: 20920468
- Yu P, Steel JC, Zhang M, Morris JC, Waldmann TA. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res 2010; 16:6019 - 28; http://dx.doi.org/10.1158/1078-0432.CCR-10-1966; PMID: 20924130
- Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumors. Cancer Res 2011; 71:3540 - 51; http://dx.doi.org/10.1158/0008-5472.CAN-11-0096; PMID: 21430066
- Pilon-Thomas S, Mackay A, Vohra N, Mulé JJ. Blockade of programmed death ligand 1 enhances the therapeutic efficacy of combination immunotherapy against melanoma. J Immunol 2010; 184:3442 - 9; http://dx.doi.org/10.4049/jimmunol.0904114; PMID: 20194714
- Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A 2006; 103:13132 - 7; http://dx.doi.org/10.1073/pnas.0605251103; PMID: 16916931
- Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, Shin T, Curiel TJ, Zhang B. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res 2010; 70:2245 - 55; http://dx.doi.org/10.1158/0008-5472.CAN-09-3109; PMID: 20179192
- Wang L, Fan J, Thompson LF, Zhang Y, Shin T, Curiel TJ, Zhang B. CD73 has distinct roles in nonhematopoietic and hematopoietic cells to promote tumor growth in mice. J Clin Invest 2011; 121:2371 - 82; http://dx.doi.org/10.1172/JCI45559; PMID: 21537079
- Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res 2007; 67:4507 - 13; http://dx.doi.org/10.1158/0008-5472.CAN-06-4174; PMID: 17483367
- Veltman JD, Lambers ME, van Nimwegen M, Hendriks RW, Hoogsteden HC, Aerts JG, Hegmans JP. COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer 2010; 10:464; http://dx.doi.org/10.1186/1471-2407-10-464; PMID: 20804550
- Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush R, Gabrilovich D. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res 2003; 63:4441 - 9; PMID: 12907617
- Hoelzinger DB, Smith SE, Mirza N, Dominguez AL, Manrique SZ, Lustgarten J. Blockade of CCL1 inhibits T regulatory cell suppressive function enhancing tumor immunity without affecting T effector responses. J Immunol 2010; 184:6833 - 42; http://dx.doi.org/10.4049/jimmunol.0904084; PMID: 20483762
- Fridlender ZG, Buchlis G, Kapoor V, Cheng G, Sun J, Singhal S, Crisanti MC, Wang LC, Heitjan D, Snyder LA, et al. CCL2 blockade augments cancer immunotherapy. Cancer Res 2010; 70:109 - 18; http://dx.doi.org/10.1158/0008-5472.CAN-09-2326; PMID: 20028856
- Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, De Palma A, Mauri P, Monegal A, Rescigno M, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med 2011; 208:1949 - 62; http://dx.doi.org/10.1084/jem.20101956; PMID: 21930770
- Pere H, Montier Y, Bayry J, Quintin-Colonna F, Merillon N, Dransart E, Badoual C, Gey A, Ravel P, Marcheteau E, et al. A CCR4 antagonist combined with vaccines induces antigen-specific CD8+ T cells and tumor immunity against self antigens. Blood 2011; 118:4853 - 62; http://dx.doi.org/10.1182/blood-2011-01-329656; PMID: 21908423
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162 - 74; http://dx.doi.org/10.1038/nri2506; PMID: 19197294
- Munn DH. Blocking IDO activity to enhance anti-tumor immunity. [Elite Ed] Front Biosci (Elite Ed) 2012; 4:734 - 45; PMID: 22201909
