Abstract
We recently dissected how senescent tumors can trigger complementing signaling pathways that mobilize natural killer (NK) cells to eliminate malignant cells. In addition to cell-intrinsic effects on proliferation, senescence induces the production of chemokine (C-C motif) ligand 2 (CCL2), which recruits NK cells to mediate direct tumoricidal effects. Hence, senescence activates a cancer cell-extrinsic oncosuppression program.
Keywords: :
Cellular senescence is a complex biological program during which cells remain biologically active but permanently lose their ability to divide. In healthy cells experiencing oncogenic signaling, the oncosuppressive pathways mediated by tumor protein TP53 (best known as p53) and cyclin-dependent kinase inhibitor 1A (CDKN1A, best known as p21CIP1) and/or by CDKN2A (best known as p16INK4A) and retinoblastoma 1 (RB1) are activated to trigger oncogene-induced senescence (OIS), mainly owing to the transactivation of genes that block cell cycle progression and promote the senescent state.Citation1,Citation2 It is now accepted that OIS plays an important role in preventing the development of malignant lesions from pre-malignant cells.Citation1,Citation3 Our laboratory has recently been interested in understanding the relationship between the senescent state and the activation of innate immune responses that have the potential to control oncogenesis.Citation4 Although senescence involves an intrinsic block in cell division, it may also cause the mobilization of cancer cell-extrinsic antitumor mechanisms, including antitumor immune responses. Indeed, previous studies suggested that the destruction of senescent cells by the immune system plays a role in cancer immunosurveillance as well as in the prevention of non-malignant diseases such as liver fibrosis.Citation5-Citation7 These studies implicated natural killer (NK) cells, macrophages, granulocytes and CD4+ T cells in the elimination of senescent cells.
Using the model of hepatocellular carcinoma developed by Xue et al.,Citation5 we have recently investigated how p53-expressing senescent tumors mobilize the NK-cell response and how NK cells recognize senescent malignancies. In this study, we were able to dissect one of the mechanisms that specifically link the senescence program to NK cell-mediated cancer immunosurveillance.Citation4 Using killer cell lectin-like receptor subfamily K, member 1 (NKG2D, known as NKG2D or KLRK1 in humans)-deficient hosts and antibody blockade strategies, we demonstrated that the NKG2D NK cell-activating receptor is largely responsible for the NK cell-dependent elimination of senescent tumors. Interestingly, however, the amounts of NKG2D ligands expressed on the surface of cells undergoing senescence upon p53 restoration were not increased, in line with our previous findings suggesting that the expression of mouse NKG2D ligands is controlled by a variety of pathways that are independent of p53.Citation8 Thus, both proliferating and senescent cancer cells expressed robust amounts of the NKG2D ligand retinoic acid early transcript 1e (Raet1e), and p53 restoration did not increase the sensitivity of malignant cells to elimination by NK cells. Instead, our study revealed that p53-induced senescence causes a dramatic infiltration of NK cells (as well as many myeloid cells) into the tumor mass, resulting in progressive tumor elimination. Interestingly, such a response did not rely on NKG2D, suggesting that p53 causes tumor infiltration by NK cells independently of their ability to directly recognize malignant cells.Citation4
The senescent state is associated with a number of phenotypic and functional alterations, including the secretion of soluble factors involved in the maintenance of senescence itself and other biological processes.Citation9 In particular, senescence promotes the so-called “senescence-associated secretory phenotype” (SASP), a state that is marked by the secretion of numerous pro-inflammatory cytokines and chemokines involved in the regulation of immune cells, which might promote or repress cancer progression in a context-dependent manner.Citation9 In our study, p53 restoration induced the expression of several chemokines that are known to recruit immune cells, including chemokine (C-C motif) ligand 2 (CCL2), CCL3, CCL4, CCL5 as well as chemokine (C-X-C motif) ligand 1 (CXCL1) and CXCL2. p53 restoration also induced the expression of cytokines that activate NK cells against cancer cells, including interleukin (IL)-12, IL-15 and IL-18. Among the chemokines produced upon p53 restoration, CCL2, CCL3, CCL4, and CCL5 have all been involved in the recruitment of NK cells in various experimental settings.Citation10 By means of an antibody-mediated neutralizing strategy, we demonstrated that CCL2 plays a major role in the infiltration of senescent tumors by NK cells. Conversely, neutralizing CCL3, CCL4, and CCL5 had no effect in this setting. Moreover, neutralization of CCL2, but not neutralization of CCL3, CCL4, and CCL5, resulted in a significant decrease in tumor elimination, suggesting a non-redundant role for the p53-dependent expression of CCL2 in the NK cell-dependent elimination of senescent tumors in vivo. Taken together, our data suggest that the secretion of CCL2 by senescent tumors is required for their elimination by NK cells that recognize NKG2D ligands on the surface of malignant cells ().
Interestingly, our study suggests that different pathways activated in senescent neoplastic cells cooperate to trigger their NK cell-dependent elimination. In particular, while p53-independent pathways lead to the expression of NKG2D ligands on the surface of malignant cells, tumor infiltration by large numbers of NK cells depends on the p53-induced secretion of CCL2 by senescent tumors. Both these pathways, which are involved in the mobilization of NK cells and their ability to recognize malignant cells, turned out to be required for NK cell-dependent tumor elimination.
