Abstract
Tyrosine kinase inhibitors (TKIs) have dramatically improved the outcome of chronic myeloid leukemia (CML). Besides inhibiting target kinases in leukemic cells, 2nd generation TKI dasatinib also inhibits off-targets in immune effector cells resulting in atypical immune responses in some patients. Dasatinib has been described to increase the proportion of late effector memory T-cells, however, to date no follow-up studies have been performed in first-line patients. In this study, we explored the functional properties of T-cells using primary samples from CML patients (n = 28) on TKI therapy. Granzyme B (GrB) was used as a marker for late phase antigen experienced CD4+ and CD8+ T-cells. Dasatinib treatment increased the numbers of both GrB expressing memory CD4+ and CD8+ T-cells when compared with healthy controls. Functionally, the GrB+CD4+ T-cells were highly active and differentiated into Th1-type T-cells capable of producing IFN-γ, which is important for tumor control. Similar kind of increase was not observed during imatinib or nilotinib therapy. These data support the dual mode of action of dasatinib: potent BCR-ABL1 inhibition in leukemic cells is accompanied by the enhancement of cellular immunity, which may have implications in the long-term control of leukemia.
Introduction
Chronic myeloid leukemia (CML) is a hematopoietic stem cell disorder caused by cells carrying a Philadelphia (Ph) chromosome. The Ph chromosome is a result of a translocation between chromosomes 9 and 22, forming a fusion oncogene BCR-ABL1. The oncogene results in a deregulated tyrosine kinase activity, leading to impaired apoptosis and uncontrolled proliferation causing Ph positive (Ph+) leukemias such as CML.Citation1 The invention of tyrosine kinase inhibitors (TKIs) has significantly improved the therapy outcome of CML. The first generation TKI imatinib (Glivec/Gleevec; Novartis, Basel, Switzerland) is proven to be relatively safe and result in excellent therapy responses.Citation2 However, a proportion of imatinib-treated CML patients will eventually develop resistant mutations or become intolerant. In addition, imatinib is not effective in the treatment of advanced phases of CML or Ph+ acute lymphocytic leukemia.Citation3 Therefore, second generation TKIs such as dasatinib (Sprycel; Bristol-Myers Squibb, New York, NY, USA)Citation4 and nilotinib (Tasigna; Novartis)Citation5 have replaced imatinib in poorly responding patients resulting in improved outcomes.Citation6-Citation8 Furthermore, recent clinical trials have shown that newly diagnosed CML patients treated with dasatinib or nilotinib achieve earlier deeper molecular responses than patients on imatinib therapy and progress more rarely.Citation9,Citation10
It is not entirely clear why dasatinib-treated CML patients respond faster to the treatment than imatinib-treated patients, but probably at least the increased inhibition of the oncogenic tyrosine kinase BCR-ABLCitation11,Citation12 plays a role. In addition, dasatinib may have a more profound effect on the leukemic stem and progenitor cell pool as has previously been demonstrated in newly diagnosed CML patients.Citation13 However, other mechanisms, such as the wide kinase-inhibition profile of dasatinib, could be involved. In addition to the main target BCR-ABL, dasatinib also inhibits a wide variety of kinases such as src, tec, syk and gck-families that are essential for the function of the immune system.Citation14-Citation17 Therefore, it is reasonable to believe that dasatinib may cause modifications in the immune system. It has previously been shown that dasatinib-treated CML patients display diverse T-cell populations when compared with imatinib-treated CML patients,Citation18 but these studies have mainly been done in second-line dasatinib treated patients who have received several lines of therapy (such as imatinib, interferon or even stem cell transplantation) prior to dasatinib start. In addition, the evolution and function of these cells has not been described during the therapy. Therefore, our aim was to study the changes in the T-cell phenotype and function in chronic phase CML patients during the first line treatment of dasatinib, imatinib and nilotinib in order to understand the role of immune system in the therapy responses.
Results
CML patients have increased proportion of granzyme B expressing T-cells at diagnosis, which is further increased by dasatinib therapy
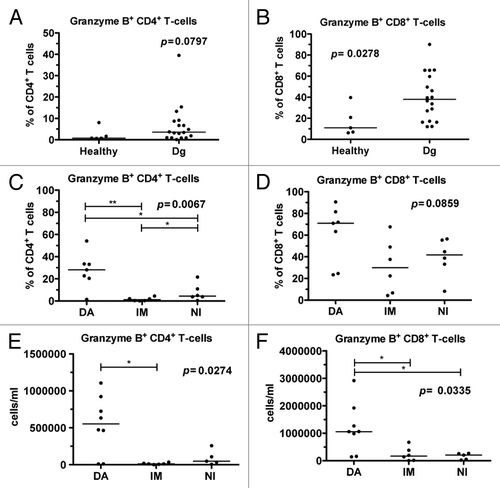
At diagnosis, CML patients had a higher proportion of Granzyme B positive (GrB+) CD4+ T-cells (median 3.6%) although the difference was not statistically significant when compared with healthy controls (median 0.8%, P = 0.08; ). Similarly, the relative amount of GrB+CD8+ T-cells was increased in untreated CML patients (median 38.0% vs. 11.0% in healthy controls, P = 0.028) (). After 6 mo of therapy, imatinib- and nilotinib-treated patients had a lower percentage of GrB+CD4+ T-cells when compared with dasatinib-treated patients (P = 0.0067; ). Moreover, the percentage of GrB+CD8+ T-cells was higher in dasatinib-treated patients (P = 0.086; ). Similarly, when absolute numbers of GrB+CD4+ and GrB+CD8+ T-cells were calculated, they were markedly higher in dasatinib treated patients, when compared with patients on imatinib or nilotinib treatment (0.54 × 106/ml vs. 0.01 × 106/ml and 0.09 × 106/ml, P = 0.0253 and 1.19 × 106/ml vs. 0.24 × 106/ml and 0.16 × 106/ml, P = 0.0439, respectively, ).