- Gough MJ, Killeen N, Weinberg AD. Targeting macrophages in the tumour environment to enhance the efficacy of αOX40 therapy. Immunology 2012; 136:437 - 47; http://dx.doi.org/10.1111/j.1365-2567.2012.03600.x; PMID: 22578109
- De Santo C, Serafini P, Marigo I, Dolcetti L, Bolla M, Del Soldato P, Melani C, Guiducci C, Colombo MP, Iezzi M, et al. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc Natl Acad Sci U S A 2005; 102:4185 - 90; http://dx.doi.org/10.1073/pnas.0409783102; PMID: 15753302
- Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med 2006; 203:2691 - 702; http://dx.doi.org/10.1084/jem.20061104; PMID: 17101732
- Ou X, Cai S, Liu P, Zeng J, He Y, Wu X, Du J. Enhancement of dendritic cell-tumor fusion vaccine potency by indoleamine-pyrrole 2,3-dioxygenase inhibitor, 1-MT. J Cancer Res Clin Oncol 2008; 134:525 - 33; http://dx.doi.org/10.1007/s00432-007-0315-9; PMID: 17909857
- Zeng J, Cai S, Yi Y, He Y, Wang Z, Jiang G, Li X, Du J. Prevention of spontaneous tumor development in a ret transgenic mouse model by ret peptide vaccination with indoleamine 2,3-dioxygenase inhibitor 1-methyl tryptophan. Cancer Res 2009; 69:3963 - 70; http://dx.doi.org/10.1158/0008-5472.CAN-08-2476; PMID: 19383920
- Yi YM, Zhang G, Zeng J, Huang SC, Li LL, Fang R, Jiang GM, Bu XZ, Cai SH, Du J. A new tumor vaccine: FAPτ-MT elicits effective antitumor response by targeting indolamine2,3-dioxygenase in antigen presenting cells. Cancer Biol Ther 2011; 11:866 - 73; http://dx.doi.org/10.4161/cbt.11.10.15179; PMID: 21372637
- Gu T, Rowswell-Turner RB, Kilinc MO, Egilmez NK. Central role of IFNgamma-indoleamine 2,3-dioxygenase axis in regulation of interleukin-12-mediated antitumor immunity. Cancer Res 2010; 70:129 - 38; http://dx.doi.org/10.1158/0008-5472.CAN-09-3170; PMID: 20028855
- Gasparri AM, Jachetti E, Colombo B, Sacchi A, Curnis F, Rizzardi GP, Traversari C, Bellone M, Corti A. Critical role of indoleamine 2,3-dioxygenase in tumor resistance to repeated treatments with targeted IFNgamma. Mol Cancer Ther 2008; 7:3859 - 66; http://dx.doi.org/10.1158/1535-7163.MCT-08-0538; PMID: 19074858
- Zheng X, Koropatnick J, Chen D, Velenosi T, Ling H, Zhang X, Jiang N, Navarro B, Ichim TE, Urquhart B, et al. Silencing IDO in dendritic cells: a novel approach to enhance cancer immunotherapy in a murine breast cancer model. Int J Cancer 2013; 132:967 - 77; http://dx.doi.org/10.1002/ijc.27710; PMID: 22870862
- Yen MC, Lin CC, Chen YL, Huang SS, Yang HJ, Chang CP, Lei HY, Lai MD. A novel cancer therapy by skin delivery of indoleamine 2,3-dioxygenase siRNA. Clin Cancer Res 2009; 15:641 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-08-1988; PMID: 19147770
- Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjällman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer 2006; 95:272 - 81; http://dx.doi.org/10.1038/sj.bjc.6603240; PMID: 16832418
- Westwood JA, Haynes NM, Sharkey J, McLaughlin N, Pegram HJ, Schwendener RA, Smyth MJ, Darcy PK, Kershaw MH. Toll-Like Receptor Triggering and T-Cell Costimulation Induce Potent Antitumor Immunity in Mice. Clin Cancer Res 2009; 15:7624 - 33; http://dx.doi.org/10.1158/1078-0432.CCR-09-2201; PMID: 19996209
