Abstract
Proper immunostimulation (“push”) and immune checkpoint blockade (“release”) are both critical for the efficacy of anticancer immunotherapy. We have recently shown that activating Toll-like receptor 9 (TLR9) while specifically blocking signal transducer and activator of transcription 3 (STAT3) in leukemic cells enhances their immunogenicity, allowing for CD8+ T cell-mediated tumor eradication. These findings underscore the therapeutic potential of such a “Push & Release” strategy against hematological malignancies.
Completely restoring the potential of the immune system to identify and selectively destroy cancer cells requires the disruption of a multilayered self-defense system established by the latter.Citation1 Targeting molecules that shield malignant cells from immunosurveillance has shown promise in clinical trials, and renewed the interest of the scientific and medical community in cancer immunotherapy.Citation1 Signal transducer and activator of transcription 3 (STAT3) is an oncogenic transcription factor commonly activated in tumors of various origin, including hematologic malignancies.Citation2 In addition, STAT3 operates in multiple immune cells that infiltrate neoplastic lesions, constituting a central mediator of tumor-induced immunosuppression.Citation2 Since STAT3 is activated both in malignant and tumor-associated immune cells, it is a unique target for therapeutic interventions. However, the pharmacological inhibition of STAT3 is challenging, calling for alternative targeting strategies. We have recently developed a novel approach for the cell-specific delivery of small-interfering RNAs (siRNAs) based on their targeting to intracellular Toll-like receptor 9 (TLR9) via TLR9 agonists, notably CpG oligodeoxynucleotides (CpG-ODNs) ().Citation3 Target cells quickly internalize CpG-ODN-coupled siRNAs into endosomes, where such conjugates undergo cleavage. In contrast to other siRNA delivery methods, which are associated with limited endosomal release, in our system TLR9 promotes the transport of uncoupled siRNAs to the RNA interference (RNAi) machinery of the endoplasmic reticulum ().Citation4 Thus, bi-functional CpG-ODN-coupled Stat3-targeting siRNAs (hereafter referred to as CpG-Stat3 siRNAs) operate as TLR9 agonists (“push”) while inhibiting STAT3 signaling (“release”), focusing immunostimulatory effects within the same target cell. We demonstrated that CpG-Stat3 siRNAs targeting tumor-associated myeloid cells, such as dendritic cells (DCs) and macrophages, significantly amplify the effects of TLR9 agonists employed as standalone interventions, hence restoring antitumor immune responses in mice. In hematological malignancies, such as acute myeloid leukemia (AML), B-cell lymphoma (BCL) or multiple myeloma (MM), TLR9 expression and STAT3 activation often involve both cancer cells and non-malignant immune cells.Citation5,Citation6 Based on these findings, we recently set out to explore the effects of systemic STAT3 blocking coupled to TLR9 activation in both such TLR9+ cell compartments, using syngeneic murine models of disseminated AML, including a genetic model that recapitulates a common cytogenetic abnormality of human AML, inv(16).Citation7
Figure 1. A “Push&Release” strategy for increasing the immunogenicity of acute myeloid leukemia cells. CpG oligodeoxynucleotides (CpG-ODN)-coupled signal transducer and activator of transcription 3 (STAT3)-targeting small-interfering RNAs (CpG-Stat3 siRNAs) are internalized by scavenger receptors and bind to Toll-like receptor 9 (TLR9) within endosomes. Therein, siRNAs are cleaved off from the conjugate by dicer 1, ribonuclease type III (DICER1). TLR9 facilitates the release and transport of STAT3-targeting siRNAs to argonaute RISC catalytic component 2 (AGO2) in the endoplasmic reticulum (ER), thereby initiating RNA interference (RNAi) and limiting STAT3 activity. The inhibition of STAT3 augments the immunostimulatory effects of TLR9 signaling, shifting the balance toward the production of pro-inflammatory cytokines and chemokines. At the same time, blocking STAT3 and stimulating TLR9 enhances the presentation of leukemia-specific antigens. Altogether, these effects generate systemic leukemia-specific CD8+ T cell-mediated immune responses and result in disease regression.