Figure 1. The proportion of granzyme B positive (GrB+) T-cells is increased in CML patients at diagnosis and further expands during dasatinib therapy. Fresh or frozen PBMNCs were first stained for surface markers (α-CD45, α-CD3, α-CD4 and α-CD8), and after fixation and permeabilization intracellular GrB was stained, and cells were analyzed with flow cytometry. (A) The relative proportions of GrB+CD4+ T-cells and (B) GrB+CD8+ T-cells in samples obtained from healthy controls (n = 5) and CML patients at diagnosis (dg) (n = 18). (C) The proportion of GrB+CD4+ T-cells and (D) GrB+CD8+ T-cells 6 mo after start on dasatinib (DA, n = 7), imatinib (IM, n = 6), or nilotinib (NI, n = 6) therapy. The absolute number of GrB+CD4+ (E) and GrB+CD8+ T-cells (F) was measured in CML patients 6 mo after the start of DA (n = 8), IM (n = 6), or NI (n = 5) therapy. Panels A and B were analyzed by nonparametric Mann Whitney t test and panels C, D and E by 1way ANOVA.
Dasatinib-treated CML patients have a higher proportion of effector CD4+ T-cells
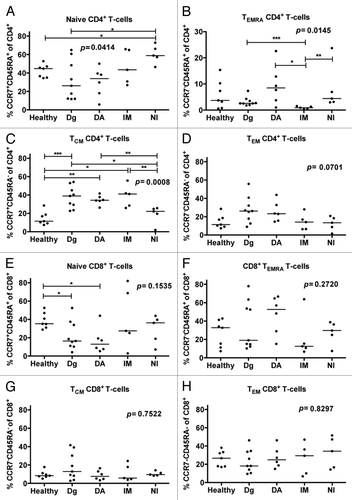
The memory cell subsets of CD4+ and CD8+ T-cells were studied in healthy, untreated CML patients, and in patients who had been treated for over 12 mo with dasatinib, imatinib, or nilotinib. These groups had significant differences in the proportions of the different CD4+ T-cell memory subsets; naïve (CCR7+CD45RA+, P = 0.04; ), central memory (CCR7+CD45RA-; P = 0.0008; ), CD45RA+ effector memory (CCR7-CD45RA+; P = 0.01; ), and effector memory (CCR7-CD45RA-, P = 0.07) CD4+ T-cells. In particular, dasatinib-treated patients had increased proportion of CD45RA+ effector memory CD4+ T-cells when compared with untreated patients (P = 0.07) and imatinib-treated patients (P = 0.017). In addition, dasatinib-treated patients had a trend of higher percentage of effector memory CD4+ T-cells when compared with nilotinib-treated patients (P = 0.052) and healthy (P = 0.07). No differences were observed between the different TKI treated patients regarding the maturation state of CD8+ T-cells (), although there was a trend for increased amount of terminal effector memory cells (TEMRA) in dasatinib patients ().
Figure 2. Patients treated with dasatinib have increased proportions of memory CD4+ T-cells. The memory cell subsets of CD4+ and CD8+ T-cells were examined by staining the fresh or frozen PBMNCs with α-CD45, α-CD3, α-CD4-, α-CD45RA and α-CCR7-PE antibodies after which they were analyzed by flow cytometry. The proportion of (A) naïve (CCR7+CD45RA+), (B) CD45RA+ effector memory (TEMRA, CCR7negCD45RA+) (C) central memory (TCM, CCR7+CD45RAneg) and (D) effector memory TEM (CCR7negCD45RAneg) CD4+ T-cells from the whole CD4+ T-cell population was measured from healthy (n = 7), untreated CML patients (n = 9) and patients treated with dasatinib (DA, n = 6), imatinib (IM, n = 5) or nilotinib (NI, n = 5) at least 12 mo after start of treatment. Same analysis was performed on CD8+ T-cells (E–H). Statistical analyses in all panels were done by 1way ANOVA.
Dasatinib therapy differentiates T-cells for Th1-type cytokine production
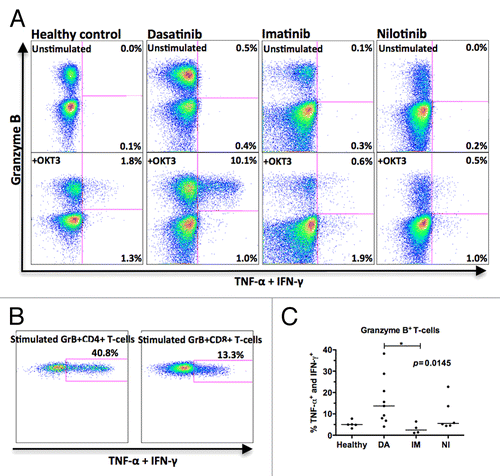
To study the function of GrB+ T-cells in dasatinib-treated patients, Th1-type cytokine production (TNF-α and IFN-γ) was measured by flow cytometry. Samples were collected from patients who had been on dasatinib therapy for 6–72 mo (median 30 mo), on imatinib for 12–30 mo (median 27), or on nilotinib for 6–42 mo (median 9 mo).
