Abstract
Single-nucleotide polymorphisms in Toll-like receptor 5 (TLR5), encoding a sensor for flagellin, have been shown to influence cytokine responses to intestinal bacteria and to be associated with significant alterations in the survival of colorectal carcinoma (CRC) patients. These findings point to a link between TLRs and CRC that may have both therapeutic and prognostic/predictive implications.
Introduction
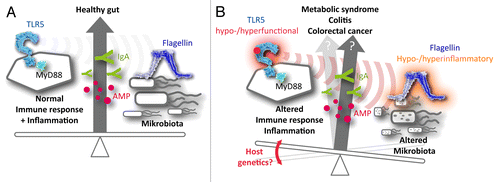
With 1.23 million new cases and 609 000 deaths per year, colorectal carcinoma (CRC) nowadays represents the third most frequent cancer worldwide,Citation1 causing a considerable socio-economic burden. Numerous preclinical and clinical studies indicate that the host immune system plays a key role in the development and progression of CRC.Citation2,Citation3 Recently, so-called “pattern recognition receptors” (PRRs), which are able to detect microbial components,Citation4 have emerged as central regulators of intestinal homeostasis and CRC.Citation2 Additionally, the infiltration of CRC lesions by immune cells correlates with disease progression. We have recently found that single nucleotide polymorphisms (SNPs) in the genes encoding Toll-like receptors (TLRs), a subset of PRR, are associated with the survival of CRC patients and impact on the secretion of inflammatory mediators in response to intestinal bacteria.Citation5 The gut microbiota have previously been implicated in colorectal carcinogenesis.Citation2 Thus, genetic variations affecting the TLR system may significantly decelerate or accelerate disease progression, hence altering the survival of CRC patients ().
Figure 1. Hypothetical influence of Toll-like receptor genetics on intestinal diseases. (A) Under physiological conditions, the immune system and the intestinal microbiota are in mutual equilibrium. In this scenario, Toll-like receptor (TLRs) sense intestinal bacteria and exert a selective pressure on the microbiota by promoting the secretion of antimicrobial effectors, including IgAs and antimicrobial peptides (AMPs). (B) In a genetically predisposed host (or due to other causes not discussed here), the activity of pattern-recognition receptors (PRRs) such as TLR5 may be altered, impacting on the release of several immunomodulatory molecules. This affects the selective pressure exerted by the host immune system on the intestinal microbiota, changing its composition in terms of phylotype and taxonomy. In turn, this favors a shift in the inflammatory potential of microbe-associated molecular patterns like flagellin, further altering the activity of TLR5 and other PRRs. Thus, it is conceivable that functionally relevant genetic variations affecting the PRR system alter the delicate equilibrium that normally exists between the host immune system and the gut microbiota, favoring the insurgence of intestinal diseases including colorectal carcinoma.
TLRs as Sentinels of Infection
Microbes are generally detected by the innate immune system via germ-line encoded PRRs, which are capable to sense not only microbe-associated molecular patterns (MAMPs) but also endogenous danger-associated molecular patterns (DAMPs). The activity of PRRs thus influences the host response to inflammatory, autoimmune, infectious, and malignant diseases, but also regulates tissue homeostasis. TLRs constitute the PRR family best characterized to date and include TLR4 and TLR5, which are sensors for bacterial lipopolysaccharide and flagellin, respectively. TLR4 and TLR5 signal via the adaptor myeloid differentiation primary response 88 (MyD88), resulting in the activation of NF-κB, activator protein 1 (AP1) and various interferon-regulatory factors (IRFs) and hence in the expression of numerous genes that control immune responses. Thus, TLR signaling leads to the secretion of various cytokines that establish a tightly regulated inflammatory state.Citation4
Role of TLRs in the Gut: Sensors of Microbial Colonization
The intestine represents a unique environment for host-pathogen interactions, mostly as it contains a commensal microflora in close proximity with intestinal epithelial cells, stromal cells, and tissue-infiltrating immune cells (all of which express TLRs).Citation4 Murine models have illustrated the importance of TLRs for normal gut functions, dissecting the mechanisms whereby the dysregulation of one or more TLRs contributes to intestinal inflammation. This process also depends on the presence of intestinal bacteria.Citation2 Thus, the ability of TLRs to sense the intestinal microbiota may be critical in the regulation of the balance between commensal microbes and immune system ().
