Abstract
The physiology of paracellular permeation of ions and solutes in the kidney is pivotally important but poorly understood. Claudins are the key components of the paracellular pathway. Defects in claudin function result in a broad range of renal diseases, including hypomagnesemia, hypercalciuria and nephrolithiasis. This review describes recent findings on the physiological function of claudins underlying paracellular transport mechanisms with a focus on renal Ca2+ handling. We have uncovered a molecular mechanism underlying paracellular Ca2+ transport in the thick ascending limb of Henle (TAL) that involves the functional interplay of three important claudin genes: claudin-14, -16 and -19, all of which are associated with human kidney diseases with hypercalciuria, nephrolithiasis and bone mineral loss. The Ca2+ sensing receptor (CaSR) signaling in the kidney has long been a mystery. By analyzing small non-coding RNA molecules in the kidney, we have uncovered a novel microRNA based signaling pathway downstream of CaSR that directly regulates claudin-14 gene expression and establishes the claudin-14 molecule as a key regulator for renal Ca2+ homeostasis. The molecular cascade of CaSR-microRNAs-claudins forms a regulatory loop to maintain proper Ca2+ homeostasis in the kidney.
Introduction
Kidneys function by initially excreting many salts and small molecules found in the blood, then selectively reabsorbing those that need to be conserved while allowing others to be excreted in the urine. The traditional view of the renal reabsorption process is that of a tandem array of ion channels and transporters located in the cell plasma membrane conducting ion transport in a coordinated manner at the expense of energy. Evidence accumulated during the last decade supports the existence of a previously unrecognized, yet pivotally important mechanism by which the kidney utilizes the cell-cell junctions to conduct ion transport. The junctional organelle is known as the tight junction (zonula occludens). It is found in vertebrate epithelia responsible for the barrier to movement of ions and molecules between apical and basal compartments.Citation1,Citation2 The integral membrane proteins of the tight junction include occludin (a 65 kDa membrane protein bearing four transmembrane domains and two uncharged extracellular loops),Citation3 the junctional adhesion molecules (JAMs),Citation4 a four-member group of glycosylated proteins and the claudins.
The Claudin Family
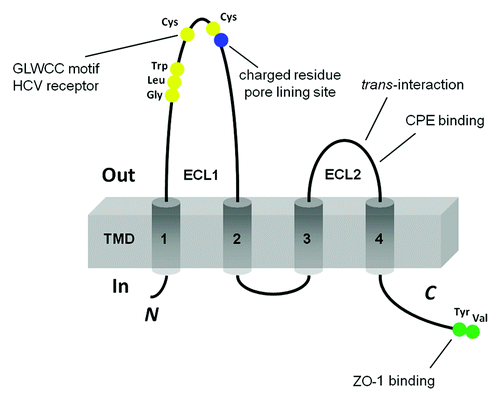
Claudins (CLDNs) are tetraspan proteins consisting of a family with at least 26 members,Citation5-Citation7 ranging in molecular mass from 20–28 kD. Claudins have four transmembrane domains, two extracellular loops, amino- and C-terminal cytoplasmic domains and a short cytoplasmic turn (). The first extracellular loop (ECL1) of claudin consists of ~50 amino acids with a common motif (-GLWCC; PROSITE ID: PS01346),Citation8 and intercalating negativeCitation9,Citation10 and positiveCitation11,Citation12 charges that contribute to paracellular ion selectivity. The GLWCC motif is critical as a receptor for Hepatitis C virus (HCV) entry.Citation13 The charges in ECL1 regulate paracellular ion selectivity through electrostatic effects. The second extracellular loop (ECL2) consists of ~25 amino acids with a predicted helix-turn-helix motif that mediates trans-claudin interactions (vide infra) and claudin interactions with the Clostridium perfringens enterotoxin (CPE).Citation14 The C-terminal domain of claudin contains a PDZ (postsynaptic density 95/discs large/zonula occludens-1) binding domain (YV) that is critical for interaction with the submembrane scaffold protein ZO-1 and correct localization in the TJ.Citation15,Citation16
Figure 1. Schematic presentation of the topology of claudin monomer. The model depicts the conserved structural features of claudin and the known interaction sites. ECL1 and ECL2 denote the extracellular loops 1 and 2, respectively. The transmembrane domains 1 to 4 (TM1-TM4) and the regions important for hepatitis C virus (HCV) entry, paracellular ion selectivity, Clostridium perfringens enterotoxin (CPE) binding, and ZO-1 binding are shown.