In unstimulated samples collected in the morning before the patient had taken the drug (12 or 24h after last capsule), no significant amount of cytokine production by T-cells was observed (, upper panels). In stimulated samples (, lower panels) 13.7% of the GrB+ T-cells in dasatinib-treated patients produced TNF-α and IFN-γ (median value of 9 patients, range 4.1–38.2%) whereas only 2.5% of GrBneg T-cells produced these cytokines (median value of 9 patients, range 0.9–4.2%). The GrB+ T-cells were the major cytokine producers and accounted for more than 90% of all cytokine-producing T-cells. When studied separately, both GrB+CD4+ and GrB+CD8+ T-cells produced cytokines (). In healthy volunteers (n = 5, median 5.0%, range 3.2–7.8%), imatinib- (n = 4, median 2.4%, range 1.1–6.4%) and nilotinib- (n = 6, median 5.5%, range 4.4–22.7%) treated patients the GrB+ T-cells were significantly less active Th1-type cytokine producers compared with same cells in dasatinib-treated patients (P = 0.0145).
Figure 3. Granzyme B (GrB+) positive T-cells in dasatinib-treated CML patients are sensitized to produce Th1-type cytokines upon stimulation. Fresh PBMNCs were stimulated with OKT3 and co-stimulatory molecules (α-CD28 and α-CD49d) for 6 h in the presence of Golgi STOP. After the stimulation, Th1-type cytokine (TNF-α and IFN-γ) production in T-cells was measured by flow cytometry. Panel (A) presents representative cases showing one healthy volunteer, one dasatinib-treated patient (patient nr 7 in ), one imatinib-treated patient (patient nr 15), and one nilotinib-treated patient (patient nr 17). Each plot shows 20 000 events. (B) Cytokine production by GrB+CD4+ and GrB+CD8+ T-cells in a representative dasatinib-treated patient (patient nr 7 in ). (C) The percentage of cytokine-producing GrB+ T-cells in healthy volunteers (n = 5) and patients treated with dasatinib (DA, n = 9), imatinib (IM, n = 4) or nilotinib (NI, n = 6) were compared by 1way ANOVA.
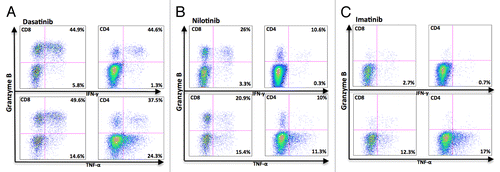
To study the TNF-α and IFN-γ production separately, samples from 5 patients (frozen PBMNCs from 1 imatinib-, 2 nilotinib-, and 2 dasatinib-treated patients) were analyzed with the antibodies separately. In both dasatinib- and nilotinib-treated patients GrB+ T-cells (both CD4 and CD8) were responsible for IFN-γ production (, upper panels), and GrBneg T-cells produced considerably less IFN-γ. In the imatinib-treated patient, a very few T-cells produced IFN-γ upon stimulation (, upper panels). In all TKI groups, both GrB+ and GrBneg produced TNF-α (, lower panels).
Figure 4. Granzyme B positive T-cells in dasatinib-treated CML patients produce mainly IFN-γ. Fresh PBMNCs were stimulated with OKT3 and co-stimulatory molecules (α-CD28 and α-CD49d) for 6 h in the presence of Golgi STOP. After the stimulation, Th1-type cytokine (TNF-α and IFN-γ) production in T-cells was measured separately by flow cytometry. The figure presents a representative dasatinib- (A), nilotinib- (B), and imatinib- (C) treated patient (patient nr 6, 23, and 14 in ).
TKI intake decreases cytokine production in T-cells, but does not inhibit it completely
As TKIs are reported to inhibit T-cell function in vitro,Citation19-Citation21 we studied the effect of TKI intake on cytokine production. PB samples were collected before the patients took their daily drug dose and 1h after dasatinib, and 2h after nilotinib and imatinib intake (approximated peak plasma levels). In parallel, the absolute number of GrB+CD4+ and GrB+CD8+ T-cells were determined. As a control, we also studied the cytokine production of T-cells in in vitro cultures were dasatinib was constantly present.
Dasatinib intake significantly increased the absolute number of GrB+CD4+ (P = 0.002) and GrB+CD8+ (P = 0.0039) T-cells in the blood (). No significant increase was observed after imatinib intake, but nilotinib decreased the number of GrB+CD4+ T-cells (P = 0.03) ().
Figure 5. Dasatinib intake decreases the absolute number of Granzyme B positive (GrB+) CD4+ and CD8+ T-cells and decreases cytokine production. Fresh PBMNCs were stimulated with OKT3 and co-stimulatory molecules (α-CD28 and α-CD49d) for 6 h in the presence of Golgi STOP. After the stimulation, Th1-type cytokine (TNF-α and IFN-γ) production in T-cells was measured by flow cytometry. The absolute counts of GrB+CD4+ and GrB+CD8+ T-cells in CML patients before (0h) and after (1 or 2 h) the patients’ daily dose of dasatinib (DA, panel A), imatinib (IM, panel B), and nilotinib (NI, panel C). Panel (D) shows the percentage of cytokine-producing GrB+ T-cells in these patients before and after the drug intake. Cytokine production before and after drug intake was analyzed with paired t test.
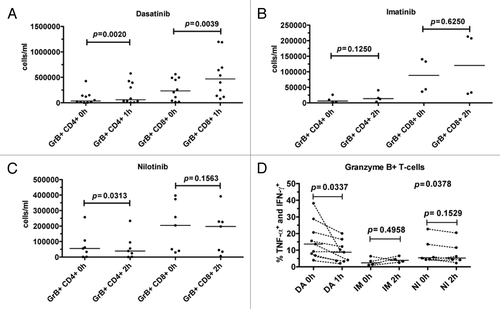
In contrast, the proportion of cytokine producing GrB+ T-cells reduced after dasatinib intake. This was accordant with the in vitro results showing that when dasatinib is constantly present, the cytokine production is inhibited (data not shown). However, despite the relative decrease of cytokine producing GrB+ T-cells, dasatinib-treated patients still had a significant population of active, cytokine producing GrB+ T-cells in the blood as the absolute number of GrB+ T-cells was increased 1h post drug intake. No significant differences in the T-cell function were observed after imatinib (P = 0.50) or nilotinib (P = 0.15) intake ().
