Abstract
Although high levels of tumor-infiltrating lymphocytes (TILs) generally correlate with good prognosis in high-grade serous ovarian cancer (HGSC) patients, little is known about the phenotype or specificity of these cells. We have recently demonstrated that TIL expressing the intra-epithelial lymphocyte marker CD103 (official name, integrin αE, ITGAE) abundantly infiltrate HGSCs, strongly correlating with increased disease-specific survival.
In recent years, an ever growing number of studies has revealed that high levels of tumor-infiltrating lymphocytes (TILs) correlate with a favorable long-term prognosis in patients affected by various epithelial neoplasms.Citation1 Although TILs comprise a complex mixture of immune cells, CD8+ T cells stand out as the TILs associated with the most robust prognostic value in a majority of settings.Citation2 In ovarian cancer patients, the survival benefit is generally more pronounced when CD8+ TILs are located within the neoplastic epithelium rather than in the tumor-associated stroma.Citation3,Citation4 This epithelial/stromal distinction makes the precise quantification of TILs challenging as the epithelial and stromal areas of the tumor are often in close juxtaposition. Moreover, the molecular mechanisms that are responsible for the epithelial localization of TILs remain poorly defined.
Our group has recently described a subset of CD8+ T cells infiltrating human ovarian cancers that express CD103 (official name integrin αE, ITGAE) on the cell surface.Citation5,Citation6 CD103 is the αE subunit of the dimeric αE/β7 integrin, which binds to the epithelial cell surface molecule E-cadherin. Adhesive interactions between αE/β7 and E-cadherin play an important role in the retention of antigen-specific lymphocytes within epithelial tissues. Accordingly, CD103 is expressed by < 2% of circulating T cells but is widely expressed on intra-epithelial lymphocytes (IELs) of the gut mucosa and skin.Citation7 CD103 is also expressed by the majority of tissue-infiltrating, alloreactive CD8+ T cells in the course of transplant rejectionCitation8 and graft-vs.-host reactions.Citation9 Furthermore, CD103 is increasingly being recognized as a definitive marker of so-called tissue resident memory CD8+ T cells (CD8+ TRM cells) in the setting of infectious diseases.Citation10
Although prior anecdotal reports have documented the presence of CD103+ TILs within epithelial neoplasms, their precise intratumoral location and prognostic significance has been difficult to ascertain, mostly due to the lack of a CD103-specific antibody suitable for use on paraffin-embedded tissue sections. Fortuitously, such a reagent has recently been developed for the detection of hairy cell leukemia cells, which coincidentally also express CD103 on their surface.Citation11 Using this new antibody, we found that CD103+ cells infiltrate all 4 major histological subtypes of ovarian cancer, reaching the highest densities in high-grade serous ovarian cancer (HGSC) lesions.Citation6 Importantly, CD103+ TILs preferentially localize to the tumor epithelium in both ovarian and breast carcinoma lesions, and although most CD103+ TILs turned out to be CD8+ T cells, some tumors also contained high amounts of CD103+CD56+ natural killer (NK) cells. By univariate and multivariate analysis, CD103+ TILs were significantly associated with disease-specific survival in HGSC patients, a prognostic effect that was attributable to CD103+CD8+ T cells rather than to CD103+CD56+ NK cells.
Given their striking clinical significance, what can be inferred about the functional properties of CD103+ TILs? Earlier studies showed that CD8+ T cells upregulate CD103 upon antigenic stimulation in the presence of transforming growth factor-β (TGF-β).Citation9 This requirement for dual antigen/TGF-β co-stimulation suggests that CD103+ TILs might be actively responding to tumor-associated antigens (TAAs) in a TGF-β-rich tumor microenvironment. This hypothesis would also be consistent with our finding that CD103+ TILs display an activated effector memory phenotype (CD62L-CD27+CD28-HLA-DRhi) and that the frequency of CD103+ TILs in ovarian cancer-associated ascites correlates with the level of TGF-β in the ascites fluid.Citation5 In addition, virtually all CD103+ TILs express TIA1, a marker of cytolytic activity that also has a high prognostic significance for ovarian cancer patients.Citation12 Thus, CD103 demarcates a subset of intraepithelial, activated, cytolytic CD8+ T cells exhibiting a highly favorable phenotype.
Despite their desirable phenotype, however, CD103+ TILs failed to impede primary tumor progression, as our patients presented with advanced disease requiring clinical intervention. Thus, we further speculate that tumor-specific CD103+ TILs were functional earlier in the oncogenic process, but have become “trapped” in the tumor bed as a consequence of CD103/E-cadherin adhesive interactions. This would result in a state of chronic antigen stimulation over a period of weeks to months, potentially leading to functional exhaustion. Consistent with this scenario, we have recently found that the vast majority of CD103+ cells infiltrating ovarian cancer lesions express the T-cell exhaustion marker programmed cell death 1 (PDCD1, best known as PD-1),Citation6 suggesting that they are under the control of this well-characterized, peripheral immune checkpoint mechanism. To explain the association between CD103+ TILs with favorable disease outcome among ovarian cancer patients, one must remember that these cells are actually removed during surgical de-bulking, implying that they themselves cannot be responsible for improved prognosis. Therefore, we propose that CD103+ TILs act as a surrogate indicator of either a highly immunogenic tumor, or a patient with residual anti-tumor immunity in the periphery post-surgery, or both. Antitumor immunity could be successfully re-engaged upon cytoreductive surgery or chemotherapy and could impede tumor re-growth for some period, until the above described exhaustion scenario eventually repeats itself. Looking ahead, the fact that most CD103+ TILs express PD-1 raises the encouraging prospect that the functional activity of these cells could be enhanced by PD-1-blocking interventions. Indeed, based on results from our group and others, CD103 warrants investigation as a predictive biomarker for clinical responses to checkpoint-blocking approaches. CD103 might also prove useful to isolate specific TIL subsets for functional studies, antigen discovery, or adoptive cell transfer-based therapeutic interventions.
