Abstract
Monoclonal antibodies that target immune checkpoints are undoubtedly changing the therapeutic landscape of different human malignancies. Here we comment on the effects of blocking cytotoxic T lymphocyte-associated protein 4 (CTLA4) by means of the monoclonal antibody tremelimumab in patients with refractory malignant mesothelioma, a deadly disease with no effective therapeutic options.
“Immunotherapists” are well acquainted with the notion that achieving a long-term disease control and improving survival by modulating the immune system is a reachable goal in a proportion of cancer patients.Citation1 The significant clinical success obtained by a novel class of therapeutic monoclonal antibodies (mAb) that target immune checkpoints is spreading this knowledge and conviction among an ever larger community of oncologists. Along similar lines, the clear-cut therapeutic success achieved in cutaneous melanoma patients by ipilimumab, a mAb specific for blocking cytotoxic T lymphocyte-associated protein 4 (CTLA4), has broadened its therapeutic indications on one hand,Citation2 and has prompted the clinical development of additional checkpoint- blocking mAbs, including molecules that target programmed cell death 1 (PDCD1, best known as PD-1) and its main ligand CD274 (best known as PD-L1), on the other hand.Citation3 Based on accumulating clinical data, a further step into the future will undoubtedly involve the combinatorial and/or sequential administration of multiple checkpoints-blocking mAbs, as well as their association with conventional chemotherapeutic and/or active immunotherapy (e.g., anticancer vaccines).
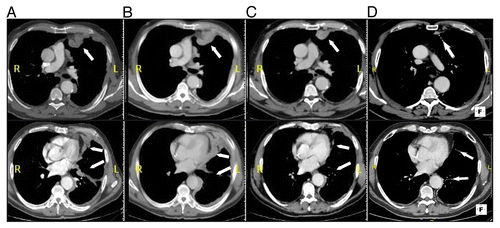
In spite of the steadily increasing incidence of this disease, the current therapeutic options against malignant mesothelioma (MM) are largely unsatisfactory. The combination of platinum-based chemotherapy and pemetrexed constitutes indeed the standard of care, being associated with a median survival of only 12 mo.Citation4 Furthermore, second-line treatments remain an unsolved issue, since different cytotoxic chemotherapeutic and targeted anticancer agents have failed to exert clinical activity in MM patients.Citation5 Seeking to assess the therapeutic potential of blocking CTLA4 in malignancies previously unexplored in this sense and generally associated with dismal prognosis, we designed the MESOT-TREM-2008 study. In this context, we investigated the clinical and immunological activity of the anti-CTLA4 mAb tremelimumab in unresectable MM patients who failed a first-line platinum-containing chemotherapeutic regimen. The preliminary results of this clinical trial have recently been published in The Lancet Oncology.Citation6 Though the primary endpoint of the study (i.e., objective response rate) was not met, 2 out of 29 patients treated with tremelimumab achieved a durable partial response, and 7 had a prolonged stabilization of disease, with a comprehensive disease control rate of 31%. Initial disease progression followed by a long-lasting partial response was observed in one patient (). Such an “unusual” pattern of response has been previously documented in metastatic melanoma patients receiving ipilimumab.Citation7 Thus, our findings corroborate the notion that blocking CTLA4 can lead to “peculiar” clinical responses in patients affected by various tumor types. This implies that a careful assessment of disease progression is mandatory before the discontinuation of anti-CTLA4 therapy, as clinical benefits may be delayed.Citation7 Such an aspect is highly relevant for patients with MM, a setting in which radiological assessments are central in the evaluation of disease progression and response to treatment.Citation8 An additional typical feature of CTLA4 blockade is the induction of persistent disease stabilization. Indeed, a long-lasting stable disease was achieved by 24% of the MM patients enrolled in our study. The 1- and 2-y survival rates that we observed, 48.3% and 36.7%, respectively, compare quite favorably with available data for MM patients.Citation5 Though limited by the small cohort size, our study identified a proportion of MM patients who may obtain long-term clinical benefits from tremelimumab, which is consistent with extensive clinical data collected in metastatic melanoma patients receiving ipilimumab.Citation9
Figure 1. CT scan of a malignant pleural mesothelioma patient receiving tremelimumab. (A–D) Computer tomography (CT) scans were performed at baseline (A) and after the second (day 180, B), fourth (day 470, C), and seventh (day 630, D) dose of tremelimumab. The patient achieved a partial response (C and D) after initial disease progression was noted at the first (data not shown) and second assessments (B).