Discussion
Increasing evidence suggests that TKIs have pronounced immunomodulatory off-target effects, which may play a role in their therapeutic efficacy both in patients with hematological malignancies and solid tumors (such as gastrointestinal stromal tumors).Citation22-Citation28 The results, however, are still controversial as recently reviewed by us.Citation29 In vitro studies have pointed out that the effects on the immune cells are merely suppressive or inhibitory,Citation19,Citation20,Citation30-Citation38 whereas we and others have reported opposite immunostimulatory effects in vivo.Citation22,Citation23,Citation25,Citation27,Citation37,Citation39-Citation43 The results presented in this paper support the immunostimulatory role of dasatinib and show that dasatinib therapy not only increases the amount of cytotoxic memory GrB+CD8+ T-cells, but also increases the amount of highly active GrB+CD4+ T-cells in opposite to the other TKIs (imatinib, nilotinib). These results suggest that the promotion of Th1-type immune responses together with increased amount of cytotoxic cells may play a role in the therapy outcome of dasatinib treated patients.
CML is known to be one of the most immunogenic cancers,Citation44 and anti-CML-specific T-cells can be found in untreated patients.Citation45-Citation48 Our current observations confirm that newly-diagnosed CML patients have increased amount of late, antigen experienced CD8+ and CD4+ T-cells defined by the expression of GrB.Citation49 Similar results were obtained by the analysis of different memory subsets of T-cells characterized by CCR7 and CD45RA expression as untreated CML patients had an increased proportion of the memory T-cell subsets when compared with healthy donors. These both observations are signs of cytotoxic immunoactivation in CML patients. Interestingly, when these T-cell subsets were studied in follow-up samples after the patients began on dasatinib, imatinib or nilotinib therapy, we observed that marked changes occurred only during dasatinib therapy. The proportion of circulating GrB+CD8 T-cells further increased from the diagnostic phase situation almost 2-fold by the 6 mo after the start of dasatinib therapy. The most prominent changes, however, occurred in the number and type of GrB+CD4+ T-cells. Six months treatment with dasatinib was associated with the 3-fold higher proportion and 5-fold increase in the absolute number of circulating GrB+CD4+ T-cells compared with the situation at diagnosis. Similar results were obtained when the memory status of the T-cells was studied by CCR7 and CD45RA expression, as dasatinib treatment further increased the percentage of TEMRA CD4+ T-cells.
In dasatinib-treated patients the GrB+CD4+ T-cells were highly active and responded to stimulation by secreting considerable amounts of Th1-type cytokines IFN-γ and TNF-α. In healthy individuals, GrB+CD4+ T-cells are rarely seen.Citation50 In other malignancies, there are a few reports suggesting that these cells play a role in anti-tumor activity. In a melanoma mice model it has been reported that CD4+ T-cells are able to expand and differentiate into IFN-γ-secreting cytotoxic CD4+ T-cells. In addition, the cytotoxic activity correlated with the high levels of GrB.Citation51 Similarly, in the myeloma mice model it was shown that CD4+ T-cells were able to mediate primary anti-tumor immune responses by secreting IFN-γ and TNF-α.Citation52 Furthermore, in humans, CD4+ T-cells have been described to recognize and lyse foreign leukemic target cells without the lysis of nonmalignant remission cells from the same patient.Citation53
Interestingly, GrB+CD4+ T-cells may also have an effect on the other cells involved in tumor immunosurveillance. We have previously shown that long-term dasatinib therapy decreases the number of regulatory T-cells (Tregs), which is especially prominent in patients with the expansion of large granular lymphocytes.Citation42,Citation54,Citation55 Earlier publications suggest that CD4+ responder T-cells may alter the function of Tregs. These cells produce GrB in response to strong T-cell receptor stimulation and in this manner they are able to kill effector Tregs.Citation56 Similarly, a study by Janikashvili et al. showed that effector-memory CD4+ T-cells are capable of impairing tumor-induced immunosuppressive Tregs,Citation57 which was dependent on the production of IFN-γ.Citation57 This might be one of the explanations why Tregs are downregulated in dasatinib-treated CML patients as we found that GrB+ T-cells produce considerable amounts of IFN-γ in dasatinib-treated patients, which was not the case in imatinib- or nilotinib-treated patients.
To our knowledge, this is the first report describing the effects of short-term TKI exposure in vivo on T-cell function. Similarly as in the previous studies,Citation19,Citation30,Citation31,Citation36 we noticed that dasatinib inhibits the T-cell function when constantly present in in vitro cultures. However, in vivo in patients the pharmacokinetics of dasatinib differs markedly from the other TKIs.Citation39,Citation58 The drug half-life is considerably short, only a few hours, and the peak plasma concentrations occur already at 1 h after the drug intake. It could be argued that the constant presence of dasatinib in in vitro cultures does not mimic the real situation in patients. Therefore, we collected blood samples both before and after TKI intake, and the functional assays were performed on freshly isolated cells. Our results show that the short-term in vivo dasatinib exposure decrease the cytokine production of GrB+ T-cells concordant with the earlier in vitro findings, but it did not inhibit it completely. Further, even though the proportion of cytokine producing T-cells decreased, the absolute number of these cells remained the same (ie. above the level seen in other TKI-treated patients or healthy controls) as lymphocyte counts increased after 1h of dasatinib intake. Together with increased NK-cell cytotoxicity observed after dasatinib intake in our previous studies,Citation39 this could result in enhanced anti-leukemia immune activity in dasatinib-treated patients.
