Abstract
The extracellular matrix (ECM) plays an essential role in organizing tissues, defining their shapes or in presenting growth factors. Their components have been well described in most species, but our understanding of the mechanisms that control ECM remodeling remains limited. Likewise, how the ECM contributes to cellular mechanical responses has been examined in few cases. Here, I review how studies performed in C. elegans have brought several significant advances on those topics. Focusing only on epithelial cells, I discuss basement membrane invasion by the anchor cell during vulva morphogenesis, a process that has greatly expanded our knowledge of ECM remodeling in vivo. I then discuss the ECM role in a novel mechanotransduction process, whereby muscle contractions stimulate the remodeling of hemidesmosome-like junctions in the epidermis, which highlights that these junctions are mechanosensitive. Finally, I discuss progress in defining the composition and potential roles of the apical ECM covering epidermal cells in embryos.
In this article, I will examine recent findings concerning the cellular and developmental functions of the extracellular matrix (ECM) surrounding epithelial tissues in C. elegans. I will focus on three areas of direct relevance to other systems where important advances have been made: basement membrane (BM) breaching during tissue invasion, mechanotransduction and apical extracellular matrix. I will present these topics in the context of two developmental events, the formation of an open BM-free connection between the uterus and the vulva during larval development and the elongation of the embryo. Both processes are presented in separate sections. This short review starts with a brief update on proteins found in the C. elegans extracellular matrix. For more comprehensive coverage in other systems of the processes discussed herein, readers are referred to references Citation1–Citation3.
Composition of the Basement Membrane Surrounding C. elegans Epithelia
Like in other metazoans, C. elegans epithelial and mesodermal tissues are covered with a basement membrane (BM). Its composition, which has been described in a comprehensive review, is quite similar in structure to its vertebrate counterpart (except for the notable absence of fibronectin);Citation4 it is not produced by fibroblasts, as is often the case in vertebrates. Of particular relevance in the framework of the present discussion are laminin, collagen IV, SPARC and the heparan sulfate proteoglycan perlecan. The first three are present in the two BMs separating the somatic gonad from the vulva, although it is not always known which tissue produces each of them, and are essential for development.Citation4 Perlecan is found at the muscle-epidermis interface and is secreted by the epidermis.Citation5 It provides a polarizing signal to initiate the assembly of muscle sarcomeres and impacts on the assembly of epidermal hemidesmosomes.Citation5-Citation8
Among classical ECM receptors, C. elegans has a much simpler repertoire of integrins since its genome encodes only two α-integrin chains (INA-1 and PAT-2) and one β-integrin chain (PAT-3). The BM receptor at the vulva-uterus interface is INA-1/PAT-3 (see below), whereas the main perlecan receptor is PAT-2/PAT-3 in body wall muscles. The perlecan receptor in the epidermis presumably corresponds to the C. elegans-specific protein myotactin.Citation11,Citation12 Myotactin contains numerous extra-cellular EGF repeats, and an intracellular domain without any recognizable domain.Citation12 Perlecan and myotactin null mutations arrest embryonic elongation midway and disrupt the muscle-epidermis connection.Citation5,Citation7,Citation12
Since the review mentioned above,Citation4 A. Chisholm and his collaborators characterized two additional BM components located at the muscle-epidermis interface: an ECM spondin-family homolog (SPON-1) and a peroxidasin homolog (PXN-2).Citation9,Citation10 A conserved catalytic residue among peroxidases is essential for PXN-2 function, suggesting that PXN-2 has peroxidase activity, but its endogenous substrates remain unknown.Citation10 Strong mutations in spon-1 or pxn-2 disrupt muscle-epidermis attachments but at a much later stage of embryonic elongation than perlecan (see above), suggesting a role in maintaining these attachments. In spon-1 mutants, the BM at the muscle-epidermis interface becomes convoluted, whereas in pxn-2 mutants it appears unstructured and very thick.Citation9,Citation10 The significance of the muscle-epidermis detachment and embryonic elongation defects in such mutants is further discussed in the third part of this review.
Cell Invasion by Breaching the Basement Membrane
Remodeling of the ECM plays a fundamental role in restructuring tissue architecture.Citation2 The processes and proteins that influence ECM modifications include transcription factors, metalloproteases that degrade the ECM, oxidating enzymes that modify its properties and novel ECM components whose presence alters the ECM mechanical properties.Citation2
The formation of the vulva-uterus connection in C. elegans through the invasion of the BM by the anchor cell (AC) provides a powerful system to analyze these events using sophisticated genetic tools. The vulva proper originates from a set of three vulval precursors called P5.p through P7.p, which are located in the ventral part of the larval mid-body. During the third larval stage, a signal secreted by the overlying AC in the somatic gonad induces these precursors to divide up to three times to generate 22 cells, which invaginate, fuse and form a stack of seven toroids (labeled vulA ventrally to vulF dorsally in contact with the somatic gonad). Midway through the third larval stage, the AC breaches and invades the BM separating vulval precursors from the somatic gonad which later provides a passageway for egg-laying ().