The identification of robust biomarkers that might predict the response of cancer patients to CTLA4-blocking agents is still a major, unsettled topic. In our study, we prospectively demonstrated that the increase in the absolute number of circulating CD4+ICOS+ T lymphocytes in the very early phases of treatment is predictive of an improved survival. However, the levels of CD4+ICOS+ T lymphocytes before therapy did not allow for the identification of patients who would experience a favorable disease outcome upon the administration of tremelimumab.Citation6 This finding, which is in line with retrospective data obtained in metastatic melanoma patients treated with ipilimumab,Citation9 suggests that the analysis of circulating CD4+ICOS+ T lymphocytes might represent a general, dynamic, tool to guide the use of anti-CTLA4 mAbs in patients affected by different tumor types.Citation10
Though limited by the restricted size of the patient cohort, the MESOT-TREM-2008 study provided initial, and in our view highly encouraging, evidence in support of the clinical and immunological activity of tremelimumab in pre-treated MM patients. To further corroborate these findings, we launched the MESOT-TREM-2012 study (NCT01655888), exploring a more intensive tremelimumab administration schedule in second-line MM patients. The results of this clinical trial are eagerly awaited.
Disclosure of Potential Conflicts of Interest
Luana Calabrò declares no conflict of interest; Michele Maio is consultant, advisor, or both, to Bristol-Myers Squibb, Roche-Genetech, and Medimmune.
Acknowledgments
This work was supported by unrestricted grants from the Associazione Italiana per la Ricerca sul Cancro, Istituto Toscano Tumori.
Citation: Calabró L, Maio M. Immune checkpoint blockade in malignant mesothelioma: A novel therapeutic strategy against a deadly disease?. OncoImmunology 2013; 2:e27482; 10.4161/onci.27482
References
- Eggermont AM, Kroemer G, Zitvogel L. Immunotherapy and the concept of a clinical cure. Eur J Cancer 2013; 49:2965 - 7; http://dx.doi.org/10.1016/j.ejca.2013.06.019; PMID: 23890942
- Calabrò L, Danielli R, Sigalotti L, Maio M. Clinical studies with anti-CTLA-4 antibodies in non-melanoma indications. Semin Oncol 2010; 37:460 - 7; http://dx.doi.org/10.1053/j.seminoncol.2010.09.006; PMID: 21074061
- Mullard A. New checkpoint inhibitors ride the immunotherapy tsunami. Nat Rev Drug Discov 2013; 12:489 - 92; http://dx.doi.org/10.1038/nrd4066; PMID: 23812256
- Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003; 21:2636 - 44; http://dx.doi.org/10.1200/JCO.2003.11.136; PMID: 12860938
- Zucali PA, Simonelli M, Michetti G, Tiseo M, Ceresoli GL, Collovà E, Follador A, Lo Dico M, Moretti A, De Vincenzo F, et al. Second-line chemotherapy in malignant pleural mesothelioma: results of a retrospective multicenter survey. Lung Cancer 2012; 75:360 - 7; http://dx.doi.org/10.1016/j.lungcan.2011.08.011; PMID: 21937142
- Calabrò L, Morra A, Fonsatti E, Cutaia O, Amato G, Giannarelli D, Di Giacomo AM, Danielli R, Altomonte M, Mutti L, et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol 2013; 14:1104 - 11; http://dx.doi.org/10.1016/S1470-2045(13)70381-4; PMID: 24035405
- Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009; 15:7412 - 20; http://dx.doi.org/10.1158/1078-0432.CCR-09-1624; PMID: 19934295
- Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol 2004; 15:257 - 60; http://dx.doi.org/10.1093/annonc/mdh059; PMID: 14760119
- Di Giacomo AM, Calabrò L, Danielli R, Fonsatti E, Bertocci E, Pesce I, Fazio C, Cutaia O, Giannarelli D, Miracco C, et al. Long-term survival and immunological parameters in metastatic melanoma patients who responded to ipilimumab 10 mg/kg within an expanded access programme. Cancer Immunol Immunother 2013; 62:1021 - 8; http://dx.doi.org/10.1007/s00262-013-1418-6; PMID: 23591982
- Calabrò L, Maio M. Biomarkers for immune checkpoint inhibitors. Lancet Oncol 2014; 15:e1; http://dx.doi.org/10.1016/S1470-2045(13)70573-4