In conclusion, 2nd generation TKI dasatinib therapy does not only increase the number of memory CD4+ and CD8+ T-cells, but also generates a strong Th1-type immune response in these cells. These data support the dual mode of action of dasatinib: the BCR-ABL1 inhibition in leukemic cells is accompanied by enhancement of cellular immunity, which may have implications in the long-term control of Ph+ leukemia.
Patients and Methods
Study patients and samples
The study included chronic phase CML patients on imatinib, dasatinib, or nilotinib treatment (). The study was conducted in accordance with the principles of the Helsinki declaration and was approved by the Helsinki University Central Hospital Ethics Committee. Written informed consents were obtained from all patients included in this study.
Table 1. Patient characteristics
Fresh peripheral blood (PB) EDTA samples were collected from the patients and healthy controls. Samples from the patients were collected before and 1 or 2 h after the daily dose of their drug (dasatinib, imatinib, or nilotinib). Mononuclear cells (MNCs) were separated by Ficoll gradient centrifugation (GE healthcare). Results of routine laboratory tests (blood cell counts, differential analysis of leukocytes) were obtained from all patients before and after the intake of the daily drug dose.
T-cell phenotyping
T-cell phenotyping was performed on fresh PBMNC or follow-up samples stored in liquid nitrogen. For the analysis of GrB+ T-cells, the cells were first stained for cell surface markers; α-CD45 APC-H7 (BD 641417), α-CD3 APC (BD 345767), α-CD4 PerCP (BD 345770), and α-CD8 PE-Cy7 (BD 335822), and then fixed and permeabilized with Fix/Perm (BD 554714), and intracellular GrB was stained (Alexa Fluor 700, BD 561016)). The memory cell subsets of CD4+ and CD8+ T-cells were studied by the following panel: α-CD45-APC-H7, α-CD3-PeCy7 (BD 557851), α-CD4-PerCP, α-CD45RA AlexaFluor700 (BD 560673) and α-CCR7-PE (R&D Systems FAB197P). 50´000 CD45+ cells were acquired with FACSAria (BD) and analyzed with FlowJo.
Activation assay of peripheral T-cells
Fresh PBMNCs were re-suspended in RPMI (10% FBS, penicillin, 1% streptomycin and 1% l-glutamin; all Lonza) and stimulated with OKT3 (5μg/ml, BD 555329), α-CD28 (1μg/ml, BD 340975) and α-CD49d (1μg/ml, BD 340976) in the presence of Golgi STOP (BD 554724). After 6 h of incubation in 37 °C, the cells were harvested and washed. The cells were stained as following: α-CD45 APC-H7, α-CD3 APC, α-CD4 PerCP, and α-CD8 PE-Cy7. After the staining of surface markers, the cells were fixed and permeabilized with Fix/Perm according to manufacturer’s instructions. Intracellular TNF-α (FITC, BD 554512), IFN-γ (FITC, BD 554700), and GrB (Alexa 700) were stained and 50´000 CD45+ cells were acquired with FACSAria (BD) and analyzed with FlowJo (version 9.1, TreeStar).
Statistical analysis
All statistics were done with GraphPad Prism software (version 5.0c; GraphPad) using nonparametric Mann–Whitney t test to compare two groups, 1way ANOVA to compare several groups, and paired t test to study the significance between paired observations before and after drug intake.
| Abbreviations: | ||
| CML | = | chronic myeloid leukemia |
| GrB | = | Granzyme B |
| TKI | = | tyrosine kinase inhibitor |
| PB | = | peripheral blood |
| MNC | = | mononuclear cells |
| Ph | = | Philadelphia |
| TNF-α | = | tumor necrosis factor alpha |
| IFN-γ | = | interferon gamma |
Disclosure of Potential Conflicts of Interest
K.P. has received research funding and honoraria from Novartis, Bristol-Myers Squibb and Pfizer. S.M. has received honoraria and research funding from Novartis and Bristol-Myers Squibb.