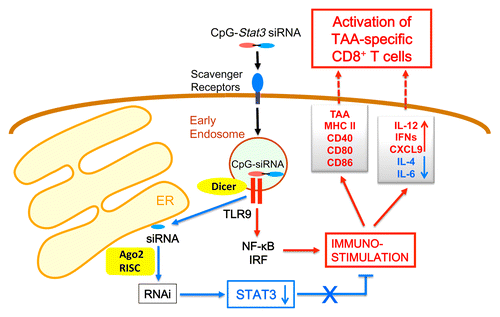
Our studies demonstrate that STAT3 negatively regulates antigen presentation not only by normal DCs but also by malignant leukemic cells. The systemic administration of our CpG-Stat3 siRNA alleviated the immunosuppressive effects of persistent STAT3 signaling in both these cell compartments. The concomitant inhibition of STAT3 and activation of TLR9 increased the expression of MHC class II and co-stimulatory molecules on the surface of both DCs and AML cells. This said, experiments involving the administration of CpG-Stat3 siRNAs to TLR9-deficient mice demonstrated that improving the immunogenicity of AML cells is sufficient to induce potent antitumor responses. Of note, the antineoplastic activity of CpG-Stat3 siRNAs was completely abrogated in immunodeficient mice, indicating that such therapeutic effects are primarily mediated by the immune system. In line with this notion, CpG-Stat3 siRNAs were shown to increase the circulating levels of interferon γ (IFNγ) and interleukin (IL)-12, 2 critical mediators of TH1 immune responses, while limiting the abundance of TH2 cytokines, such as IL-4 and IL-6. Antibody-mediated neutralization experiments and tumor-associated antigen recall assays confirmed the critical role of CD8+ T cells in the antitumor activity of CpG-Stat3 siRNAs. The targeted blocking of STAT3 coupled to the activation of TLR9 resulted in systemic cancer-specific immune responses that led to tumor eradication in multiple organs and prolonged the survival of the majority of disease mice.
Enduring AML remission requires the elimination of quiescent but self-renewable leukemia-initiating cells.Citation8 In serial transplantation experiments, we demonstrated that the repeated systemic administration of CpG-Stat3 siRNAs significantly reduces the engraftment and leukemia-initiating potential of AML cells. In general, these findings imply that immune responses induced by specifically blocking STAT3 and stimulating TLR9 in leukemia cells may have a broad effect against various AML cell subpopulations. Presumably, this reflect the ubiquitous expression of TLR9 in AML cells, irrespective of their cytogenetic subtype and hierarchy.Citation6 In addition, accumulating evidence demonstrates that TLR9 levels are upregulated in response to cellular or microenvironmental stress, even in cell populations that fail to express TLR9 in baseline conditions.Citation6,Citation9 Our preliminary studies confirmed that TLR9 is expressed by all cell compartments of primary human AML, including leukemic progenitors and stem cells, which are all sensitive to CpG-STAT3 siRNAs (Kortylewski, unpublished data). Other types of hematological cancers that express TLR9 and exhibit constitute STAT3 activation could potentially respond to this strategy.Citation6 In fact, it has recently been demonstrated that STAT3 inhibition increases the immunogenicity of mouse BCL cells, resulting in limited T-cell tolerance.Citation10 Our own results support the role of STAT3 as a regulator of immune checkpoints in BCL and underscore the feasibility of concomitantly blocking STAT3 and stimulating TLR9 for the immunotherapy of this hematological malignancy. We observed that the administration of CpG-Stat3 siRNAs combined with radiotherapy induces the regression of BCLs in immunocompetent mice, but only moderately inhibits lymphoma growth in immunodeficient animals.Citation6 Finally, human TLR9+ multiple myelomas are known for their elevated levels of constitute STAT3 activity, and were previously validated as a target for the CpG-siRNA strategy.Citation6 Whether inhibiting STAT3 while activating TLR9 enhances the immunogenicity of myeloma cells besides limiting their viability is still unknown. The availability of FDA-approved CpG ODNs and pharmacological JAK inhibitors provides an opportunity for combinatorial systemic treatments. However, the lack of specificity of both reagents for TLR9+ immune cells may limit the overall efficacy of this approach and result in off-target effects, which constitutes a concern especially for JAK inhibitors. Our proof-of-principle study indicates that even with a limited uptake of CpG-Stat3 siRNAs by leukemic cells (~15–50% of total AML cells, depending on location), this strategy cancer generate enduring systemic antitumor immunity. Nevertheless, the use of CpG-Stat3 siRNAs with an improved serum half-life can further enhance their efficacy and allow for a reduced administration frequency (Zhang and Kortylewski, unpublished data).
