Abstract
Metastatic cell heterogeneity presents a significant obstacle to the development of targeted molecular and immunotherapeutics. Profiling of melanocyte differentiation antigens has revealed a nonstochastic, site-specific pattern of expression in metastases that was highest in brain, intermediate in soft tissues/lymph nodes, and lowest in visceral sites. Site-specific antigen heterogeneity, thus, is an important confounding factor to consider when assessing the potential efficacy of antigen-specific therapies.
Review
Cancer metastases demonstrate both intra- and interlesional heterogeneity, which presents a significant obstacle to the current developmental paradigm for highly targeted molecular and immune-based therapeutics.Citation1 Cutaneous melanoma represents a model histology to gain further insight into the nature of human metastasis heterogeneity. Melanoma metastases exhibit a high mutation frequency,Citation2 diverse phenotype,Citation3 diffuse dissemination pattern, and a unique ability to elicit spontaneous host immune responses.Citation4 As highly targeted immune therapies promise to play an increasing role in the treatment of metastatic melanoma, an improved understanding of metastasis heterogeneity is critical to assessing potential tumor susceptibility in future clinical studies.
To profile interlesional heterogeneity among melanoma metastases, we performed a semi-quantitative immunohistochemical assessment of a panel of prototypic melanocyte differentiation antigens (MDAs) including gp100, MART-1, and tyrosinase (TYR). The role of MDAs as targets for immunotherapy has been studied extensively. These antigens are favorable targets for the profiling of heterogeneity due to their high expression level in normal melanocytes and primary melanomas but loss in a substantial proportion of metastatic lesions.Citation5 Immunoediting, whereby T cells recognize and clear MDA expressing cells, has been implicated as the mechanism for antigen heterogeneity among metastases based upon pre-clinical studies. Thus, we further characterized both the melanoma expression of MHC I and II as well as the CD4+ and CD8+ T cell infiltrates present within the tumors.
In this analysis of over 3000 human melanoma metastases, we confirmed the interlesional heterogeneity of MDA expression. Interestingly, when MDA expression was analyzed by anatomic site, a site-specific pattern was apparent with the highest expression levels seen in brain, intermediate levels in soft tissues and lymph nodes, and lowest levels in visceral (lung and liver) metastases ().Citation6 Classically, the heterogeneity associated with the metastatic process has been explained with Paget’s “seed and soil” hypothesis.Citation7 Indeed, the anatomic heterogeneity we observed may be partially explained by site-specific interactions. Preclinical work by Fidler et al. found that murine melanoma brain metastases were uniformly pigmented compared with variable pigmentation observed at other metastatic sitesCitation8 – consistent with the higher MDA expression that we observed in human brain metastases.
Figure 1. Melanoma differentiation antigen expression varies by anatomic site. Highest expression was observed in brain, intermediate expression in soft tissue and lymph node, and lowest expression in visceral (lung and liver) metastases.
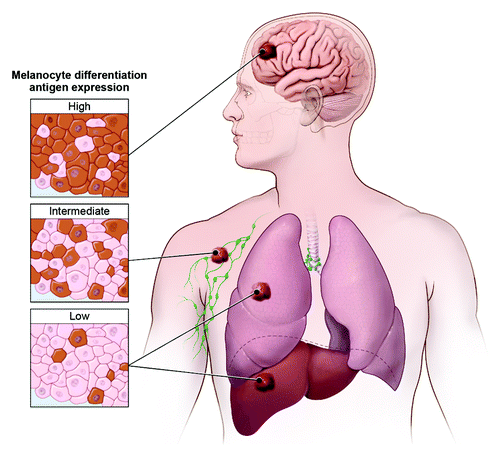
Our additional findings, however, suggest the potentially active role of the immune system in further sculpting the metastatic phenotype.Citation6 We found that TYR expression was disproportionately absent as compared with gp100 and MART expression. Furthermore, although loss of MART and MHC II expression both correlated with CD8+ T-cell infiltration, TYR was uniquely correlated with the levels of both endogenous CD8+ and CD4+ infiltrating T cells, suggesting that TYR expression in metastases may be naturally and selectively edited by antigen-specific T cells. Interestingly, in our analysis of site-specific antigen expression, the brain was the sole site of metastatic lesions in which TYR expression was not preferentially lost relative to the other antigens. This finding would suggest that the process driving differential TYR loss is mitigated within the brain, potentially consistent with the immune-privileged status of the central nervous system.