References
- Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood 2000; 96:3343 - 56; PMID: 11071626
- Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, et al, IRIS Investigators. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 2006; 355:2408 - 17; http://dx.doi.org/10.1056/NEJMoa062867; PMID: 17151364
- Ottmann OG, Pfeifer H. First-line treatment of Philadelphia chromosome-positive acute lymphoblastic leukaemia in adults. Curr Opin Oncol 2009; 21:Suppl 1 S43 - 6; http://dx.doi.org/10.1097/01.cco.0000357476.43164.6b; PMID: 19561414
- Hochhaus A, Baccarani M, Deininger M, Apperley JF, Lipton JH, Goldberg SL, Corm S, Shah NP, Cervantes F, Silver RT, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia 2008; 22:1200 - 6; http://dx.doi.org/10.1038/leu.2008.84; PMID: 18401416
- Kantarjian HM, Giles F, Gattermann N, Bhalla K, Alimena G, Palandri F, Ossenkoppele GJ, Nicolini FE, O’Brien SG, Litzow M, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood 2007; 110:3540 - 6; http://dx.doi.org/10.1182/blood-2007-03-080689; PMID: 17715389
- Kantarjian H, Giles F, Wunderle L, Bhalla K, O’Brien S, Wassmann B, Tanaka C, Manley P, Rae P, Mietlowski W, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med 2006; 354:2542 - 51; http://dx.doi.org/10.1056/NEJMoa055104; PMID: 16775235
- Ottmann O, Dombret H, Martinelli G, Simonsson B, Guilhot F, Larson RA, Rege-Cambrin G, Radich J, Hochhaus A, Apanovitch AM, et al. Dasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a phase 2 study. Blood 2007; 110:2309 - 15; http://dx.doi.org/10.1182/blood-2007-02-073528; PMID: 17496201
- Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, Cortes J, O’Brien S, Nicaise C, Bleickardt E, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med 2006; 354:2531 - 41; http://dx.doi.org/10.1056/NEJMoa055229; PMID: 16775234
- Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, Pasquini R, Clark RE, Hochhaus A, Hughes TP, et al, ENESTnd Investigators. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010; 362:2251 - 9; http://dx.doi.org/10.1056/NEJMoa0912614; PMID: 20525993
- Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, Moiraghi B, Shen Z, Mayer J, Pasquini R, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2010; 362:2260 - 70; http://dx.doi.org/10.1056/NEJMoa1002315; PMID: 20525995
- Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, Castaneda S, Cornelius LA, Das J, Doweyko AM, et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem 2004; 47:6658 - 61; http://dx.doi.org/10.1021/jm049486a; PMID: 15615512
- Weisberg E, Manley P, Mestan J, Cowan-Jacob S, Ray A, Griffin JD. AMN107 (nilotinib): a novel and selective inhibitor of BCR-ABL. Br J Cancer 2006; 94:1765 - 9; http://dx.doi.org/10.1038/sj.bjc.6603170; PMID: 16721371
- Mustjoki S, Richter J, Barbany G, Ehrencrona H, Fioretos T, Gedde-Dahl T, Gjertsen BT, Hovland R, Hernesniemi S, Josefsen D, et al, Nordic CML Study Group (NCMLSG). Impact of malignant stem cell burden on therapy outcome in newly diagnosed chronic myeloid leukemia patients. Leukemia 2013; 27:1520 - 6; http://dx.doi.org/10.1038/leu.2013.19; PMID: 23328954
- Hantschel O, Rix U, Superti-Furga G. Target spectrum of the BCR-ABL inhibitors imatinib, nilotinib and dasatinib. Leuk Lymphoma 2008; 49:615 - 9; http://dx.doi.org/10.1080/10428190801896103; PMID: 18398720
- Rix U, Hantschel O, Dürnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV, Kaupe I, Bennett KL, Valent P, Colinge J, et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood 2007; 110:4055 - 63; http://dx.doi.org/10.1182/blood-2007-07-102061; PMID: 17720881
- Hantschel O, Rix U, Schmidt U, Bürckstümmer T, Kneidinger M, Schütze G, Colinge J, Bennett KL, Ellmeier W, Valent P, et al. The Btk tyrosine kinase is a major target of the Bcr-Abl inhibitor dasatinib. Proc Natl Acad Sci U S A 2007; 104:13283 - 8; http://dx.doi.org/10.1073/pnas.0702654104; PMID: 17684099
- Bantscheff M, Eberhard D, Abraham Y, Bastuck S, Boesche M, Hobson S, Mathieson T, Perrin J, Raida M, Rau C, et al. Quantitative chemical proteomics reveals mechanisms of action of clinical ABL kinase inhibitors. Nat Biotechnol 2007; 25:1035 - 44; http://dx.doi.org/10.1038/nbt1328; PMID: 17721511
- Rohon P, Porkka K, Mustjoki S. Immunoprofiling of patients with chronic myeloid leukemia at diagnosis and during tyrosine kinase inhibitor therapy. Eur J Haematol 2010; 85:387 - 98; http://dx.doi.org/10.1111/j.1600-0609.2010.01501.x; PMID: 20662899
- Blake S, Hughes TP, Mayrhofer G, Lyons AB. The Src/ABL kinase inhibitor dasatinib (BMS-354825) inhibits function of normal human T-lymphocytes in vitro. Clin Immunol 2008; 127:330 - 9; http://dx.doi.org/10.1016/j.clim.2008.02.006; PMID: 18395492
- Seggewiss R, Loré K, Greiner E, Magnusson MK, Price DA, Douek DC, Dunbar CE, Wiestner A. Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood 2005; 105:2473 - 9; http://dx.doi.org/10.1182/blood-2004-07-2527; PMID: 15572591