Functional Role of TLR5 and MYD88 SNPs in CRC and Beyond
The signaling pathways emanating from TLRs and other PRRs have been implicated in colorectal carcinogenesis, yet the underlying mechanisms remain to be elucidated. In murine models of CRC, the genetic loss of MYD88, TLR4, or TLR5 has been associated with either increased or decreased tumor growth.Citation5 Our data demonstrate that SNPs affecting TLR3, TLR5, MYD88, Toll-interleukin 1 receptor (TIR) domain containing adaptor protein (TIRAP) and interleukin-10 (IL10) correlate with the survival of CRC patients and other clinico-pathological parameters.Citation5,Citation6 As these SNPs as well as SNPs affecting other PRR-coding genes are functionally interconnected, the genetics of the PRR system appears to profoundly influence the etiology of human CRC. Some of the SNPs that we investigated result in functional alterations of note. For instance, the TLR5 variants rs5744174 and rs2072493 are associated with alterations in the sensing of commensal and pathogenic bacterial flagellin, in turn impacting on the secretion of IL-1β and IL-6 (both of which play a critical role in colorectal carcinogenesis).Citation5,Citation7 Interestingly, rs5744174 has also been identified as a major genetic adaptation in humans, and may thus have a relevance beyond the CRC setting.Citation8 Moreover, Tlr5−/− mice are characterized by altered levels of circulating IL-1β as well as by changes in the gut microbiota.Citation9 It appears plausible that SNPs affecting the TLR system modify the intestinal inflammatory milieu and hence promote or inhibit colorectal carcinogenesis, either directly or upon alterations of the intestinal microbiome.
Clinical Application for Functional TLR SNPs
Two applications may originate from our recent findings. First, functional TLR SNPs may constitute an easily screenable biomarker to direct individuals that are at high-risk for succumbing to CRC toward diagnostic colonoscopy. Colonoscopy is highly effective in reducing CRC incidence as it allows for the identification and removal of pre-malignant lesions.Citation10 In ~1–2% of the individuals who do not participate in colonoscopy screening programs, such lesions go unnoticed and develop into overt neoplasms. CRC patients have a 5-y survival rate of approximately 60% (~90% for Stage I lesions, ~10% for Stage IV disease). The SNPs that we studied are attractive for screening purposes as they are relatively frequent (> 10% of the Caucasian population), as opposed to the rare APC, MLH1, or MSH2 germline mutations that cause hereditary CRC, and thus relevant for widespread use.Citation5 An unambiguous and straightforward SNP-based screening program might raise both the awareness and compliance of the population, and thus effectively prevent premature death by CRC in thousands of individuals (in Europe, the life-time risk of developing CRC is ~5%). Second, if TLRs actively regulate CRC progression, specific pharmacological or probiotic interventions may provide benefits in a prophylactic setting by tuning the TLR-activating potential of the intestinal microbiota down.
Outlook
Further basic and translational research into the connection between TLR, the intestinal microbiota and colorectal carcinogenesis is warranted. Appropriate mouse models will undoubtedly be vital to uncover additional leads on the etiology and progression of CRC. However, to meet the challenge posed by this considerable sanitary problem and truly ameliorate the course of disease for current and future CRC patients, we contend that research efforts should concentrate on the human system.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Weber AN, Försti A. Toll-like receptor genetic variants. OncoImmunology 2014; 3:e27763; 10.4161/onci.27763
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69 - 90; http://dx.doi.org/10.3322/caac.20107; PMID: 21296855
- Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 2010; 10:131 - 44; http://dx.doi.org/10.1038/nri2707; PMID: 20098461
- Pagès F, Galon J, Fridman WH. The essential role of the in situ immune reaction in human colorectal cancer. J Leukoc Biol 2008; 84:981 - 7; http://dx.doi.org/10.1189/jlb.1107773; PMID: 18559950
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011; 34:637 - 50; http://dx.doi.org/10.1016/j.immuni.2011.05.006; PMID: 21616434
- Klimosch SN, Försti A, Eckert J, Knezevic J, Bevier M, von Schönfels W, Heits N, Walter J, Hinz S, Lascorz J, et al. Functional TLR5 genetic variants affect human colorectal cancer survival. Cancer Res 2013; 73:7232 - 42; http://dx.doi.org/10.1158/0008-5472.CAN-13-1746; PMID: 24154872
- Castro FA, Försti A, Buch S, Kalthoff H, Krauss C, Bauer M, Egberts J, Schniewind B, Broering DC, Schreiber S, et al. TLR-3 polymorphism is an independent prognostic marker for stage II colorectal cancer. Eur J Cancer 2011; 47:1203 - 10; http://dx.doi.org/10.1016/j.ejca.2010.12.011; PMID: 21239167
- Waldner MJ, Foersch S, Neurath MF. Interleukin-6--a key regulator of colorectal cancer development. Int J Biol Sci 2012; 8:1248 - 53; http://dx.doi.org/10.7150/ijbs.4614; PMID: 23136553
- Grossman SR, Andersen KG, Shlyakhter I, Tabrizi S, Winnicki S, Yen A, Park DJ, Griesemer D, Karlsson EK, Wong SH, et al, 1000 Genomes Project. Identifying recent adaptations in large-scale genomic data. Cell 2013; 152:703 - 13; http://dx.doi.org/10.1016/j.cell.2013.01.035; PMID: 23415221
- Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 2010; 328:228 - 31; http://dx.doi.org/10.1126/science.1179721; PMID: 20203013
- Brenner H, Altenhofen L, Hoffmeister M. Eight years of colonoscopic bowel cancer screening in Germany: initial findings and projections. Dtsch Arztebl Int 2010; 107:753 - 9; PMID: 21085544