Claudin mutations have serious consequences, consistent with defects in epithelial ion flux. Mutations in CLDN14 cause nonsyndromic recessive deafness DFNB29,Citation17 ostensibly due to a failure in ion balance in the organ of Corti.Citation18 CLDN1-deficient mice die within one day of birth and show a loss of the water barrier function of skin.Citation19 Targeted deletion of CLDN5, which is known to be expressed in vascular endothelia as well as other locations,Citation20 results in a selective increase in brain vascular permeability to molecules < 800 daltons.Citation21 Targeted disruption of the CLDN11 gene results in severe demyelination and male sterility, consistent with the presence of this protein at the nodes of Ranvier and in Sertoli tight junctions, leading to disrupted ionic balances.Citation22 Mutations in CLDN16 have been associated with human FHHNC (familial hypomagnesemia with hypercalciuria and nephrocalcinosis; OMIM 248250).Citation23 Transgenic RNAi depletion of CLDN16 demonstrated severe renal Mg2+ and Ca2+ losses in mice.Citation24
In renal epithelia, claudins have been shown to confer ion selectivity to the paracellular pathway resulting in differences in TER and paracellular permeabilities. Studies have shown that CLDN4, -5, -8, -11 and -14 selectively decrease the permeability of cations through tight junctions,Citation25-Citation29 specifically to Na+, K+, H+ and ammonium. CLDN2 and -15 increase cation permeability.Citation30-Citation32 These properties have been attributed to charged amino acids in the first extracellular domain.Citation9 These and other studiesCitation33 have led to models of the claudins forming the paracellular channel (), a novel class of channels oriented perpendicular to the membrane plane and serving to join two extracellular compartments.Citation34 Measurement of paracellular permeability using cell membrane impermeable tracers indicates that there are 4–7 Å diameter channels in the TJ.Citation33,Citation35,Citation36 The paracellular channels in the tight junction have properties of ion selectivity, pH dependence and anomalous mole fraction effects, similar to conventional transmembrane channels.Citation33
Claudin-Claudin Interaction
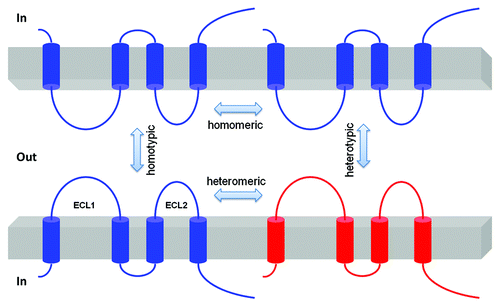
Claudins cis associate within the plasma membrane of the cell into dimers, or higher oligomeric state. These associations are followed by trans interactions between claudins in adjacent cells, and additional cis interactions to assemble claudin oligomers into TJ strands. The cis interaction can involve a single type of claudin (homomeric interaction) or different types of claudins (heteromeric interaction); the trans interaction can have homotypic or heterotypic mode ().Citation37 There are few data available allowing an understanding of the molecular interactions between the claudins. One study has shown that heterotypic interactions of CLDN1 and CLDN3 are permitted, but that interactions between CLDN1 and CLDN2 are not observed.Citation37 Co-culture of HeLa cells expressing different claudin genes revealed that while CLDN1 and CLDN5 were heterotypically interacting with CLDN3, they would not heterotypically bind to CLDN4, demonstrating considerable selectivity in heterotypic claudin-claudin interactions.Citation38 While different claudins can assemble into the same TJ strand, current limitations of resolution do not permit a clear understanding of what heteromeric interactions (within cells) are favored. Efforts have been made to demonstrate the oligomerization properties of CLDN4 in cultured insect cells with ambiguous results.Citation39 FRAP studies suggest that claudin molecules assembled in tight junctions have limited mobility,Citation40 consistent with their known heteromeric interactions with scaffold proteins in the tight junction.
The Renal Localization of Claudins
There are segment-specific claudin expression profiles along the length of the nephron. Northern analysis of mouse kidneys using probes specific for CLDN1–16 reveal that only CLDN6, -9, -13 and -14 are not detectable. CLDN5 and -15 are present only in endothelial cells; the rest are specifically expressed in different segments of the nephron.Citation41 Using antisera available at the time to perform immunostaining on mouse kidneys,Citation41 CLDN-3, -10, -11 and -16 were observed in the thick ascending limb (TAL), CLDN-3 and -8 in the distal convoluted tubule, and CLDN-3, -4 and -8 in the collecting duct (CLDN4 was also observed in the thick ascending limbCitation41 although absent in bovine TALCitation42). CLDN2 is highly expressed in the “leaky” proximal nephronCitation43 consistent with its high cation selectivity when expressed in MDCK cells.Citation30,Citation31 CLDN4 and CLDN8 are expressed primarily along the aldosterone-sensitive distal nephron, and in inner medullary segments of the thin descending limbs of juxtamedullary nephrons.Citation44,Citation45 Immunofluorescence analysis has shown that CLDN7 is expressed in the TAL and collecting ducts of porcine and rat kidneys,Citation46 although another study described CLDN7 in the distal nephron as located primarily on the basolateral membrane.Citation45 In summary, while there are still some conflicting published data, CLDN-2, -10, and -11 are expressed in the proximal tubule; CLDN-3, -4, -7, -8, -10 and -16 are expressed in the thick ascending limb and the distal nephron. It is also clear that the patterns of claudin expression along the nephron changes with development, with CLDN-7 and -8 upregulated postnatally.Citation47
Claudin-16