- Chen J, Schmitt A, Chen B, Rojewski M, Rübeler V, Fei F, Yu Y, Yu X, Ringhoffer M, von Harsdorf S, et al. Nilotinib hampers the proliferation and function of CD8+ T lymphocytes through inhibition of T cell receptor signalling. J Cell Mol Med 2008; 12:5B 2107 - 18; http://dx.doi.org/10.1111/j.1582-4934.2008.00234.x; PMID: 18194453
- Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, Sorenson EC, Popow R, Ariyan C, Rossi F, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med 2011; 17:1094 - 100; http://dx.doi.org/10.1038/nm.2438; PMID: 21873989
- Zitvogel L, Kroemer G. Anticancer effects of imatinib via immunostimulation. Nat Med 2011; 17:1050 - 1; http://dx.doi.org/10.1038/nm.2429; PMID: 21900920
- Borg C, Terme M, Taïeb J, Ménard C, Flament C, Robert C, Maruyama K, Wakasugi H, Angevin E, Thielemans K, et al. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J Clin Invest 2004; 114:379 - 88; http://dx.doi.org/10.1172/JCI21102; PMID: 15286804
- Chaput N, Flament C, Locher C, Desbois M, Rey A, Rusakiewicz S, Poirier-Colame V, Pautier P, Le Cesne A, Soria JC, et al. Phase I clinical trial combining imatinib mesylate and IL-2: HLA-DR(+) NK cell levels correlate with disease outcome. Oncoimmunology 2013; 2:e23080; http://dx.doi.org/10.4161/onci.23080; PMID: 23525357
- Yang Y, Liu C, Peng W, Lizée G, Overwijk WW, Liu Y, Woodman SE, Hwu P. Antitumor T-cell responses contribute to the effects of dasatinib on c-KIT mutant murine mastocytoma and are potentiated by anti-OX40. Blood 2012; 120:4533 - 43; http://dx.doi.org/10.1182/blood-2012-02-407163; PMID: 22936666
- Poggi A, Zocchi MR. Imatinib mesylate can help to direct natural immunity toward an anti-leukemic reactivity by acting on the bone marrow microenvironment. Oncoimmunology 2012; 1:214 - 6; http://dx.doi.org/10.4161/onci.1.2.18112; PMID: 22720246
- Yang Y, Lizée G, Hwu P. Strong emerging rationale for combining oncogene-targeted agents with immunotherapy. Oncoimmunology 2013; 2:e22730; http://dx.doi.org/10.4161/onci.22730; PMID: 23524978
- Kreutzman A, Porkka K, Mustjoki S. Immunomodulatory Effects of Tyrosine Kinase Inhibitors. International Trends in Immunity 2013; 01:22 - 33
- Schade AE, Schieven GL, Townsend R, Jankowska AM, Susulic V, Zhang R, Szpurka H, Maciejewski JP. Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood 2008; 111:1366 - 77; http://dx.doi.org/10.1182/blood-2007-04-084814; PMID: 17962511
- Weichsel R, Dix C, Wooldridge L, Clement M, Fenton-May A, Sewell AK, Zezula J, Greiner E, Gostick E, Price DA, et al. Profound inhibition of antigen-specific T-cell effector functions by dasatinib. Clin Cancer Res 2008; 14:2484 - 91; http://dx.doi.org/10.1158/1078-0432.CCR-07-4393; PMID: 18413841
- Seggewiss R, Price DA, Purbhoo MA. Immunomodulatory effects of imatinib and second-generation tyrosine kinase inhibitors on T cells and dendritic cells: an update. Cytotherapy 2008; 10:633 - 41; http://dx.doi.org/10.1080/14653240802317639; PMID: 18836918
- Dietz AB, Souan L, Knutson GJ, Bulur PA, Litzow MR, Vuk-Pavlovic S. Imatinib mesylate inhibits T-cell proliferation in vitro and delayed-type hypersensitivity in vivo. Blood 2004; 104:1094 - 9; http://dx.doi.org/10.1182/blood-2003-12-4266; PMID: 15100154
- Chen J, Schmitt A, Chen B, Rojewski M, Ringhoffer M, von Harsdorf S, Greiner J, Guillaume P, Döhner H, Bunjes D, et al. Imatinib impairs CD8+ T lymphocytes specifically directed against the leukemia-associated antigen RHAMM/CD168 in vitro. Cancer Immunol Immunother 2007; 56:849 - 61; http://dx.doi.org/10.1007/s00262-006-0232-9; PMID: 17009043
- Blake SJ, Bruce Lyons A, Fraser CK, Hayball JD, Hughes TP. Dasatinib suppresses in vitro natural killer cell cytotoxicity. Blood 2008; 111:4415 - 6; http://dx.doi.org/10.1182/blood-2008-02-138701; PMID: 18398058
- Fei F, Yu Y, Schmitt A, Rojewski MT, Chen B, Greiner J, Götz M, Guillaume P, Döhner H, Bunjes D, et al. Dasatinib exerts an immunosuppressive effect on CD8+ T cells specific for viral and leukemia antigens. Exp Hematol 2008; 36:1297 - 308; http://dx.doi.org/10.1016/j.exphem.2008.05.002; PMID: 18619726
- Nagata Y, Ohashi K, Fukuda S, Kamata N, Akiyama H, Sakamaki H. Clinical features of dasatinib-induced large granular lymphocytosis and pleural effusion. Int J Hematol 2010; 91:799 - 807; http://dx.doi.org/10.1007/s12185-010-0565-1; PMID: 20405252
- Hassold N, Seystahl K, Kempf K, Urlaub D, Zekl M, Einsele H, Watzl C, Wischhusen J, Seggewiss-Bernhardt R. Enhancement of natural killer cell effector functions against selected lymphoma and leukemia cell lines by dasatinib. Int J Cancer 2012; 131:E916 - 27; http://dx.doi.org/10.1002/ijc.27537; PMID: 22419518
- Mustjoki S, Auvinen K, Kreutzman A, Rousselot P, Hernesniemi S, Melo T, Lahesmaa-Korpinen AM, Hautaniemi S, Bouchet S, Molimard M, et al. Rapid mobilization of cytotoxic lymphocytes induced by dasatinib therapy. Leukemia 2013; 27:914 - 24; http://dx.doi.org/10.1038/leu.2012.348; PMID: 23192016
- Valent JN, Schiffer CA. Prevalence of large granular lymphocytosis in patients with chronic myelogenous leukemia (CML) treated with dasatinib. Leuk Res 2011; 35:e1 - 3; http://dx.doi.org/10.1016/j.leukres.2010.08.022; PMID: 20888043