In conclusion, our findings strongly suggest that CD103 is a definitive marker of intraepithelial, tumor-specific TILs in ovarian cancer, paving the way to the discovery of novel TAAs and perhaps the development of novel therapeutic strategies. We also find that intraepithelial CD103+ TILs are present in a small proportion of breast cancers, implying that CD103 may be a universal feature of TIL in epithelial cancers. If this were indeed the case, CD103+ cells might constitute prominent targets for the development of novel therapeutic regimens against these dreadful neoplasms. ()
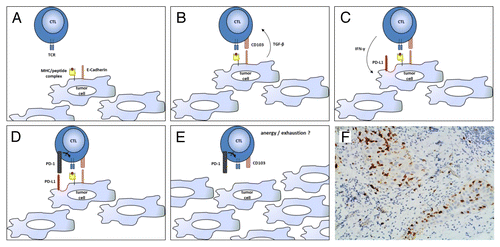
Figure 1. CD103+ lymphocytes infiltrating human ovarian carcinoma. (A–E) Ovarian cancer-infiltrating lymphocytes (TILs) express CD103 in response to tumor-associated antigen (TAAs) and transforming growth factor-β (TGF-β). CD103+ tumor-infiltrating lymphocytes (TILs) efficiently control tumor growth for a while, but then become trapped within neoplastic lesions as a consequence of CD103 expression, eventually becoming exhausted (PD-1+) owing to chronic antigen stimulation. (F) CD103+ TILs in a high-grade serous ovarian cancer specimen obtained from cytoreductive surgery. The section was stained with an anti-CD103 rabbit monoclonal antibody (Epitomics clone EPR4166Citation2), an anti-rabbit horseradish peroxidase-conjugate antibody and diaminobenzidine (DAB). CD103+ TILs (stained in brown) can be seen clustering within epithelial tumor regions. CTL, cytotoxic T lymphocyte; IFNγ, interferon γ; PD-L1, PD-1 ligand 1; TCR, T-cell receptor.
Citation: Webb JR, Milne K, Nelson BH. Location, location, location: CD103 demarcates intraepithelial, prognostically favorable CD8+ tumor-infiltrating lymphocytes in ovarian cancer. OncoImmunology 2013; 2:e27668; 10.4161/onci.27668
References
- Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 2011; 105:93 - 103; http://dx.doi.org/10.1038/bjc.2011.189; PMID: 21629244
- Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005; 102:18538 - 43; http://dx.doi.org/10.1073/pnas.0509182102; PMID: 16344461
- Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A 2007; 104:3360 - 5; http://dx.doi.org/10.1073/pnas.0611533104; PMID: 17360651
- Clarke B, Tinker AV, Lee CH, Subramanian S, van de Rijn M, Turbin D, Kalloger S, Han G, Ceballos K, Cadungog MG, et al. Intraepithelial T cells and prognosis in ovarian carcinoma: novel associations with stage, tumor type, and BRCA1 loss. Mod Pathol 2009; 22:393 - 402; http://dx.doi.org/10.1038/modpathol.2008.191; PMID: 19060844
- Webb JR, Wick DA, Nielsen JS, Tran E, Milne K, McMurtrie E, Nelson BH. Profound elevation of CD8+ T cells expressing the intraepithelial lymphocyte marker CD103 (alphaE/beta7 Integrin) in high-grade serous ovarian cancer. Gynecol Oncol 2010; 118:228 - 36; http://dx.doi.org/10.1016/j.ygyno.2010.05.016; PMID: 20541243
- Webb JR, Milne K, Watson PH, Deleeuw RJ, Nelson BH. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res 2013; Forthcoming PMID: 24190978
- Cerf-Bensussan N, Jarry A, Brousse N, Lisowska-Grospierre B, Guy-Grand D, Griscelli C. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur J Immunol 1987; 17:1279 - 85; http://dx.doi.org/10.1002/eji.1830170910; PMID: 3498635
- Hadley G. Role of integrin CD103 in promoting destruction of renal allografts by CD8 T cells. Am J Transplant 2004; 4:1026 - 32; http://dx.doi.org/10.1111/j.1600-6143.2004.00465.x; PMID: 15196058
- El-Asady R, Yuan R, Liu K, Wang D, Gress RE, Lucas PJ, Drachenberg CB, Hadley GA. TGF-beta-dependent CD103 expression by CD8(+) T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med 2005; 201:1647 - 57; http://dx.doi.org/10.1084/jem.20041044; PMID: 15897278
- Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol 2013; 31:137 - 61; http://dx.doi.org/10.1146/annurev-immunol-032712-095954; PMID: 23215646
- Morgan EA, Yu H, Pinkus JL, Pinkus GS. Immunohistochemical detection of hairy cell leukemia in paraffin sections using a highly effective CD103 rabbit monoclonal antibody. Am J Clin Pathol 2013; 139:220 - 30; http://dx.doi.org/10.1309/AJCPHW7RULIZT2GB; PMID: 23355207
- Milne K, Köbel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, Watson PH, Nelson BH. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One 2009; 4:e6412; http://dx.doi.org/10.1371/journal.pone.0006412; PMID: 19641607