CLDN16, also known as paracellin-1, has been identified as a renal tight junction protein that is mutated in patients with the inherited disorder FHHNC (familial hypomagnesemia with hypercalciuria and nephrocalcinosis OMIM 248250).Citation23 Many different FHHNC mutations have been identified in the human CLDN16 gene.Citation48,Citation49 The expression of CLDN16 is restricted to the thick ascending limb (TAL) of the nephron.Citation23 We transfected a renal model cell line LLC-PK1 with CLDN16 and found a large increase in Na+ permeability (PNa) accompanied by an only moderately enhanced Mg2+ permeability (PMg).Citation10 The increase in PNa was not affected by the Na+/K+-ATPase inhibitor (1 mM ouabain). However it was greatly reduced or completely disappeared in all FHHNC relevant CLDN16 mutants.Citation10 CLDN16 deficient knockdown (KD) mice show significantly reduced plasma Mg2+ levels and increased urinary excretions (approximately four-fold) of Mg2+ and Ca2+.Citation50 Calcium deposits were readily observed in the basement membranes of the medullary tubules and the interstitium in CLDN16 KD mouse kidney.Citation50 These phenotypes of CLDN16 KD mice are very similar to the symptoms in human FHHNC patients. When TAL segments were isolated and perfused ex vivo, the paracellular ion selectivity (PNa/PCl) of the TAL was significantly reduced from 3.1 ± 0.3 in WT to 1.5 ± 0.1 in CLDN16 KD.Citation50
Claudin-19
CLDN19 mutations have also been associated with human FHHNC and renal Mg2+ loss.Citation51 While targeted deletion of CLDN19 in mice initially focused on its role in peripheral myelin,Citation52 promoter analysisCitation53 and subsequent studiesCitation54 have emphasized the localization of CLDN19 in the TAL of the kidney (colocalizing with CLDN16). Using the LLC-PK1 cells, we found that CLDN19 profoundly decreased the Cl− permeability (PCl) and functioned as a Cl− blocker.Citation55 The FHHNC mutations from human patients either partially or completely abolish the CLDN19 effects on PCl.Citation55 Coexpression of CLDN16 and CLDN19 in LLC-PK1 cells resulted in a dramatic upregulation of PNa and downregulation of PCl, generating a highly cation-selective paracellular pathway.Citation55 CLDN19 KD animals phenocopy CLDN16 KD and develop the FHHNC symptoms of reduced plasma Mg2+ levels and excessive renal wasting of Mg2+ and Ca2+.Citation56
Claudin-14
A recent genome-wide association study (GWAS) has identified CLDN14 as a major risk gene of hypercalciuric nephrolithiasis.Citation57 The renal localization of CLDN14 has been controversial. Immunofluorescence analysis showed CLDN14 gene expression in the TAL and the proximal tubules of mouse kidneys,Citation58 while another study reported no CLDN14 expression in the kidney.Citation41 In a CLDN14 lacZ reporter mouse, Gong et al. have found the promoter activity and the mRNA level of CLDN14 highly localized in the TAL segment.Citation59 The protein level of CLDN14, however, was extremely low in kidneys of mice fed a basal or low Ca2+ diet. Feeding mice a high Ca2+ diet profoundly upregulated the protein levels of CLDN14 in the TAL segment, suggesting a regulatory role for CLDN14 in renal function.Citation59 When expressed alone in LLC-PK1 cells, CLDN14 was without any significant effect on PNa or PCl. Coexpression of CLDN14 with CLDN16 abolished the cation permeability of CLDN16 channel by reducing PNa.Citation59 CLDN14 expression produced no difference in CLDN19 channel function. CLDN14 knockout (KO) mice show normal renal function under basal dietary condition.Citation27,Citation58 When fed with a high Ca2+ diet, CLDN14 KO animals excreted significantly less Ca2+ and Mg2+ than wild-type (WT) animals.Citation59 The plasma Mg2+ level was significantly higher in CLDN14 KO than in WT.Citation59 Together, CLDN14 KO animals develop the renal phenotypes exactly opposite to those in CLDN16 KD, which supports the in vitro finding that CLDN14 blocks CLDN16 channel permeability.
The Dynamic Trio in Renal Disease
The phenotypic similarities of CLDN19 KD with CLDN16 KD can be explained by the cis interaction between the two claudins. Using a split-ubiquitin yeast 2-hybrid (Y2H) assay, we found strong CLDN16 and CLDN19 heteromeric interaction.Citation55 In mammalian cells such as human embryonic kidney (HEK) 293 cells, CLDN16 can be coimmunoprecipitated with CLDN19.Citation55 Freeze-fracture replicas revealed the assembly of tight junction strands in L cells coexpressing CLDN16 and CLDN19, supporting their heteromeric interaction. The point mutations in CLDN16 (L145P, L151F, G191R, A209T and F232C) or CLDN19 (L90P and G123R) that are known to cause human FHHNC disrupt the CLDN16 and CLDN19 heteromeric interaction.Citation55 The same mutations also abolish the cation selectivity generated by CLDN16 and CLDN19 in LLC-PK1 cells, suggesting a mechanism for the role of claudin mutations in the development of FHHNC.Citation55 In vivo, knockdown of CLDN19 causes a loss of CLDN16 from tight junctions in the TAL without a decrease in CLDN16 expression level, whereas knockdown of CLDN16 produces a similar effect on CLDN19.Citation56 CLDN14 was observed to interact with CLDN16 but not with CLDN19 using several criteria.Citation59 Y2H analysis showed strong CLDN14–16 interaction the strength of which was similar to the CLDN16–19 interaction.Citation59 In doubly transfected HEK293 cells, CLDN14 coprecipitated with CLDN16 but not with CLDN19. Intriguingly, in triply transfected HEK293 cells, CLDN14 coprecipitated not only with CLDN16 but also with CLDN19.Citation59 There are two explanations for these findings relating to the CLDN14 assembly mechanism: (1) CLDN14 integrates into the CLDN16–19 channel to form a higher oligomeric complex with novel physiological signature; (2) CLDN14 replaces CLDN19 to form an independent channel with CLDN16 that coexists with the CLDN16–19 channel. The available biochemical data clearly favor the first hypothesis.