Overall, our results highlight the unique role of STAT3 as a regulator of immune checkpoints, while suggesting that its effects on the survival of leukemia cells may functionally overlap with those of other signal transducers. Additional efforts are needed to understand the molecular mechanisms that are activated in leukemic cells as a result of the inhibition of STAT3 coupled to the activation of TLR9. The negative impact of STAT3 on antigen presentation and TLR signalingCitation2 is well-established but there is much to learn about the pool of tumor-specific antigenic epitopes that are exposed as a result of STAT3 inhibition. Whether CpG-Stat3 siRNAs can expand the range of tumor-associated antigens presented on the surface of leukemic cells also remains to be explored. Such an effect could have important implications for designing new tumor-specific immunotherapies against leukemia and possibly other hematologic malignancies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Kortylewski M, Kuo Y. Push and release: TLR9 activation plus STAT3 blockade for systemic antitumor immunity. OncoImmunology 2013; 2:e27441; 10.4161/onci.27441
References
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252 - 64; http://dx.doi.org/10.1038/nrc3239; PMID: 22437870
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009; 9:798 - 809; http://dx.doi.org/10.1038/nrc2734; PMID: 19851315
- Kortylewski M, Swiderski P, Herrmann A, Wang L, Kowolik C, Kujawski M, Lee H, Scuto A, Liu Y, Yang C, et al. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat Biotechnol 2009; 27:925 - 32; http://dx.doi.org/10.1038/nbt.1564; PMID: 19749770
- Nechaev S, Gao C, Moreira D, Swiderski P, Jozwiak A, Kowolik CM, Zhou J, Armstrong B, Raubitschek A, Rossi JJ, et al. Intracellular processing of immunostimulatory CpG-siRNA: Toll-like receptor 9 facilitates siRNA dicing and endosomal escape. J Control Release 2013; 170:307 - 15; http://dx.doi.org/10.1016/j.jconrel.2013.06.007; PMID: 23777886
- Benekli M, Baumann H, Wetzler M. Targeting signal transducer and activator of transcription signaling pathway in leukemias. J Clin Oncol 2009; 27:4422 - 32; http://dx.doi.org/10.1200/JCO.2008.21.3264; PMID: 19667270
- Zhang Q, Hossain DM, Nechaev S, Kozlowska A, Zhang W, Liu Y, Kowolik CM, Swiderski P, Rossi JJ, Forman S, et al. TLR9-mediated siRNA delivery for targeting of normal and malignant human hematopoietic cells in vivo. Blood 2013; 121:1304 - 15; http://dx.doi.org/10.1182/blood-2012-07-442590; PMID: 23287859
- Hossain DM, Dos Santos C, Zhang Q, Kozlowska A, Liu H, Gao C, Moreira D, Swiderski P, Jozwiak A, Kline J, et al. Leukemia cell-targeted STAT3 silencing and TLR9-triggering generate systemic antitumor immunity. Blood 2013; Forthcoming PMID: 24169824
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997; 3:730 - 7; http://dx.doi.org/10.1038/nm0797-730; PMID: 9212098
- Shatz M, Menendez D, Resnick MA. The human TLR innate immune gene family is differentially influenced by DNA stress and p53 status in cancer cells. Cancer Res 2012; 72:3948 - 57; http://dx.doi.org/10.1158/0008-5472.CAN-11-4134; PMID: 22673234
- Cheng F, Wang H, Horna P, Wang Z, Shah B, Sahakian E, Woan KV, Villagra A, Pinilla-Ibarz J, Sebti S, et al. Stat3 inhibition augments the immunogenicity of B-cell lymphoma cells, leading to effective antitumor immunity. Cancer Res 2012; 72:4440 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-11-3619; PMID: 22728650
