Abstract
The mechanisms by which retinoblastoma 1 (RB1) mediates oncosuppressive functions are still being elucidated. We found that radiation-induced senescence in the bone depends on RB1 and is associated with the secretion of multiple bioactive factors, including interleukin-6 (IL-6), as well as with the infiltration of natural killer T (NKT) cells. Importantly, the inhibition of RB1, IL-6 or NKT cells predisposed mice to radiation-induced osteosarcomas, unveiling a cancer cell-extrinsic mechanisms that underlie the oncosuppressive activity of RB1.
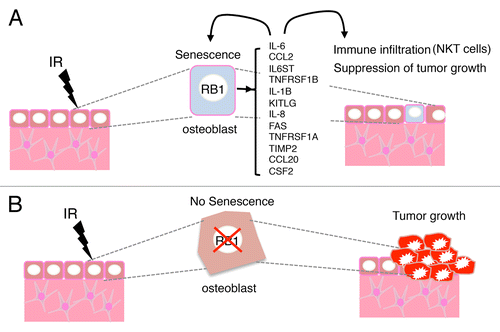
Germline mutations in retinoblastoma 1 (RB1) and ionizing radiation are the major risk factors for the development of osteosarcoma. We have recently demonstrated that normal bone cells sustaining radiation injury enter a state of permanent proliferative arrest known as cellular senescence.Citation1 This state is associated with the secretion of a large panel of growth factor and cytokines, including interleukin (IL)-6, that reinforce senescence in an autocrine/paracrine fashion. Interestingly, these mediators also recruit various components of the immune system including natural killer T (NKT) cells, are play a prominent role in cancer immunosurveillance. Altogether, the soluble mediators released by senescent bone cells and the immune effector cells they recruit contribute to tumor suppression upon the exposure of osteoblasts to carcinogenic doses of ionizing radiation in vivo ().
Figure 1. Cancer cell-extrinsic oncosuppressive functions of RB1. (A) The irradiation of osteoblasts with functional retinoblastoma 1 (RB1) results in the emission of an alarm signal that comprise (among other molecules) interleukin (IL)-1β, IL-6 and IL-8. This signal reinforces senescence in an autocrine or paracrine fashion, but also recruits immune cells, including natural killer T (NKT) cells. (B) In osteoblasts lacking RB1 irradiation fails to induce the secretion of IL-6 and the consequent recruitment of NKT cells, hence favoring oncogenesis.
Our findings add to a wealth of data linking senescence, once thought to be a purely cell-autonomous oncosuppressive process like apoptosis, to cancer immunosurveillance. In osteosarcoma cell lines, the reintroduction of functional RB1 results in a permanent cell cycle exit and senescence. Senescence is also induced in normal cells by the ectopic expression of oncogenes. Generally, this results not only in a definite growth arrest, but also in the release of a large panel of bioactive molecules, a process referred to as “senescence-associated secretory phenotype” (SASP). Our data place these observations into a context that go beyond radiation-induced osteosarcoma formation.
Another interesting implication of our findings relates to the in vivo model systems that are available for the study of osteosarcoma. These generally include xenograft models, whereby human cell lines are implanted into immunocompromised mice, genetically engineered or carcinogen-induced murine models, and large canine breeds, in which spontaneous osteosarcoma is relatively common.Citation2 The use of xenografts is associated with obvious limitations, especially in an era in which the contribution of the immune system to the efficacy of anticancer therapy is being increasingly recognized. Genetically engineered mouse models come in 2 flavors: those in which an oncogene (e.g., Fos) is activated,Citation3 and those in which one or more oncosuppressor gene(s) (e.g., Rb1 or Trp53) are deleted, in both cases either conditionally within osteoblasts or at the whole-body level.Citation4 Since we have shown that the deletion of Rb1 abrogates senescence as induced in osteoblasts by carcinogenic stimuli, and presumably also by ectopic oncogene activation, murine osteosarcoma modes based on the inactivation of Trp53, either alone or together with Rb1, probably fail to engage the immune system in the way we observed in our study. It is difficult to know how this relates to human osteosarcoma. However, a limited fraction of osteosarcoma cases have been documented in families with the Li-Fraumeni syndrome (bearing germline mutations in TP53) or familial retinoblastoma (bearing germline mutations in RB1).
Moreover, we found that the SASP and RB1 expression correlate with improved clinical outcome in patients affected by primary osteosarcomas, most of whom have not been exposed to ionizing radiation. Taken together, these observations suggest that murine models of osteosarcoma driven by the deletion of key genes involved in senescence may not recapitulate the immunological aspects of the human disease.
The molecular circuitries that underpin the complex features of senescence are not well understood. The spectrum of bioactive molecules released by a given cell undergoing senescence is dependent not only on tissue type but also on the initiating insult. In the irradiated bone, RB1 promotes the expression of IL-1β, IL-6, IL-8, KIT ligand (KITLG), FAS, chemokine (C-C motif) ligand 2 (CCL2), CCL20, and tumor necrosis factor receptor superfamily, member 11B (TNFRSF11B, best known as OPG). The genes coding for all these mediators are direct targets of the transcription factor NF-κB. RB1 has previously been shown to activate NF-κB, although we did not test this in our paper.Citation5 In addition, the inhibition of NF-κB appears to delay DNA damage-induced senescence in aging mice.Citation6 In the bone, RB1 is likely to play a role in the E2F-mediated repression of genes controlling cellular proliferation as well as in the activation of NF-κB-regulated SASP-underlying genes, thus promoting senescence and the activation of an immune response.