MicroRNA as Guardian Molecule for Claudin
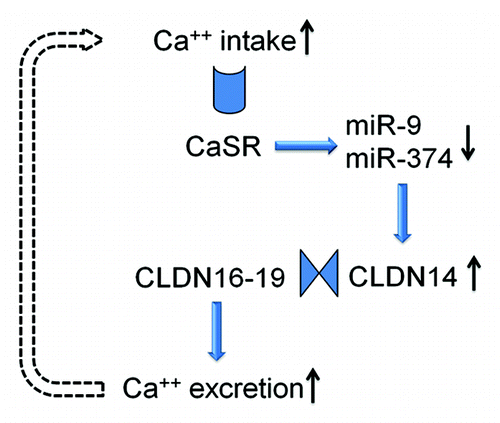
Although the promoter activity and the mRNA level of CLDN14 are very high in the kidney, its protein level is surprisingly low, suggesting post-transcriptional regulation. MicroRNAs are key regulatory molecules that regulate gene expression on the post-transcriptional level by inducing target mRNA decay or translational repression.Citation60 Gong et al. have identified two microRNA molecules—miR-9 and miR-374 from TAL cells, both of which recognize partially complementary binding sites located in 3′-UTRs of CLDN14 mRNA.Citation59 MiR-9 and miR-374 suppress CLDN14 translation and induce its mRNA decay in a synergistic manner.Citation59 Under normal dietary conditions, miR-9 and miR-374 tightly regulate the gene expression level of CLDN14 and protect CLDN16–19 channel function. The observed association between CLDN14 and hypercalciuric nephrolithiasisCitation57 can be explained by CLDN14 deregulation that escapes microRNA suppression, inhibits CLDN16–19 channel permeabilites and phenocopies FHHNC to variable degrees. High Ca2+ intake significantly downregulates the expression levels of miR-9 and miR-374 in TAL cells,Citation59 which in turn causes an increase in CLDN14 expression level discussed elsewhere in this review. The microRNA-CLDN14 cascade is under direct regulation of the Ca2+ sensing receptor (CaSR) in the TAL, a site at which the CaSR monitors the circulating Ca2+ levels and adjusts excretion rates accordingly.Citation59 The dietary regulation of microRNA suggests a physiological role for microRNA based signaling in the kidney. A physiological role of microRNA signaling provides a rationale for studying pathological changes such as nephrolithiasis, because any pathological abnormality must have a physiological origin.
An Integrated Signaling Pathway
The thick ascending limb (TAL) is a predominant renal tubular segment responsible for Mg2+ and Ca2+ reabsorption.Citation61,Citation62 The epithelial cells in the TAL form a water-impermeable barrier, actively transport Na+ and Cl− via the transcellular route, and provide a paracellular pathway for the selective reabsorption of Mg2+ and Ca2+.Citation63–Citation64 The paracellular reabsorption of Mg2+ and Ca2+ is driven by a lumen-positive transepithelial voltage (Vte). The generation of Vte can be attributed to: (1) the active transport Vte due to apical K+ secretion through ROMK and basolateral Cl− exit through ClC-Kb/barttin channels; (2) the diffusion Vte generated because of the transepithelial NaCl concentration gradient and the cation selectivity of the tight junction.Citation65 In the early TAL segment, it is the first mechanism that provides a voltage around +8 mV. There is minimal contribution of diffusion potential at this early segment since the concentration gradient has not yet been built up (). With continuous NaCl reabsorption along the axis of the TAL, the lumen fluid is diluted and a large NaCl gradient is generated in the late TAL segment. Because the paracellular permeability of the TAL is cation-selective, the diffusion Vte is superimposed onto the active transport Vte and becomes the major source of the lumen-positive Vte, which now increases substantially—up to +30 mV ().
Figure 4. Transepithelial ion transport in the thick ascending limb segment. (A) When similar salt concentrations are present at the luminal and basolateral sides, the luminal spontaneous potential Vte of +8mV is generated by the concerted action of luminal K+ channels, basolateral Cl− channels, the Na+2Cl−K+ cotransporter, and the Na+K+-ATPase. Vte drives Na+ absorption through the paracellular pathway. (B) When a dilute luminal fluid is present after NaCl absorption along the water-tight TAL, the luminal potential Vte of +30 mV is now generated as a diffusion voltage by the ‘backleak’ of Na+. The diffusion voltage depends on the permselectivity of the tight junction. The membrane voltage (Vm) trace depicts the virtual measurement by an electrode that is pushed from the basolateral side through the cell to the luminal side.
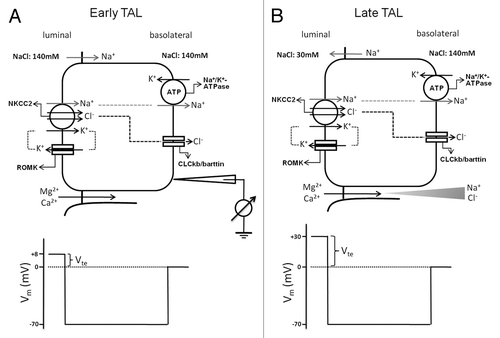
It is clear that two basic prerequisites are required for the paracellular Mg2+ and Ca2+ reabsorption in the TAL: the lumen-positive Vte as the driving force and the paracellular cation permeability. The CLDN16 channel provides cation permeability to the tight junction. The CLDN19 channel increases the cation selectivity of the tight junction and the diffusional Vte by: (1) selectively blocking anion permeation; (2) interacting with CLDN16 to increase the overall cation selectivity of the tight junction. Removal of either claudin would cause the tight junction to lose its cation selectivity and generate renal defects in Mg2+ and Ca2+ reabsorption. The phenotypic similarities between CLDN16 KD and CLDN19 KD animals can be explained by the molecular interaction between CLDN16 and CLDN19. This molecular interaction provides a mechanistic basis for the role of claudin mutations in the development of FHHNC. The discovery of CLDN14 as a regulatory molecule for renal Ca2+ homeostasis is particularly important. First, accumulating data have demonstrated that paracellular Ca2+ reabsorption in the TAL can be directly regulated by CaSR activation during hypercalcemia.Citation66,Citation67 CLDN14 is the “underlying factor” sought for many years. Through physical interactions, CLDN14 blocks the paracellular cation channel made of CLDN16 and CLDN19, suggesting a mechanism for its role in nephrolithiasis. Second, tight junction proteins were previously considered as constitutive and structural molecules. CLDN14 is the first TJ molecule the expression level of which can be regulated in response to pathophysiological changes. The tight junction is not as ‘lethargic’ as previously thought. The renal regulation of Ca2+ excretion involves the functional interplay of three important claudin genes: CLDN14, 16 and 19, all of which are associated with human kidney diseases. The claudin channel molecules are part of the CaSR signaling cascade that employs two microRNA molecules to transduce signals. The molecular axis of CaSR-microRNA-CLDN14-CLDN16/19 provides a feedback mechanism to counterbalance extracellular Ca2+ variations. Increases in extracellular Ca2+ levels activate CaSR → CaSR activation downregulates miR-9 and miR-374 expression → decreases in microRNA levels relieve their suppression of CLDN14 → increases in CLDN14 levels suppress CLDN16–19 permeabilities, promoting Ca2+ excretion by the kidney; and vice versa ().