- Morales JK, Kmieciak M, Graham L, Feldmesser M, Bear HD, Manjili MH. Adoptive transfer of HER2/neu-specific T cells expanded with alternating gamma chain cytokines mediate tumor regression when combined with the depletion of myeloid-derived suppressor cells. Cancer Immunol Immunother 2009; 58:941 - 53; http://dx.doi.org/10.1007/s00262-008-0609-z; PMID: 18979098
- Srivastava MK, Zhu L, Harris-White M, Kar UK, Huang M, Johnson MF, Lee JM, Elashoff D, Strieter R, Dubinett S, et al. Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS One 2012; 7:e40677; http://dx.doi.org/10.1371/journal.pone.0040677; PMID: 22815789
- Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol 2007; 25:911 - 20; http://dx.doi.org/10.1038/nbt1323; PMID: 17664940
- Welford AF, Biziato D, Coffelt SB, Nucera S, Fisher M, Pucci F, Di Serio C, Naldini L, De Palma M, Tozer GM, et al. TIE2-expressing macrophages limit the therapeutic efficacy of the vascular-disrupting agent combretastatin A4 phosphate in mice. J Clin Invest 2011; 121:1969 - 73; http://dx.doi.org/10.1172/JCI44562; PMID: 21490397
- Kudo-Saito C, Schlom J, Camphausen K, Coleman CN, Hodge JW. The requirement of multimodal therapy (vaccine, local tumor radiation, and reduction of suppressor cells) to eliminate established tumors. Clin Cancer Res 2005; 11:4533 - 44; http://dx.doi.org/10.1158/1078-0432.CCR-04-2237; PMID: 15958639
- Li J, Hu P, Khawli LA, Epstein AL. Complete regression of experimental solid tumors by combination LEC/chTNT-3 immunotherapy and CD25(+) T-cell depletion. Cancer Res 2003; 63:8384 - 92; PMID: 14679000
- Mandl SJ, Rountree RB, Dalpozzo K, Do L, Lombardo JR, Schoonmaker PL, Dirmeier U, Steigerwald R, Giffon T, Laus R, et al. Immunotherapy with MVA-BN®-HER2 induces HER-2-specific Th1 immunity and alters the intratumoral balance of effector and regulatory T cells. Cancer Immunol Immunother 2012; 61:19 - 29; http://dx.doi.org/10.1007/s00262-011-1077-4; PMID: 21822917
- Klages K, Mayer CT, Lahl K, Loddenkemper C, Teng MW, Ngiow SF, Smyth MJ, Hamann A, Huehn J, Sparwasser T. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res 2010; 70:7788 - 99; http://dx.doi.org/10.1158/0008-5472.CAN-10-1736; PMID: 20924102
- Mattarollo SR, Steegh K, Li M, Duret H, Foong Ngiow S, Smyth MJ. Transient Foxp3(+) regulatory T-cell depletion enhances therapeutic anticancer vaccination targeting the immune-stimulatory properties of NKT cells. Immunol Cell Biol 2013; 91:105 - 14; http://dx.doi.org/10.1038/icb.2012.58; PMID: 23090488
- Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res 2005; 65:3437 - 46; PMID: 15833879
- Buhtoiarov IN, Sondel PM, Wigginton JM, Buhtoiarova TN, Yanke EM, Mahvi DA, Rakhmilevich AL. Anti-tumour synergy of cytotoxic chemotherapy and anti-CD40 plus CpG-ODN immunotherapy through repolarization of tumour-associated macrophages. Immunology 2011; 132:226 - 39; http://dx.doi.org/10.1111/j.1365-2567.2010.03357.x; PMID: 21039467
- Alderson KL, Luangrath M, Elsenheimer MM, Gillies SD, Navid F, Rakhmilevich AL, Sondel PM. Enhancement of the anti-melanoma response of Hu14.18K322A by αCD40 + CpG. Cancer Immunol Immunother 2013; 62:665 - 75; http://dx.doi.org/10.1007/s00262-012-1372-8; PMID: 23151945
- Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011; 331:1612 - 6; http://dx.doi.org/10.1126/science.1198443; PMID: 21436454
