Abstract
Local immunotherapy resurfaces in the field of cancer as a potential way to cure localized and metastatic disease with limited toxic effects. We have recently demonstrated that local administration of agonistic CD40 antibodies can cure localized as well as disseminated bladder neoplasms. This approach reduces the circulating concentrations of antibodies that would result from systemic delivery, hence resulting in limited toxicity.
Keywords: :
While non-muscle invasive bladder cancer (NMIBC) can be treated with the intravesical instillation of chemotherapeutic agents or bacillus Calmette–Guérin (BCG), patients with invasive bladder neoplasms are generally subject to aggressive systemic treatments or radical surgery. For NMIBC patients, a BCG-based maintenance regimen prevents disease recurrence better than mitomycin C. BCG is also the therapeutic option of choice for patients with high-grade pTa and pT1 bladder cancer, but these individuals still experience relapse.Citation1 Novel immunotherapeutic strategies to improve disease outcome among bladder cancer patients involve the co-administration of BCG and interferon α2b (IFNα2b), intralesional or intravesical IL-2 therapy,Citation2 as well as other approaches that so far have been investigated only in experimental models.
Localized bladder cancer is by definition simple to access with intravesical instillations of BCG or chemotherapeutic agents. The interaction of BCG with the urothelium triggers a release of cytokines that attract innate and adaptive immune cells, targeting both the bacterial infection and malignant cells. Our group has previously investigated alternatives to local BCG therapy in an experimental model of bladder cancer, finding that both CpG oligodeoxynucleotides (ODNs)Citation3,Citation4 and adenoviral vectors expressing CD40 ligand (CD40L)Citation5,Citation6 can eradicate orthotopically growing tumors and elicit long-lived immunological memory. Additionally, we have tested the intravesical instillation of CD40L-expressing adenoviruses in a Phase I/IIa clinical trial. This immunotherapeutic approach was well tolerated, promoted immune activation and mediated antitumor effects.Citation7 The use of monoclonal antibodies in cancer therapy is rapidly expanding and immunostimulatory antibodies are generally administered systemically, perhaps because this approach initially involved tumor-targeting antibodies only. The latter home to neoplastic lesions, where they affect tumorigenic signaling pathways and/or induce antibody-dependent immune effector functions, including phagocytosis and cell-mediated cytotoxicity. Therefore tumor-targeting antibodies, although administered systemically, exert localized effects, since their targets are normally expressed in a tissue- or tumor-restricted pattern. Recently, we have witnessed various attempts to use immunostimulatory antibodies in the clinic. In this setting, CP-870,893 (a human IgG2 CD40 agonist antibody) exhibited systemic side effects. The maximum tolerated dose (MTD) of CP-870 893 was estimated to 0.2 mg/kg, thereby limiting its usefulness for the initiation of effective antitumor responses.Citation8 CD40, as well as other immune cell receptors, is widely expressed throughout the body, implying that the intravenous injection of CD40 agonistic antibodies is intrinsically prone to result in off-target side effects.
It is attractive to target proteins in a highly selective manner, and we, as well as others, have begun to assess the efficacy of monoclonal antibodies administered locally. The rationale behind local immunotherapy with monoclonal antibodies is that this approach may allow for the direct targeting of immune cells within neoplastic lesions while restricting the diffusion of antibodies to sites where adverse events would be induced. In this setting, metastatic lesions would be targeted by the cellular arm of the immune system upon local activation of tumor-targeting immune responses rather than by the drug itself. Today, there is no empirical evidence in support of the notion that breaking peripheral tolerance in all organs is required to achieve robust antitumor responses. Accumulating data, including our own, demonstrate that efficient antitumor responses can be achieved local immunomodulation.
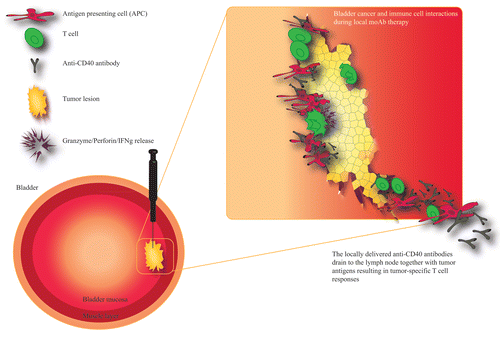
Others have observed that the local delivery of monoclonal antibodies by a slow-release system exerts robust antineoplastic effects with limited toxicity.Citation9 We have recently demonstrated that the peritumoral injection of CD40 agonistic antibodies promotes antitumor immune responses that are superior to those elicited by the same dose of the antibody delivered systemically, but results in reduced side effects. Bio-distribution studies of the CD40 agonistic antibody revealed that the peritumoral route of administration avoids a peak in serum antibody concentration. This was paralleled by increased CD40 expression on antigen-presenting cells that expanded in the tumor-draining lymph node. In addition, our findings demonstrate tumor-specific long term protection in animals experiencing complete disease regression and that the antineoplastic activity of local anti-CD40 therapy is dependent on CD8+ T cells. We also demonstrated that a relatively low dose of anti-CD40 antibodies administered to a given tumor (to minimize the leakage of antibodies) can mediate antineoplastic effects on distant lesions, suggesting that the local therapy concept could benefit bladder cancer patients with metastatic tumors ().Citation10
Figure 1. Localized or disseminated bladder cancer can be treated with peritumoral or intratumoral injections of CD40 agonistic antibodies. Various immunotherapeutics, including CD40 agonistic antibodies, can be easily administered into neoplastic lesions growing in the bladder urothelium by ultrasound-guided or transurethral injections. CD40 agonistic antibodies can then activate tumor-infiltrating immune cells as well as immune cells in the tumor-draining lymph node. The drainage of these antibodies is paralleled by that of tumor debris, resulting in the efficient priming and/or activation of tumor-specific T cells. These T lymphocyte can home to the tumor and exert antineoplastic effects by multiple mechanisms, including perforin/granzyme-induced apoptosis. Tumor-specific T cells can also control metastatic lesions and prevent disease recurrence.