The Journey Ahead
The GWAS study established a genetic link between CLDN14 sequence variants and nephrolithiasis.Citation57 However, none of the identified sequence variants in CLDN14 appear to be causative. Because two of the four identified variants are located in the last exon of CLDN14 gene, this exon may contain rare causative variations related to microRNA regulation, mRNA stability and translational efficiency. Gong et al. have established a physiological role for CLDN14 in renal Ca2+ handling.Citation59 It will be essential to understand the pathogenic role of CLDN14 in nephrolithiasis. There are two interrelating hypotheses: (1) CLDN14 overexpression in the kidney leads to hypercalciuria and nephrolithiasis. Because CLDN14 is a negative regulator of the CLDN16–19 channel, overexpression of CLDN14 will lead to renal Ca2+ wasting. This hypothesis can be tested by generating a CLDN14 overexpressing (CLDN14OX) mouse model. (2) The kidney will restore normal Ca2+ homeostasis through other nephron segments when the TAL is deregulated. Because the distal convoluted tubule (DCT) expresses a Ca2+ channel—TRPV5, Ca2+ excretion changes in the TAL will be compensated for in the DCT. This hypothesis can be tested by crossing CLDN14OX with CLDN14−/− to generate a TAL deregulated animal model—CLDN14OX−/−. The microRNAs identified by Gong et al. are of critical importance.Citation59 Owing to the short seed sequence of microRNA, a cognate microRNA regulates multiple target genes. Although miR-9 and miR-374 converge onto CLDN14, they could extend extracellular Ca2+ signaling to cellular functions beyond the paracellular channel in the TAL. What remain largely unknown are the microRNA targeted genes in the kidney. From a therapeutic point of view, small RNA molecules such as antagomirs that repress microRNA function in vivo in the kidney may represent a novel tool for clinical intervention in renal Ca2+ excretion. Nevertheless, manipulation of microRNAs in vivo may induce unwanted side effects if their downstream target genes have not been thoroughly studied.
Questions and Answers
Dr Eduardo Slatopolsky, Joseph Friedman Professor of Medicine:
You showed very nicely the importance of the thick ascending limb of Henle (TAL) and claudin on magnesium reabsorption. But still there is a significant amount of magnesium that escapes the reabsorption and goes into the distal tubule where the epithelial magnesium channel, Transient Receptor Potential Melastatin subtype 6 (TRPM6) participates in its reabsorption. Do defects in magnesium reabsorption in more distal nephron segments result in stone disease?
Dr Jianghui Hou, Assistant Professor of Medicine:
Indeed, the TRPM6 channel plays an important role in the kidney, in addition to its role in the colon. Mutations in the TRPM6 channel are linked to HSH syndrome: hypomagnesemia with secondary hypocalcemia. I am not aware of a TRPM6 knockout mouse model that is not an embryonic lethal.Citation68 The human patient data suggest hypocalcemia and possibly hypercalciuria are caused by parathyroid gland failure induced by hypomagnesemia. When there is hypercalciuria, the risk of developing kidney stones will be higher. The mystery is where calcium loss occurs. In contrast to the TAL where calcium and magnesium handling is coupled, the distal convoluted tubule employs a different mechanism to handle calcium, through another channel: TRPV5.
Dr Slatopolsky:
So your point is that they get hypermagnesuria but they do not get kidney stones.
Dr Hou:
They may develop kidney stones or have higher risks. The underlying mechanism may be an indirect effect through claudin-14 or TRPV5 or other molecules.
Dr Aubrey Morrison, Professor of Medicine:
How do you think the calcium sensing receptor (CaSR) functions in the presence of the lower magnesium? Are you implying that the calcium sensory receptor is able to discriminate between the calcium and magnesium?
Dr Hou:
That is a very good question. Unlike in the parathyroid gland where CaSR shows higher affinity toward calcium than magnesium (approximately 5:1), kidney tubular cells seem to show a different affinity pattern. Our in vitro measurements found a rather equal affinity of CaSR toward calcium and magnesium. Actually, there is an independent study published a few years ago that supports our data and shows slightly higher affinity of CaSR toward magnesium than calcium in kidney tubular cells.Citation69
Dr Slatopolsky:
Alex, are you aware of two different types of calcium receptors?
Dr Alex Brown, Associate Professor of Medicine:
No. The calcium receptor turns on different signaling pathway in different cell types.
Dr Slatopolsky:
We joke and we call the calcium receptor a promiscuous receptor because it can detect not only calcium and magnesium, but also many other agonists. Have you tried other CaSR agonists?
Dr Hou:
Yes, we have tried another CaSR agonist, Gadolinium, and found a similar effect on claudin-14 and microRNA expression. In addition, we tested the effects of PTH on claudin expression but found none, suggesting the observed effects are mediated through the CaSR but not PTH.
Dr Jeffrey Miner, Professor of Medicine:
Have you tried expressing the claudin-14 either in vitro or in vivo without the 3′ untranslated region?
Dr Hou:
I haven’t done it. We are generating mutations in the microRNA binding sites in claudin-14 3′UTR in order to determine whether microRNA effects are mediated through the 3′UTR of claudin-14.