Our study highlights one mechanism whereby p53-induced cellular senescence exerts oncosuppressive effects by a cancer cell-extrinsic pathway that relies on the immune system. These findings demonstrate the importance of NK cells at the early stages of tumorigenesis, when p53 has not yet been lost or inactivated (an event that occurs in the majority of developing tumors). Our study may also explain how the loss of p53 can impair cancer immunosurveillance, i.e., as it limits the oncosuppressive functions mediated by NK cells. Novel strategies aimed at restoring p53 expression and activation might therefore be fruitful for promoting the infiltration of neoplastic lesions by immune effectors, notably NK cells, and tumor elimination. Moreover, the therapeutic restoration of p53 in a fraction of malignant cells may be sufficient to mobilize NK cells, which would kill both p53-proficient and p53-deficient neoplastic cells. Conversely, the cell-intrinsic oncosuppressive effects of p53 mediated by a cell cycle arrest or apoptosis would presumably require p53 restoration in all neoplastic cells.
Further studies are required to understand the senescence-associated events that underlie the activation of innate immune responses against senescent cancer cells. Characterizing the SASP and its role in the regulation of immune cell effector functions represents a new avenue of investigation in the field of senescence research. Such a line of investigation will certainly provide a better understanding of immunosurveillance and may uncover new immunotherapeutic strategies against a broad variety of cancers.
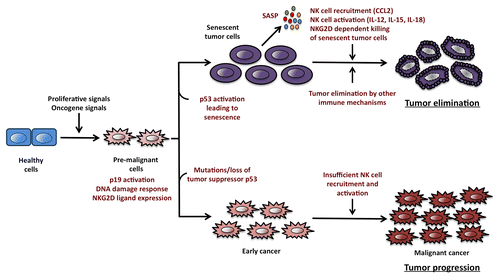
Figure 1. Oncogene-induced senescence promotes immunosurveillance by natural killer cells. The activation of oncogenes and the consequent delivery of proliferative signals to healthy cells generate a pool of pre-malignant cells expressing ligands for the NKG2D receptor (also called killer cell lectin-like receptor subfamily K, member 1, KLRK1, in humans). In this setting, cyclin-dependent kinase inhibitor 2A, isoform 4 (also called p19ARF in mice or p16ARF in humans) and the DNA damage response (DDR) can activate p53 and other oncosuppressive factors that promote cellular senescence. Senescent cells stop proliferating and mobilize an oncosuppressive mechanism mediated by immune cells. The so-called “senescence-associated secretory phenotype” (SASP) is associated with the release of chemokines such as chemokine (C-C motif) ligand 2 (CCL2) and cytokines like interleukin (IL-12), IL-15 and IL-18, which are involved in the recruitment and activation of natural killer (NK) cells. Once activated, NK cells infiltrate neoplastic lesions and kill senescent cancer cells upon the recognition of NKG2D ligands displayed on their surface. These processes result in the progressive elimination of malignant cells and hence inhibit tumor progression. In contrast, Tp53 mutations allow cancer cells to bypass the senescence program and hence form tumors that are not subjected to NK cell-dependent immunosurveillance. Indeed, although p53-deficient malignant cells express near-to-normal levels of NKG2D ligands on their surface, they are unable to efficiently recruit and activate NK cells, which allows tumors to escape immunosurveillance.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We gratefully acknowledge discussions and research efforts of members of the Raulet laboratory. The work discussed herein was supported by grant R01 AI039642 from the National Institutes of Health to D.H. Raulet. A. Iannello was supported by the Canadian Institutes for Health Research (CIHR) Banting postdoctoral scholarship and a Special Fellow Award from the Leukemia and Lymphoma Society.
Citation: Iannello A, Raulet DH. Immunosurveillance of senescent cancer cells by natural killer cells. OncoImmunology 2013; 2:e27616; 10.4161/onci.27616
References
- Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat Rev Cancer 2010; 10:51 - 7; http://dx.doi.org/10.1038/nrc2772; PMID: 20029423
- Zilfou JT, Lowe SW. Tumor suppressive functions of p53. Cold Spring Harb Perspect Biol 2009; 1:a001883; http://dx.doi.org/10.1101/cshperspect.a001883; PMID: 20066118
- Braig M, Schmitt CA. Oncogene-induced senescence: putting the brakes on tumor development. Cancer Res 2006; 66:2881 - 4; http://dx.doi.org/10.1158/0008-5472.CAN-05-4006; PMID: 16540631
- Iannello A, Thompson TW, Ardolino M, Lowe SW, Raulet DH. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med 2013; 210:2057 - 69; http://dx.doi.org/10.1084/jem.20130783; PMID: 24043758
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 2007; 445:656 - 60; http://dx.doi.org/10.1038/nature05529; PMID: 17251933
- Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 2011; 479:547 - 51; http://dx.doi.org/10.1038/nature10599; PMID: 22080947
- Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell 2008; 134:657 - 67; http://dx.doi.org/10.1016/j.cell.2008.06.049; PMID: 18724938
- Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol 2013; 31:413 - 41; http://dx.doi.org/10.1146/annurev-immunol-032712-095951; PMID: 23298206
- Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol 2013; 75:685 - 705; http://dx.doi.org/10.1146/annurev-physiol-030212-183653; PMID: 23140366
- Grégoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunol Rev 2007; 220:169 - 82; http://dx.doi.org/10.1111/j.1600-065X.2007.00563.x; PMID: 17979846
