Abstract
Mucosal boundaries, which are immunologically tolerogenic, and is further enhanced by undergo malignant transformation at high rates. We have identified the expression of neonatal Fc receptor for IgG (FcRn) by dendritic cells as a critical mediator of mucosal anti-cancer immunosurveillance. This discovery extends our understanding of neonatal Fc receptors, defines a role for tumor-reactive IgGs, and identifies an avenue for the development of novel anti-cancer therapeutics.
Cancers arise with a disproportionately high frequency in tissues of mucosal origin such as the lung, stomach, and colon.Citation1 One important contributor to the increased risk of neoplasia at these sites is the immunosuppressive nature of the mucosal immune system. While this is required for the tolerance of innocuous foreign oral and airborne antigens (be them of microbial or non-microbial origin), but generates an ideal environment for neoplastic growth to proceed under limited control.Citation2 Understanding the local dynamics of antitumor immunosurveillance is thus critical for our ability to prevent and treat mucosal neoplasms.
We have recently demonstrated that intestinal dendritic cells (DCs) utilize Fc fragment of IgG, receptor, transporter, α (FCGRT), best known as neonatal Fc receptor for IgG (FcRn), to elicit protective immune responses against the development of colorectal carcinoma and its metastatic dissemination to the lungs.Citation1 This tumor immunosurveillance depends on the presence of tumor-reactive IgGs capable of forming immune complexes (ICs) with tumor-associated antigens (TAAs). Upon interaction with Fcγ receptors expressed on the surface of DCs, these ICs deliver TAAs to a FcRn-driven processing pathway that promotes cross-presentation and the activation of tumor-specific CD8+ T cells.Citation1,Citation3 Such FcRn-mediated priming results in the robust activation of cytotoxic T cells upon the activation of a signaling cascade within DCs that culminates in the secretion of interleukin-12 (IL-12). Thus, the delivery of TAAs to a FcRn-dependent processing pathway results in the emission of both signal 1 (TAA in complex with MHC molecules) and signal 3 (an appropriate cytokine cocktail) to CD8+ T cells in the mucosal environment ().Citation4 Importantly, our findings suggest that FcRn contributes to tumor immunosurveillance in the very early phases of the crosstalk between developing neoplasms and the immune system, in conditions in which low amounts of TAAs are available.Citation5 The expression of FcRn by DCs of mesenteric lymph nodes and the lamina propria of untreated, healthy mice is critical for the establishment of homeostatic tumor-protective immune responses in the large intestine (LI), since the LI of FcRn deficient mice contains reduced numbers of CD8+ T cells, TH1-polarized CD4+ T cells and transcripts coding for TH1 cytokines.Citation1,Citation6 Not only do these studies demonstrate a physiological role for the FcRn in integrating B-cell and T-cell immunity as it regulates the fate of internalized IgG-containing ICs but also show for the first time that FcRn is able to orchestrate an intracellular signaling cascade when cross-linked by ICs.
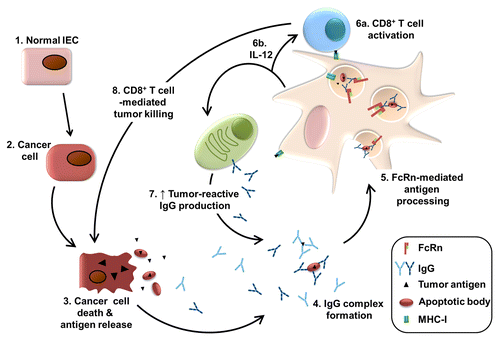
Figure 1. FcRn-mediated antitumor immunosurveillance relies on the cross-priming of CD8+ T cells in the presence of DC-derived IL-12. Normal intestinal epithelial cells (IEC) undergo neoplastic transformation in response to a variety of cues (1,2). The death of neoplastic cells results in the release of tumor-associated antigens (TAAs), either alone (during necrosis) or as part of apoptotic bodies (during apoptosis) (3). Cross-reactive natural IgGs form immune complexes (ICs) with TAAs or tumor-derived apoptotic bodies (4). These ICs are taken up by tumor-infiltrating dendritic cells (DCs), bind to the neonatal Fc receptor for IgG (FcRn) and are routed to an antigen-processing pathway that culminates in the presentation of tumor-derived epitopes onto MHC class I molecules at the cell surface (5). The interaction of naïve CD8+ T cells with the TAA/MHCI molecules results in the activation of tumor-targeting cytotoxic T cells (6a). The cross-linking of FcRn by ICs also leads to the secretion of interleukin-12 (IL-12) by DCs, which promotes the complete activation of CD8+ T cells (6b). IL-12 also stimulates local plasma cells to secrete additional tumor-reactive IgGs (7). The killing of cancer cells by cytotoxic CD8+ T lymphocytes increases the release of TAAs, thereby creating a positive feedback loop enabling potent FcRn-mediated antitumor immunity (8).