Dr Maggie Chen, Assistant Professor of Medicine:
I think the current concept is that after activation of the CaSR in the basal lateral membrane of the TAL there is inhibition of the ROMK channel in the apical membrane that can cause hypercalciuria and hypermagnesuria. Which part plays the bigger role?
Dr Hou:
I will answer your question from two perspectives. The traditional view of CaSR function in the kidney is that it will transduce signals germane to the transcellular pathway, e.g., ROMK channel. The microRNAs we have identified may also regulate the transcellular pathways and serve as a converging point for signal transduction. My colleague Markus Bleich has measured the effects of CaSR activation on both the transcellular and paracellular ion conductance in perfused TAL. He was able to capture changes in the paracellular pathway but not in the transcellular pathway, further supporting our view of paracellular channels playing a major role in the CaSR signaling pathway.Citation70
Dr Maggie Chen:
You said there are two types of CaSR: in the basolateral membranes of the TAL and also in the apical site of the proximal tubule and the collecting duct. What is the role of the apical CaSR?
Dr Hou:
That is a major mystery. Some suspect the apical CaSR senses changes of calcium in the tubular filtrate and transduces signals to cells. For example, CaSR in the proximal tubule could transduce signals to regulate calcitriol synthesis, which in turn affects calcium reabsorption in the distal nephron. The CaSR in the collecting duct has also been implicated in the regulation of water transport. However, I think the primary role of CaSR is still its classic role in the TAL—sensing the changes in circulating calcium levels.
| Abbreviations: | ||
| FHHNC | = | hypomagnesemia with hypercalciuria and nephrocalcinosis |
| TAL | = | thick ascending limb |
| TER | = | transepithelial resistance |
| TJ | = | tight junction |
| GWAS | = | genome-wide association study |
| Y2H | = | yeast 2-hybrid assay |
| CaSR | = | Ca2+ sensing receptor |
Note
Edited transcripts of research conferences sponsored by Organogenesis and the Washington University George M. O’Brien Center for Kidney Disease Research (P30 DK079333) are published in Organogenesis. These conferences cover organogenesis in all multicellular organisms including research into tissue engineering, artificial organs and organ substitutes and are participated in by faculty at Washington University School of Medicine, St. Louis, MO USA.
References
- Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol 1963; 17:375 - 412; http://dx.doi.org/10.1083/jcb.17.2.375; PMID: 13944428
- Miller F. Hemoglobin absorption by the cells of the proximal convoluted tubule in mouse kidney. J Biophys Biochem Cytol 1960; 8:689 - 718; http://dx.doi.org/10.1083/jcb.8.3.689; PMID: 13770760
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 1993; 123:1777 - 88; http://dx.doi.org/10.1083/jcb.123.6.1777; PMID: 8276896
- Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): more molecules with dual functions?. J Cell Sci 2004; 117:19 - 29; http://dx.doi.org/10.1242/jcs.00930; PMID: 14657270
- Lal-Nag M, Morin PJ. The claudins. Genome Biol 2009; 10, 235.1–7.
- Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, Saito K, et al. Predicted expansion of the claudin multigene family. FEBS Lett 2011; 585:606 - 12; http://dx.doi.org/10.1016/j.febslet.2011.01.028; PMID: 21276448
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2001; 2:285 - 93; http://dx.doi.org/10.1038/35067088; PMID: 11283726
- Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta 2008; 1778:631-45.
- Colegio OR, Van Itallie CM, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol 2003; 284:C1346 - 54; PMID: 12700140
- Hou J, Paul DL, Goodenough DA. Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci 2005; 118:5109 - 18; http://dx.doi.org/10.1242/jcs.02631; PMID: 16234325
- Alexandre MD, Jeansonne BG, Renegar RH, Tatum R, Chen YH. The first extracellular domain of claudin-7 affects paracellular Cl- permeability. Biochem Biophys Res Commun 2007; 357:87 - 91; http://dx.doi.org/10.1016/j.bbrc.2007.03.078; PMID: 17400193
- Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol 2006; 68:403 - 29; http://dx.doi.org/10.1146/annurev.physiol.68.040104.131404; PMID: 16460278
- Cukierman L, Meertens L, Bertaux C, Kajumo F, Dragic T. Residues in a highly conserved claudin-1 motif are required for hepatitis C virus entry and mediate the formation of cell-cell contacts. J Virol 2009; 83:5477 - 84; http://dx.doi.org/10.1128/JVI.02262-08; PMID: 19297469
- Fujita K, Katahira J, Horiguchi Y, Sonoda N, Furuse M, Tsukita S. Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein. FEBS Lett 2000; 476:258 - 61; http://dx.doi.org/10.1016/S0014-5793(00)01744-0; PMID: 10913624
- Hamazaki Y, Itoh M, Sasaki H, Furuse M, Tsukita S. Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J Biol Chem 2002; 277:455 - 61; http://dx.doi.org/10.1074/jbc.M109005200; PMID: 11689568
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol 1999; 147:1351 - 63; http://dx.doi.org/10.1083/jcb.147.6.1351; PMID: 10601346
- Wilcox ER, Burton QL, Naz S, Riazuddin S, Smith TN, Ploplis B, et al. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell 2001; 104:165 - 72; http://dx.doi.org/10.1016/S0092-8674(01)00200-8; PMID: 11163249