IL-6 is a key component of the SASP response to irradiation. IL-6 is known to exert pro- and antitumor functions, dependent on tumor type and stage.Citation7 We propose that the immediate response of osteoblasts to genotoxic stress results in the RB1-dependent secretion of IL-6, which mediates antineoplastic effects early in osteosarcoma development as it reinforces senescence and alerts the immune system. IL-8 and chemokine (C-X-C motif) ligand 2 (CXCL2, also known as MIP2) are also upregulated by irradiation and have been shown to reinforce senescence as part of a positive feedback loop that amplifies the DNA damage response.Citation8 We demonstrated that exogenous recombinant IL-6 can restore the senescent response of RB1-deficient cells exposed to radiation ex vivo. In mice, IL-6 regulates the trafficking of NKT cells in a paracrine manner. We also propose that the introduction of IL-6 into an IL-6-naïve microenvironment may act as an adjuvant to stimulate antitumor immune responses. Indeed, the growth of IL-6-deficient osteosarcomas was suppressed when these tumors were implanted into wild-type mice. Similarly, the administration of exogenous IL-6 may also activate antitumor immune responses to osteosarcoma. Increased IL-6 expression has been correlated with infection and inflammation following surgery, and in osteosarcoma patients post-operative infections are associated with improved survival.Citation9 Liposomal muramyl tripeptide phosphatidylethanolamine (mifamurtide, trade name Mepact®) is currently approved for the treatment of non-metastatic osteosarcoma. This synthetic analog of the mycobacterial cell wall is a non-specific immunostimulatory agent. The administration of mifamurtide is also associated with an increased secretion of several cytokines including IL-6.Citation10 Our results suggest that the RB1-mediated release of IL-6 in response to radiation promotes senescence and the recruitment of NKT cells, which contribute to cancer immunosurveillance. These findings extend our understanding on the oncosuppressive functions of RB1, and link them to emerging therapeutic opportunities whereby immunomodulators may play an important role against the development and progression of osteosarcoma.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Kansara M, Thomas D. RB1 mediated cell-autonomous and host-dependent oncosuppressor mechanisms in radiation-induced osteosarcoma. OncoImmunology 2013; 2:e27569; 10.4161/onci.27569
References
- Kansara M, Leong HS, Lin DM, Popkiss S, Pang P, Garsed DW, Walkley CR, Cullinane C, Ellul J, Haynes NM, et al. Immune response to RB1-regulated senescence limits radiation-induced osteosarcoma formation. J Clin Invest 2013; 123:5351 - 60; http://dx.doi.org/10.1172/JCI70559; PMID: 24231354
- Mohseny AB, Hogendoorn PC, Cleton-Jansen AM. Osteosarcoma models: from cell lines to zebrafish. Sarcoma 2012; 2012:417271; http://dx.doi.org/10.1155/2012/417271; PMID: 22566751
- Wang ZQ, Liang J, Schellander K, Wagner EF, Grigoriadis AE. c-fos-induced osteosarcoma formation in transgenic mice: cooperativity with c-jun and the role of endogenous c-fos. Cancer Res 1995; 55:6244 - 51; PMID: 8521421
- Walkley CR, Qudsi R, Sankaran VG, Perry JA, Gostissa M, Roth SI, Rodda SJ, Snay E, Dunning P, Fahey FH, et al. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev 2008; 22:1662 - 76; http://dx.doi.org/10.1101/gad.1656808; PMID: 18559481
- Takebayashi T, Higashi H, Sudo H, Ozawa H, Suzuki E, Shirado O, Katoh H, Hatakeyama M. NF-kappa B-dependent induction of cyclin D1 by retinoblastoma protein (pRB) family proteins and tumor-derived pRB mutants. J Biol Chem 2003; 278:14897 - 905; http://dx.doi.org/10.1074/jbc.M210849200; PMID: 12594215
- Tilstra JS, Robinson AR, Wang J, Gregg SQ, Clauson CL, Reay DP, Nasto LA, St Croix CM, Usas A, Vo N, et al. NF-κB inhibition delays DNA damage-induced senescence and aging in mice. J Clin Invest 2012; 122:2601 - 12; http://dx.doi.org/10.1172/JCI45785; PMID: 22706308
- Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for translational therapeutics. Cancer 2007; 110:1911 - 28; http://dx.doi.org/10.1002/cncr.22999; PMID: 17849470
- Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008; 133:1019 - 31; http://dx.doi.org/10.1016/j.cell.2008.03.039; PMID: 18555778
- Jeys LM, Grimer RJ, Carter SR, Tillman RM, Abudu A. Post operative infection and increased survival in osteosarcoma patients: are they associated?. Ann Surg Oncol 2007; 14:2887 - 95; http://dx.doi.org/10.1245/s10434-007-9483-8; PMID: 17653803
- Asano T, Kleinerman ES. Liposome-encapsulated MTP-PE: a novel biologic agent for cancer therapy. J Immunother Emphasis Tumor Immunol 1993; 14:286 - 92; http://dx.doi.org/10.1097/00002371-199311000-00006; PMID: 8280710