- Sharma MD, Hou DY, Baban B, Koni PA, He Y, Chandler PR, Blazar BR, Mellor AL, Munn DH. Reprogrammed foxp3(+) regulatory T cells provide essential help to support cross-presentation and CD8(+) T cell priming in naive mice. Immunity 2010; 33:942 - 54; http://dx.doi.org/10.1016/j.immuni.2010.11.022; PMID: 21145762
- Sharma MD, Hou DY, Liu Y, Koni PA, Metz R, Chandler P, Mellor AL, He Y, Munn DH. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood 2009; 113:6102 - 11; http://dx.doi.org/10.1182/blood-2008-12-195354; PMID: 19366986
- Ko HJ, Kim YJ, Kim YS, Chang WS, Ko SY, Chang SY, Sakaguchi S, Kang CY. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res 2007; 67:7477 - 86; http://dx.doi.org/10.1158/0008-5472.CAN-06-4639; PMID: 17671218
- Huang H, Li F, Gordon JR, Xiang J. Synergistic enhancement of antitumor immunity with adoptively transferred tumor-specific CD4+ and CD8+ T cells and intratumoral lymphotactin transgene expression. Cancer Res 2002; 62:2043 - 51; PMID: 11929823
- Huang H, Xiang J. Synergistic effect of lymphotactin and interferon gamma-inducible protein-10 transgene expression in T-cell localization and adoptive T-cell therapy of tumors. Int J Cancer 2004; 109:817 - 25; http://dx.doi.org/10.1002/ijc.20043; PMID: 15027114
- Narvaiza I, Mazzolini G, Barajas M, Duarte M, Zaratiegui M, Qian C, Melero I, Prieto J. Intratumoral coinjection of two adenoviruses, one encoding the chemokine IFN-gamma-inducible protein-10 and another encoding IL-12, results in marked antitumoral synergy. J Immunol 2000; 164:3112 - 22; PMID: 10706701
- Palmer K, Hitt M, Emtage PC, Gyorffy S, Gauldie J. Combined CXC chemokine and interleukin-12 gene transfer enhances antitumor immunity. Gene Ther 2001; 8:282 - 90; http://dx.doi.org/10.1038/sj.gt.3301386; PMID: 11313802
- Liu Y, Huang H, Saxena A, Xiang J. Intratumoral coinjection of two adenoviral vectors expressing functional interleukin-18 and inducible protein-10, respectively, synergizes to facilitate regression of established tumors. Cancer Gene Ther 2002; 9:533 - 42; http://dx.doi.org/10.1038/sj.cgt.7700466; PMID: 12032664
- Jiang XB, Lu XL, Hu P, Liu RE. Improved therapeutic efficacy using vaccination with glioma lysate-pulsed dendritic cells combined with IP-10 in murine glioma. Vaccine 2009; 27:6210 - 6; http://dx.doi.org/10.1016/j.vaccine.2009.08.002; PMID: 19699331
- Li J, O’Malley M, Urban J, Sampath P, Guo ZS, Kalinski P, Thorne SH, Bartlett DL. Chemokine expression from oncolytic vaccinia virus enhances vaccine therapies of cancer. Mol Ther 2011; 19:650 - 7; http://dx.doi.org/10.1038/mt.2010.312; PMID: 21266959
- Parker JN, Meleth S, Hughes KB, Gillespie GY, Whitley RJ, Markert JM. Enhanced inhibition of syngeneic murine tumors by combinatorial therapy with genetically engineered HSV-1 expressing CCL2 and IL-12. Cancer Gene Ther 2005; 12:359 - 68; http://dx.doi.org/10.1038/sj.cgt.7700784; PMID: 15678154
- Oh SM, Oh K, Lee DS. Intratumoral administration of secondary lymphoid chemokine and unmethylated cytosine-phosphorothioate-guanine oligodeoxynucleotide synergistically inhibits tumor growth in vivo. J Korean Med Sci 2011; 26:1270 - 6; http://dx.doi.org/10.3346/jkms.2011.26.10.1270; PMID: 22022177
- Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer 2009; 9:57 - 63; http://dx.doi.org/10.1038/nrc2541; PMID: 19052556
- Lou Y, Liu C, Lizée G, Peng W, Xu C, Ye Y, Rabinovich BA, Hailemichael Y, Gelbard A, Zhou D, et al. Antitumor activity mediated by CpG: the route of administration is critical. J Immunother 2011; 34:279 - 88; http://dx.doi.org/10.1097/CJI.0b013e31820d2a05; PMID: 21389870
- Amos SM, Pegram HJ, Westwood JA, John LB, Devaud C, Clarke CJ, Restifo NP, Smyth MJ, Darcy PK, Kershaw MH. Adoptive immunotherapy combined with intratumoral TLR agonist delivery eradicates established melanoma in mice. Cancer Immunol Immunother 2011; 60:671 - 83; http://dx.doi.org/10.1007/s00262-011-0984-8; PMID: 21327636
- Stone GW, Barzee S, Snarsky V, Santucci C, Tran B, Langer R, Zugates GT, Anderson DG, Kornbluth RS. Nanoparticle-delivered multimeric soluble CD40L DNA combined with Toll-Like Receptor agonists as a treatment for melanoma. PLoS One 2009; 4:e7334; http://dx.doi.org/10.1371/journal.pone.0007334; PMID: 19812695
- Kirn DH, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer 2009; 9:64 - 71; http://dx.doi.org/10.1038/nrc2545; PMID: 19104515
- John LB, Howland LJ, Flynn JK, West AC, Devaud C, Duong CP, Stewart TJ, Westwood JA, Guo ZS, Bartlett DL, et al. Oncolytic virus and anti-4-1BB combination therapy elicits strong antitumor immunity against established cancer. Cancer Res 2012; 72:1651 - 60; http://dx.doi.org/10.1158/0008-5472.CAN-11-2788; PMID: 22315352
- Thomas DL, Doty R, Tosic V, Liu J, Kranz DM, McFadden G, Macneill AL, Roy EJ. Myxoma virus combined with rapamycin treatment enhances adoptive T cell therapy for murine melanoma brain tumors. Cancer Immunol Immunother 2011; 60:1461 - 72; http://dx.doi.org/10.1007/s00262-011-1045-z; PMID: 21656158
- Qiao J, Wang H, Kottke T, Diaz RM, Willmon C, Hudacek A, Thompson J, Parato K, Bell J, Naik J, et al. Loading of oncolytic vesicular stomatitis virus onto antigen-specific T cells enhances the efficacy of adoptive T-cell therapy of tumors. Gene Ther 2008; 15:604 - 16; http://dx.doi.org/10.1038/sj.gt.3303098; PMID: 18305577
- Thorne SH, Negrin RS, Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science 2006; 311:1780 - 4; http://dx.doi.org/10.1126/science.1121411; PMID: 16556847
- Cole C, Qiao J, Kottke T, Diaz RM, Ahmed A, Sanchez-Perez L, Brunn G, Thompson J, Chester J, Vile RG. Tumor-targeted, systemic delivery of therapeutic viral vectors using hitchhiking on antigen-specific T cells. Nat Med 2005; 11:1073 - 81; http://dx.doi.org/10.1038/nm1297; PMID: 16170322
- Uno T, Takeda K, Kojima Y, Yoshizawa H, Akiba H, Mittler RS, Gejyo F, Okumura K, Yagita H, Smyth MJ. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med 2006; 12:693 - 8; http://dx.doi.org/10.1038/nm1405; PMID: 16680149
- Wilson NS, Yang A, Yang B, Couto S, Stern H, Gogineni A, Pitti R, Marsters S, Weimer RM, Singh M, et al. Proapoptotic activation of death receptor 5 on tumor endothelial cells disrupts the vasculature and reduces tumor growth. Cancer Cell 2012; 22:80 - 90; http://dx.doi.org/10.1016/j.ccr.2012.05.014; PMID: 22789540
- Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res 2011; 71:5697 - 706; http://dx.doi.org/10.1158/0008-5472.CAN-11-0103; PMID: 21742772
- Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, Leonardi AJ, Morgan RA, Wang E, Marincola FM, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest 2011; 121:4746 - 57; http://dx.doi.org/10.1172/JCI58814; PMID: 22056381
- Dubrot J, Palazón A, Alfaro C, Azpilikueta A, Ochoa MC, Rouzaut A, Martinez-Forero I, Teijeira A, Berraondo P, Le Bon A, et al. Intratumoral injection of interferon-α and systemic delivery of agonist anti-CD137 monoclonal antibodies synergize for immunotherapy. Int J Cancer 2011; 128:105 - 18; http://dx.doi.org/10.1002/ijc.25333; PMID: 20309938
- Kuwashima N, Nishimura F, Eguchi J, Sato H, Hatano M, Tsugawa T, Sakaida T, Dusak JE, Fellows-Mayle WK, Papworth GD, et al. Delivery of dendritic cells engineered to secrete IFN-alpha into central nervous system tumors enhances the efficacy of peripheral tumor cell vaccines: dependence on apoptotic pathways. J Immunol 2005; 175:2730 - 40; PMID: 16081851
- Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limón P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol 2010; 10:554 - 67; http://dx.doi.org/10.1038/nri2808; PMID: 20616810
- Llopiz D, Dotor J, Casares N, Bezunartea J, Díaz-Valdés N, Ruiz M, Aranda F, Berraondo P, Prieto J, Lasarte JJ, et al. Peptide inhibitors of transforming growth factor-beta enhance the efficacy of antitumor immunotherapy. Int J Cancer 2009; 125:2614 - 23; http://dx.doi.org/10.1002/ijc.24656; PMID: 19530254
- Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, Jay SM, Demento SL, Agawu A, Licona Limon P, et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater 2012; 11:895 - 905; http://dx.doi.org/10.1038/nmat3355; PMID: 22797827
- Berraondo P, Umansky V, Melero I. Changing the tumor microenvironment: new strategies for immunotherapy. Cancer Res 2012; 72:5159 - 64; http://dx.doi.org/10.1158/0008-5472.CAN-12-1952; PMID: 22826606
- Martinez Forero I, Okada H, Topalian SL, Gajewski TF, Korman AJ, Melero I. Workshop on immunotherapy combinations. Society for Immunotherapy of Cancer annual meeting Bethesda, November 3, 2011. J Transl Med 2012; 10:108; http://dx.doi.org/10.1186/1479-5876-10-108; PMID: 22640522
- Hodi FS, Friedlander PA, Atkins MB, McDermott DF, Lawrence DP, Ibrahim N, et al. A phase I trial of ipilimumab plus bevacizumab in patients with unresectable stage III or stage IV melanoma. [Suppl.] J Clin Oncol 2011; 29:8511
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122 - 33; http://dx.doi.org/10.1056/NEJMoa1302369; PMID: 23724867
- Picozzi VJ, Kozarek RA, Traverso LW. Interferon-based adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg 2003; 185:476 - 80; http://dx.doi.org/10.1016/S0002-9610(03)00051-5; PMID: 12727570
- Chinnasamy D, Yu Z, Kerkar SP, Zhang L, Morgan RA, Restifo NP, Rosenberg SA. Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin Cancer Res 2012; 18:1672 - 83; http://dx.doi.org/10.1158/1078-0432.CCR-11-3050; PMID: 22291136
- Zhang L, Kerkar SP, Yu Z, Zheng Z, Yang S, Restifo NP, Rosenberg SA, Morgan RA. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Mol Ther 2011; 19:751 - 9; http://dx.doi.org/10.1038/mt.2010.313; PMID: 21285960