- Kitajiri SI, Furuse M, Morita K, Saishin-Kiuchi Y, Kido H, Ito J, et al. Expression patterns of claudins, tight junction adhesion molecules, in the inner ear. Hear Res 2004; 187:25 - 34; http://dx.doi.org/10.1016/S0378-5955(03)00338-1; PMID: 14698084
- Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol 2002; 156:1099 - 111; http://dx.doi.org/10.1083/jcb.200110122; PMID: 11889141
- Morita K, Sasaki H, Furuse K, Furuse M, Tsukita S, Miyachi Y. Expression of claudin-5 in dermal vascular endothelia. Exp Dermatol 2003; 12:289 - 95; http://dx.doi.org/10.1034/j.1600-0625.2003.120309.x; PMID: 12823443
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 2003; 161:653 - 60; http://dx.doi.org/10.1083/jcb.200302070; PMID: 12743111
- Gow A, Southwood CM, Li JS, Pariali M, Riordan GP, Brodie SE, et al. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell 1999; 99:649 - 59; http://dx.doi.org/10.1016/S0092-8674(00)81553-6; PMID: 10612400
- Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 1999; 285:103 - 6; http://dx.doi.org/10.1126/science.285.5424.103; PMID: 10390358
- Hou J, Goodenough DA. Claudin-16 and claudin-19 function in the thick ascending limb. Curr Opin Nephrol Hypertens 2010; 19:483 - 8; http://dx.doi.org/10.1097/MNH.0b013e32833b7125; PMID: 20616717
- Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest 2001; 107:1319 - 27; http://dx.doi.org/10.1172/JCI12464; PMID: 11375422
- Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol 2002; 283:C142 - 7; PMID: 12055082
- Ben-Yosef T, Belyantseva IA, Saunders TL, Hughes ED, Kawamoto K, Van Itallie CM, et al. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum Mol Genet 2003; 12:2049 - 61; http://dx.doi.org/10.1093/hmg/ddg210; PMID: 12913076
- Yu AS, Enck AH, Lencer WI, Schneeberger EE. Claudin-8 expression in MDCK cells augments the paracellular barrier to cation permeation. J Biol Chem 2003; 278:17350 - 9; http://dx.doi.org/10.1074/jbc.M213286200; PMID: 12615928
- Wen H, Watry DD, Marcondes MC, Fox HS. Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Mol Cell Biol 2004; 24:8408 - 17; http://dx.doi.org/10.1128/MCB.24.19.8408-8417.2004; PMID: 15367662
- Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol 2001; 153:263 - 72; http://dx.doi.org/10.1083/jcb.153.2.263; PMID: 11309408
- Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, et al. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 2002; 115:4969 - 76; http://dx.doi.org/10.1242/jcs.00165; PMID: 12432083
- Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol 2003; 285:F1078 - 84; PMID: 13129853
- Tang VW, Goodenough DA. Paracellular ion channel at the tight junction. Biophys J 2003; 84:1660 - 73; http://dx.doi.org/10.1016/S0006-3495(03)74975-3; PMID: 12609869
- Tsukita S, Furuse M. Pores in the wall: claudins constitute tight junction strands containing aqueous pores. J Cell Biol 2000; 149:13 - 6; http://dx.doi.org/10.1083/jcb.149.1.13; PMID: 10747082
- Watson CJ, Rowland M, Warhurst G. Functional modeling of tight junctions in intestinal cell monolayers using polyethylene glycol oligomers. Am J Physiol Cell Physiol 2001; 281:C388 - 97; PMID: 11443038
- Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, et al. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci 2008; 121:298 - 305; http://dx.doi.org/10.1242/jcs.021485; PMID: 18198187
- Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol 1999; 147:891 - 903; http://dx.doi.org/10.1083/jcb.147.4.891; PMID: 10562289
- Daugherty BL, Ward C, Smith T, Ritzenthaler JD, Koval M. Regulation of heterotypic claudin compatibility. J Biol Chem 2007; 282:30005 - 13; http://dx.doi.org/10.1074/jbc.M703547200; PMID: 17699514
- Mitic LL, Unger VM, Anderson JM. Expression, solubilization, and biochemical characterization of the tight junction transmembrane protein claudin-4. Protein Sci 2003; 12:218 - 27; http://dx.doi.org/10.1110/ps.0233903; PMID: 12538885
- Sasaki H, Matsui C, Furuse K, Mimori-Kiyosue Y, Furuse M, Tsukita S. Dynamic behavior of paired claudin strands within apposing plasma membranes. Proc Natl Acad Sci U S A 2003; 100:3971 - 6; http://dx.doi.org/10.1073/pnas.0630649100; PMID: 12651952
- Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol 2002; 13:875 - 86; PMID: 11912246
- Ohta H, Adachi H, Takiguchi M, Inaba M. Restricted localization of claudin-16 at the tight junction in the thick ascending limb of Henle’s loop together with claudins 3, 4, and 10 in bovine nephrons. J Vet Med Sci 2006; 68:453 - 63; http://dx.doi.org/10.1292/jvms.68.453; PMID: 16757888
- Enck AH, Berger UV, Yu AS. Claudin-2 is selectively expressed in proximal nephron in mouse kidney. Am J Physiol Renal Physiol 2001; 281:F966 - 74; PMID: 11592954
- Le Moellic C, Boulkroun S, González-Nunez D, Dublineau I, Cluzeaud F, Fay M, et al. Aldosterone and tight junctions: modulation of claudin-4 phosphorylation in renal collecting duct cells. Am J Physiol Cell Physiol 2005; 289:C1513 - 21; http://dx.doi.org/10.1152/ajpcell.00314.2005; PMID: 16107502
- Li WY, Huey CL, Yu AS. Expression of claudin-7 and -8 along the mouse nephron. Am J Physiol Renal Physiol 2004; 286:F1063 - 71; http://dx.doi.org/10.1152/ajprenal.00384.2003; PMID: 14722018