Our study could not conclusively determine the cause for the loss of TYR compared with the other MDAs in humans. Experimental evidence, however, in animal models of melanoma has previously demonstrated remarkably similar results. Lengange et al. reported in a double-transgenic MT-ret/AAD mouse model that Tyr and tyrosinase-related protein 2 (Trp2) expression were markedly reduced in both liver and lung tumors when compared with cutaneous tumors. Further, they noted a concomitant natural induction of CD8+ T cells specific for both Tyr and Trp2, suggesting that the visceral tumors, rather than the cutaneous tumors, were preferentially subjected to immunoediting by antigen-specific T cells.Citation9
The extensive profiling of metastases involving different anatomic sites in this study may explain a long observed clinical finding in which melanoma patients with isolated cutaneous metastases demonstrate a higher response rate after the administration of interleukin-2, a non-specific immunotherapy, in comparison to patients with visceral sites of metastases.Citation10 We hypothesize that the higher antigen load in cutaneous metastases could serve as a preferential target for the endogenous immune repertoire. Further, our results would suggest that melanoma brain metastases, which have the highest MDA expression, should be susceptible to immune targeting by endogenous T cells if the immunosuppressive host environment could be appropriately altered.
Although our study involved MDAs, these findings may have broader implications for the development of highly targeted cancer therapy. As these treatments are increasingly being studied in the clinic, defining the existence and degree of site-specific target expression across a wide variety of metastatic sites may prove to be of important therapeutic consequence. Ideally, biopsy confirmation of target expression should be obtained from the sites of disease anticipated to be most influential on the eventual outcome of the patient. Further, tracking antigen loss in tumors should be performed on the same lesion to avoid the confounding variable of interlesional heterogeneity. In summary, our findings suggest that future clinical efforts utilizing targeted immunotherapies must account for site-specific antigen heterogeneity in predicting the impact of antigen-based treatments on metastatic disease.
Disclosure of Potential Conflicts of Interest
The Author states he has no conflict of interest
References
- Fidler IJ. Biological heterogeneity of cancer: implication to therapy. Hum Vaccin Immunother 2012; 8:1141 - 2; http://dx.doi.org/10.4161/hv.19643; PMID: 22854675
- Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012; 366:883 - 92; http://dx.doi.org/10.1056/NEJMoa1113205; PMID: 22397650
- Prickett TD, Wei X, Cardenas-Navia I, Teer JK, Lin JC, Walia V, Gartner J, Jiang J, Cherukuri PF, Molinolo A, et al. Exon capture analysis of G protein-coupled receptors identifies activating mutations in GRM3 in melanoma. Nat Genet 2011; 43:1119 - 26; http://dx.doi.org/10.1038/ng.950; PMID: 21946352
- Boon T, van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med 1996; 183:725 - 9; http://dx.doi.org/10.1084/jem.183.3.725; PMID: 8642276
- Fetsch PA, Steinberg SM, Riker AI, Marincola FM, Abati A. Melanoma antigen expression in serial fine-needle aspiration samples in patients with metastatic malignant melanoma participating in immunotherapy clinical trials: a preliminary look. Cancer 2001; 93:409 - 14; http://dx.doi.org/10.1002/cncr.10138; PMID: 11748581
- Bartlett EK, Fetsch PA, Filie AC, Abati A, Steinberg SM, Wunderlich JR, White DE, Stephens DJ, Marincola FM, Rosenberg SA, et al. Human melanoma metastases demonstrate nonstochastic site-specific antigen heterogeneity that correlates with T-cell infiltration. Clin Cancer Res 2014; 20:2607 - 16; http://dx.doi.org/10.1158/1078-0432.CCR-13-2690; PMID: 24647571
- Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 1989; 8:98 - 101; PMID: 2673568
- Fidler IJ, Gruys E, Cifone MA, Barnes Z, Bucana C. Demonstration of multiple phenotypic diversity in a murine melanoma of recent origin. J Natl Cancer Inst 1981; 67:947 - 56; PMID: 6944560
- Lengagne R, Graff-Dubois S, Garcette M, Renia L, Kato M, Guillet JG, Engelhard VH, Avril MF, Abastado JP, Prévost-Blondel A. Distinct role for CD8 T cells toward cutaneous tumors and visceral metastases. J Immunol 2008; 180:130 - 7; http://dx.doi.org/10.4049/jimmunol.180.1.130; PMID: 18097012
- Phan GQ, Attia P, Steinberg SM, White DE, Rosenberg SA. Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol 2001; 19:3477 - 82; PMID: 11481353