- Kim DH, Kamel-Reid S, Chang H, Sutherland R, Jung CW, Kim HJ, Lee JJ, Lipton JH. Natural killer or natural killer/T cell lineage large granular lymphocytosis associated with dasatinib therapy for Philadelphia chromosome positive leukemia. Haematologica 2009; 94:135 - 9; http://dx.doi.org/10.3324/haematol.13151; PMID: 19066329
- Kreutzman A, Juvonen V, Kairisto V, Ekblom M, Stenke L, Seggewiss R, Porkka K, Mustjoki S. Mono/oligoclonal T and NK cells are common in chronic myeloid leukemia patients at diagnosis and expand during dasatinib therapy. Blood 2010; 116:772 - 82; http://dx.doi.org/10.1182/blood-2009-12-256800; PMID: 20413659
- Lee SJ, Jung CW, Kim DY, Lee KH, Sohn SK, Kwak JY, Kim HJ, Kim IH, Park S, Kim DH. Retrospective multicenter study on the development of peripheral lymphocytosis following second-line dasatinib therapy for chronic myeloid leukemia. Am J Hematol 2011; 86:346 - 50; http://dx.doi.org/10.1002/ajh.21980; PMID: 21442637
- Ilander M, Hekim C, Mustjoki S. Immunology and immunotherapy of chronic myeloid leukemia. Curr Hematol Malig Rep 2014; 9:17 - 23; http://dx.doi.org/10.1007/s11899-013-0190-1; PMID: 24390549
- Molldrem JJ, Lee PP, Wang C, Felio K, Kantarjian HM, Champlin RE, Davis MM. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med 2000; 6:1018 - 23; http://dx.doi.org/10.1038/79526; PMID: 10973322
- Rezvani K, Grube M, Brenchley JM, Sconocchia G, Fujiwara H, Price DA, Gostick E, Yamada K, Melenhorst J, Childs R, et al. Functional leukemia-associated antigen-specific memory CD8+ T cells exist in healthy individuals and in patients with chronic myelogenous leukemia before and after stem cell transplantation. Blood 2003; 102:2892 - 900; http://dx.doi.org/10.1182/blood-2003-01-0150; PMID: 12829610
- Kreutzman A, Ladell K, Koechel C, Gostick E, Ekblom M, Stenke L, Melo T, Einsele H, Porkka K, Price DA, et al. Expansion of highly differentiated CD8+ T-cells or NK-cells in patients treated with dasatinib is associated with cytomegalovirus reactivation. Leukemia 2011; 25:1587 - 97; http://dx.doi.org/10.1038/leu.2011.135; PMID: 21647156
- Kreutzman A, Rohon P, Faber E, Indrak K, Juvonen V, Kairisto V, Voglová J, Sinisalo M, Flochová E, Vakkila J, et al. Chronic myeloid leukemia patients in prolonged remission following interferon-α monotherapy have distinct cytokine and oligoclonal lymphocyte profile. PLoS One 2011; 6:e23022; http://dx.doi.org/10.1371/journal.pone.0023022; PMID: 21857985
- Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A 2008; 73:975 - 83; http://dx.doi.org/10.1002/cyto.a.20643; PMID: 18785267
- Kummer JA, Kamp AM, Tadema TM, Vos W, Meijer CJ, Hack CE. Localization and identification of granzymes A and B-expressing cells in normal human lymphoid tissue and peripheral blood. Clin Exp Immunol 1995; 100:164 - 72; http://dx.doi.org/10.1111/j.1365-2249.1995.tb03619.x; PMID: 7697916
- Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med 2010; 207:637 - 50; http://dx.doi.org/10.1084/jem.20091918; PMID: 20156971
- Corthay A, Skovseth DK, Lundin KU, Røsjø E, Omholt H, Hofgaard PO, Haraldsen G, Bogen B. Primary antitumor immune response mediated by CD4+ T cells. Immunity 2005; 22:371 - 83; http://dx.doi.org/10.1016/j.immuni.2005.02.003; PMID: 15780993
- Sosman JA, Oettel KR, Smith SD, Hank JA, Fisch P, Sondel PM. Specific recognition of human leukemic cells by allogeneic T cells: II. Evidence for HLA-D restricted determinants on leukemic cells that are crossreactive with determinants present on unrelated nonleukemic cells. Blood 1990; 75:2005 - 16; PMID: 1692492
- Mustjoki S, Ekblom M, Arstila TP, Dybedal I, Epling-Burnette PK, Guilhot F, Hjorth-Hansen H, Höglund M, Kovanen P, Laurinolli T, et al. Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia 2009; 23:1398 - 405; http://dx.doi.org/10.1038/leu.2009.46; PMID: 19295545
- Powers JJ, Dubovsky JA, Epling-Burnette PK, Moscinski L, Zhang L, Mustjoki S, Sotomayor EM, Pinilla-Ibarz JA. A molecular and functional analysis of large granular lymphocyte expansions in patients with chronic myelogenous leukemia treated with tyrosine kinase inhibitors. Leuk Lymphoma 2011; 52:668 - 79; http://dx.doi.org/10.3109/10428194.2010.550074; PMID: 21271862
- Ashley CW, Baecher-Allan C. Cutting Edge: Responder T cells regulate human DR+ effector regulatory T cell activity via granzyme B. J Immunol 2009; 183:4843 - 7; http://dx.doi.org/10.4049/jimmunol.0900845; PMID: 19801510
- Janikashvili N, LaCasse CJ, Larmonier C, Trad M, Herrell A, Bustamante S, Bonnotte B, Har-Noy M, Larmonier N, Katsanis E. Allogeneic effector/memory Th-1 cells impair FoxP3+ regulatory T lymphocytes and synergize with chaperone-rich cell lysate vaccine to treat leukemia. Blood 2011; 117:1555 - 64; http://dx.doi.org/10.1182/blood-2010-06-288621; PMID: 21123824
- van Erp NP, Gelderblom H, Guchelaar HJ. Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev 2009; 35:692 - 706; http://dx.doi.org/10.1016/j.ctrv.2009.08.004; PMID: 19733976