Importantly, the high concentration of IgGs present at mucosal tissues is a critical prerequisite for FcRn-mediated immunosurveillance. While the exact specificity of such IgGs remains unknown, it can be speculated that the mucosal IgG repertoire will be reactive against both commensal microorganisms and self antigens. Although FcRn deficiency does not alter the composition of the LI microbiota, a contribution of the local microflora to FcRn-mediated immunosurveillance cannot be formally excluded.Citation7 With respect to self antigens, natural auto-antibodies against widely expressed molecules such as phosphatidylserine are expected to be critical for the initial delivery of TAAs, alone or as part of exosomes or apoptotic bodies, to the FcRn-dependent processing pathways.Citation8 Subsequently, the release of additional TAAs from rapidly dying cancer cells might increase the amount of local IgG-containing ICs and thus strengthen the magnitude of this response.Citation9
Our findings shed new light on the FcRn for the development of novel therapies to target established tumors.Citation1,Citation10 Indeed, targeting the FcRn-dependent antigen processing pathway of DCs, which can override the tolerogenic mucosal milieu, is a promising strategy for circumventing the robust state of immunosuppression that is orchestrated by malignant cells in the tumor microenvironment.Citation5 Exploiting the FcRn biology using IgGs of known specificity is extremely feasible, given that at least one known mutation in the Fc enhances FcRn-dependent antigen presentation.Citation1,Citation10 Furthermore, it is possible that the efficacy of blocking antibodies such as trastuzumab, a v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2 (ERBB2)-targeting molecule currently used to treat breast cancer patients, results (at least in part) from the formation of IgG-containing ICs that are delivered to the FcRn-dependent antigen processing pathways of DCs, enabling the generation of robust CD8+ T-cell response.Citation10
FcRn-mediated tumor immunosurveillance results from the integration of the humoral and cellular branches of immunity, the translation of specific antibody responses into targeted CD8+ T-cell responses and, ultimately, the breakdown of local tolerance to oncogenesis. Therapeutically manipulating this robust mechanism of immunosurveillance in appropriate models will further improve our understanding of the immunological functions of FcRn within mucosal tissues.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Baker K, Rath T, Pyzik M, Blumberg RS. Neonatal Fc receptors for IgG drive CD8+ T cell-mediated anti-cancer immunosurveillance at tolerogenic mucosal sites. OncoImmunology 2014; 3:e27844; 10.4161/onci.27844
References
- Baker K, Rath T, Flak MB, Arthur JC, Chen Z, Glickman JN, Zlobec I, Karamitopoulou E, Stachler MD, Odze RD, et al. Neonatal Fc receptor expression in dendritic cells mediates protective immunity against colorectal cancer. Immunity 2013; 39:1095 - 107; http://dx.doi.org/10.1016/j.immuni.2013.11.003; PMID: 24290911
- MacDonald TT, Monteleone I, Fantini MC, Monteleone G. Regulation of homeostasis and inflammation in the intestine. Gastroenterology 2011; 140:1768 - 75; http://dx.doi.org/10.1053/j.gastro.2011.02.047; PMID: 21530743
- Baker K, Qiao SW, Kuo TT, Aveson VG, Platzer B, Andersen JT, Sandlie I, Chen Z, de Haar C, Lencer WI, et al. Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8-CD11b+ dendritic cells. Proc Natl Acad Sci U S A 2011; 108:9927 - 32; http://dx.doi.org/10.1073/pnas.1019037108; PMID: 21628593
- Curtsinger JM, Gerner MY, Lins DC, Mescher MF. Signal 3 availability limits the CD8 T cell response to a solid tumor. J Immunol 2007; 178:6752 - 60; PMID: 17513722
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013; 39:1 - 10; http://dx.doi.org/10.1016/j.immuni.2013.07.012; PMID: 23890059
- Qiao SW, Kobayashi K, Johansen FE, Sollid LM, Andersen JT, Milford E, Roopenian DC, Lencer WI, Blumberg RS. Dependence of antibody-mediated presentation of antigen on FcRn. Proc Natl Acad Sci U S A 2008; 105:9337 - 42; http://dx.doi.org/10.1073/pnas.0801717105; PMID: 18599440
- Arthur JC, Jobin C. The struggle within: microbial influences on colorectal cancer. Inflamm Bowel Dis 2011; 17:396 - 409; http://dx.doi.org/10.1002/ibd.21354; PMID: 20848537
- Kijanka G, Hector S, Kay EW, Murray F, Cummins R, Murphy D, MacCraith BD, Prehn JH, Kenny D. Human IgG antibody profiles differentiate between symptomatic patients with and without colorectal cancer. Gut 2010; 59:69 - 78; http://dx.doi.org/10.1136/gut.2009.178574; PMID: 19828471
- Kepp O, Tesniere A, Zitvogel L, Kroemer G. The immunogenicity of tumor cell death. Curr Opin Oncol 2009; 21:71 - 6; http://dx.doi.org/10.1097/CCO.0b013e32831bc375; PMID: 19125021
- Rath T, Baker K, Dumont JA, Peters RT, Jiang H, Qiao SW, Lencer WI, Pierce GF, Blumberg RS. Fc-fusion proteins and FcRn: structural insights for longer-lasting and more effective therapeutics. Crit Rev Biotechnol 2013; http://dx.doi.org/10.3109/07388551.2013.834293; PMID: 24156398