- Alexandre MD, Lu Q, Chen YH. Overexpression of claudin-7 decreases the paracellular Cl- conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. J Cell Sci 2005; 118:2683 - 93; http://dx.doi.org/10.1242/jcs.02406; PMID: 15928046
- Reyes JL, Lamas M, Martin D, del Carmen Namorado M, Islas S, Luna J, et al. The renal segmental distribution of claudins changes with development. Kidney Int 2002; 62:476 - 87; http://dx.doi.org/10.1046/j.1523-1755.2002.00479.x; PMID: 12110008
- Weber S, Schneider L, Peters M, Misselwitz J, Rönnefarth G, Böswald M, et al. Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol 2001; 12:1872 - 81; PMID: 11518780
- Konrad M, Hou J, Weber S, Dötsch J, Kari JA, Seeman T, et al. The CLDN16 genotype predicts the progression of renal failure in familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol 2008; 19:171 - 81; http://dx.doi.org/10.1681/ASN.2007060709; PMID: 18003771
- Hou J, Shan Q, Wang T, Gomes AS, Yan Q, Paul DL, et al. Transgenic RNAi depletion of claudin-16 and the renal handling of magnesium. J Biol Chem 2007; 282:17114 - 22; http://dx.doi.org/10.1074/jbc.M700632200; PMID: 17442678
- Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, et al. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet 2006; 79:949 - 57; http://dx.doi.org/10.1086/508617; PMID: 17033971
- Miyamoto T, Morita K, Takemoto D, Takeuchi K, Kitano Y, Miyakawa T, et al. Tight junctions in Schwann cells of peripheral myelinated axons: a lesson from claudin-19-deficient mice. J Cell Biol 2005; 169:527 - 38; http://dx.doi.org/10.1083/jcb.200501154; PMID: 15883201
- Luk JM, Tong MK, Mok BW, Tam PC, Yeung WS, Lee KF. Sp1 site is crucial for the mouse claudin-19 gene expression in the kidney cells. FEBS Lett 2004; 578:251 - 6; http://dx.doi.org/10.1016/j.febslet.2004.11.010; PMID: 15589828
- Angelow S, El-Husseini R, Kanzawa SA, Yu AS. Renal localization and function of the tight junction protein, claudin-19. Am J Physiol Renal Physiol 2007; 293:F166 - 77; http://dx.doi.org/10.1152/ajprenal.00087.2007; PMID: 17389678
- Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, et al. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest 2008; 118:619 - 28; PMID: 18188451
- Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, et al. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci U S A 2009; 106:15350 - 5; http://dx.doi.org/10.1073/pnas.0907724106; PMID: 19706394
- Thorleifsson G, Holm H, Edvardsson V, Walters GB, Styrkarsdottir U, Gudbjartsson DF, et al. Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Genet 2009; 41:926 - 30; http://dx.doi.org/10.1038/ng.404; PMID: 19561606
- Elkouby-Naor L, Abassi Z, Lagziel A, Gow A, Ben-Yosef T. Double gene deletion reveals lack of cooperation between claudin 11 and claudin 14 tight junction proteins. Cell Tissue Res 2008; 333:427 - 38; http://dx.doi.org/10.1007/s00441-008-0621-9; PMID: 18663477
- Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, et al. Claudin-14 regulates renal Ca++ transport in response to CaSR signaling via a novel microRNA pathway. EMBO J 2012; In press http://dx.doi.org/10.1038/emboj.2012.49; PMID: 22373575 [Epub ahead of print]
- Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet 2011; 12:99 - 110; http://dx.doi.org/10.1038/nrg2936; PMID: 21245828
- Cole DEC, Quamme GA. Inherited disorders of renal magnesium handling. J Am Soc Nephrol 2000; 11:1937 - 47; PMID: 11004227
- Hebert SC. Calcium and salinity sensing by the thick ascending limb: a journey from mammals to fish and back again. Kidney Int Suppl 2004; 91:S28 - 33; http://dx.doi.org/10.1111/j.1523-1755.2004.09105.x; PMID: 15461699
- Hebert SC, Culpepper RM, Andreoli TE. NaCl transport in mouse medullary thick ascending limbs. I. Functional nephron heterogeneity and ADH-stimulated NaCl cotransport. Am J Physiol 1981; 241:F412 - 31; PMID: 7315965
- Hebert SC, Culpepper RM, Andreoli TE. NaCl transport in mouse medullary thick ascending limbs. II. ADH enhancement of transcellular NaCl cotransport; origin of transepithelial voltage. Am J Physiol 1981; 241:F432 - 42; PMID: 7315966
- Greger R. Ion transport mechanisms in thick ascending limb of Henle’s loop of mammalian nephron. Physiol Rev 1985; 65:760 - 97; PMID: 2409564
- Desfleurs E, Wittner M, Simeone S, Pajaud S, Moine G, Rajerison R, et al. CaSR: regulation of electrolyte transport in the thick ascending limb of Henle’s loop. Kidney Blood Press Res 1998; 1:401 - 12; http://dx.doi.org/10.1159/000025892
- Motoyama HI, Friedman PA. CaSR regulation of PTH dependent calcium absorption by mouse cortical ascending limbs. Am J Physiol Renal Physiol 2002; 283:F399 - 406; PMID: 12167589
- Walder RY, Yang B, Stokes JB, Kirby PA, Cao X, Shi P, et al. Mice defective in Trpm6 show embryonic mortality and neural tube defects. Hum Mol Genet 2009; 18:4367 - 75; http://dx.doi.org/10.1093/hmg/ddp392; PMID: 19692351
- Bapty BW, Dai LJ, Ritchie G, Canaff L, Hendy GN, Quamme GA. Activation of Mg2+/Ca2+-sensing receptors inhibits hormone-stimulated Mg2+ uptake in distal convoluted tubule cells. Am J Physiol 1998; 244:F328 - 35
- Bleich M. Calcium regulation of tight junction permeability. International Conference Berlin. “Barriers and channels formed by tight junction proteins”; September 22-24, 2011, Harnack House of the Max-Planck-Gesellschaft.