It might be provocative to argue that local immunotherapy would improve the success rate of current therapies, and this approach is probably not a complete solution to the problem posed by metastatic bladder cancer. However, the local route of administration may certainly increase the therapeutic window of several immunostimulatory agents. This in turn can increase MTDs and may represent the platform for effective combinations of immunotherapeutic agents that result in limited systemic adverse events. Can we justify a systemic break of tolerance when the therapy itself aims at improving tumor-specific cellular immune responses? We believe we can do better than that. We therefore advocate to optimize not only the targeting of malignant cells but also the delivery route of anticancer immunotherapeutic agents, including immunostimulatory antibodies. It is feasible to perform ultrasound-guided or transurethral injections of primary as well as metastatic bladder cancer lesions. Our preliminary findings suggest that immunostimulatory monoclonal antibodies do not successfully penetrate the bladder wall upon intravesical instillation, an argument in support of the use of intralesional therapy to improve clinical outcome.
Disclosure of Potential Conflicts of Interest
L.S., T.T., and S.M. have a royalty agreement with Alligator Biosciences AB. T.T. is a consultant for Alligator Bioscience AB and Immuvent Inc.
References
- Malmström PU, Sylvester RJ, Crawford DE, Friedrich M, Krege S, Rintala E, Solsona E, Di Stasi SM, Witjes JA. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol 2009; 56:247 - 56; http://dx.doi.org/10.1016/j.eururo.2009.04.038; PMID: 19409692
- Askeland EJ, Newton MR, O’Donnell MA, Luo Y. Bladder Cancer Immunotherapy: BCG and Beyond. Adv Urol 2012; 2012:181987; http://dx.doi.org/10.1155/2012/181987; PMID: 22778725
- Mangsbo SM, Sandin LC, Anger K, Korman AJ, Loskog A, Tötterman TH. Enhanced tumor eradication by combining CTLA-4 or PD-1 blockade with CpG therapy. J Immunother 2010; 33:225 - 35; http://dx.doi.org/10.1097/CJI.0b013e3181c01fcb; PMID: 20445343
- Mangsbo SM, Ninalga C, Essand M, Loskog A, Tötterman TH. CpG therapy is superior to BCG in an orthotopic bladder cancer model and generates CD4+ T-cell immunity. J Immunother 2008; 31:34 - 42; http://dx.doi.org/10.1097/CJI.0b013e3181587d29; PMID: 18157010
- Lindqvist C, Sandin LC, Fransson M, Loskog A. Local AdCD40L gene therapy is effective for disseminated murine experimental cancer by breaking T-cell tolerance and inducing tumor cell growth inhibition. J Immunother 2009; 32:785 - 92; http://dx.doi.org/10.1097/CJI.0b013e3181acea69; PMID: 19752755
- Loskog AS, Fransson ME, Totterman TT. AdCD40L gene therapy counteracts T regulatory cells and cures aggressive tumors in an orthotopic bladder cancer model. Clin Cancer Res 2005; 11:8816 - 21; http://dx.doi.org/10.1158/1078-0432.CCR-05-1817; PMID: 16361570
- Malmström PU, Loskog AS, Lindqvist CA, Mangsbo SM, Fransson M, Wanders A, Gårdmark T, Tötterman TH. AdCD40L immunogene therapy for bladder carcinoma--the first phase I/IIa trial. Clin Cancer Res 2010; 16:3279 - 87; http://dx.doi.org/10.1158/1078-0432.CCR-10-0385; PMID: 20448220
- Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, Sullivan P, Mahany JJ, Gallagher M, Kramer A, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol 2007; 25:876 - 83; http://dx.doi.org/10.1200/JCO.2006.08.3311; PMID: 17327609
- Fransen MF, Sluijter M, Morreau H, Arens R, Melief CJ. Local activation of CD8 T cells and systemic tumor eradication without toxicity via slow release and local delivery of agonistic CD40 antibody. Clin Cancer Res 2011; 17:2270 - 80; http://dx.doi.org/10.1158/1078-0432.CCR-10-2888; PMID: 21389097
- Sandin LC, et al. Locally delivered CD40 agonist antibody accumulates in secondary lymphoid organs and eradicates experimental disseminated bladder cancer. Cancer Immunology Research 2013; In press http://dx.doi.org/10.1158/2326-6066.CIR-13-0067