Figure 1. Integrin contribution to BM invasion by the anchor cell (AC). (A and B) During the late 2nd larval stage and early 3rd larval stage, a netrin signal originating from the ventral neurons (A), in parallel to a signal of unknown nature secreted by the first two daughters of the central vulval precursor P6.p (black arrows in A′) polarize the AC (graded red in A′). INA-1/PAT-3 integrin activity in the AC is essential to achieve AC polarization (B). (C–D) Once polarized, the AC starts to breach and remove the BM between the somatic gonad and the vulva (green and blue lines). Two features of AC polarity correspond to the formation of ventral finger-like protrusions that start to breach the BM, and the secretion of hemicentin (orange ovals; A′). During the mid-3rd larval stage, the AC dissolves the BM at the interface with P6.p grand-daughters (C′). INA-1/PAT-3 integrin activity in the AC is essential to achieve BM removal (D). (E–F) Vulval cells (letter A–F) invagination and toroid formation causes the BM to slide laterally, further expanding the gap between the somatic gonad and the vulva (pink arrows in E) until INA-1/PAT-3 activity acting in VulD stabilizes it. In the absence of INA-1/PAT-3 integrin, BM sliding continues (F). The utse corresponds to a small syncytium resulting from the fusion of the AC and several ventral uterine cells.
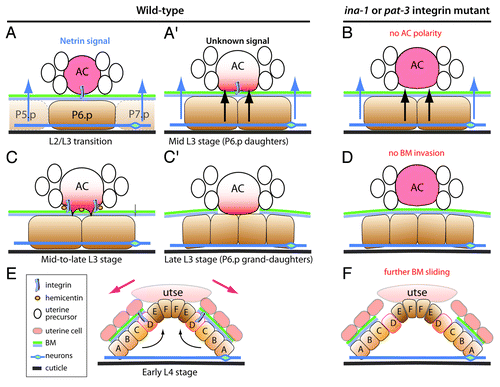
David Sherwood and his colleagues have very elegantly dissected the AC invasion process, showing that it involves four steps ().Citation13-Citation16 These events have been recently reviewed,Citation17 and my main purpose here will be to highlight some of the key findings made on the contribution of BM and its receptors in this invasion process. Their genetic analysis has shown that the INA-1/PAT-3 integrin plays two important roles during invasion. In the early phase (), INA-1/PAT-3 acts in the AC to mediate its attachment to the BM and to polarize the AC. In ina-1 or pat-3 mutants, the netrin receptor UNC-40/DCC, the phospholipid PI(4,5)P2 and the RhoG homolog MIG-2 fail to properly accumulate at the ventral surface of the AC ().Citation13 The INA-1/PAT-3 dimer is also important to regulate the assembly of hemicentin into small puncta ().Citation13 Although hemicentin mutants have only mild invasion defects, it might locally change the biophysical properties of the BM and/or position metalloproteases to initiate degradation. During the final stage, INA-1/PAT-3 acting in the vulval cell VulD limits the expansion of the gap, which invaginating vulval cells further enlarge (). In ina-1 or pat-3 mutants, the gap becomes wider ().Citation17 Interestingly, INA-1/PAT-3 works with talin and ILK in the AC, and with the Kank homolog VAB-19 in vulval cells.Citation13,Citation17 The latter is also a component of the hemidesmosome-like junction described below, and it would be interesting to determine whether other proteins of that junction contribute to stabilize the gap.
Altogether ongoing studies of the AC invasion process outline that the BM is a dynamic entity, which can be breached and expanded but needs to be stabilized. They show how integrin dimers modulate these steps, in part by polarizing the cell in cooperation with the netrin signaling pathway. Important issues for the future will be to identify the nature of the signal emanating from P6.p daughters to induce protrusions and to explore the possibility that these protrusions represent invadosomes.
Mechanotransduction Through the Basement Membrane
Numerous studies have documented how matrix stiffness influences cell differentiation, cancer progression and focal adhesion maturation through mechanotransduction.Citation18,Citation19 An emerging concept is that integrin-associated molecules act as mechanosensors, which upon changes in the internal and external mechanical environment undergo a conformational switch to attract new binding partners.Citation20 Talin is such a protein, which stretches under tension to reveal vinculin-binding sites; in turn vinculin recruitment contributes to focal adhesion maturation.Citation21 Although it is well appreciated that cells do not behave in the same way in 2D- and 3D-matrices, most of our mechanistic knowledge of mechanotransduction processes comes from studies where cells are grown in 2D-matrices.
C. elegans embryos provide a powerful integrated system to investigate the contribution of the ECM to embryonic morphogenesis. Embryos elongate along the anterior/posterior (A/P) axis in the absence of cell division, cell migration or cell intercalation,Citation22 relying mainly on cell shape changes (). Cellular and genetic analyses have shown that epithelial structures such as adherens junctions and the actomyosin cytoskeleton, as well as body wall muscle myofilaments, are required to achieve elongation.Citation22 In particular, when muscles cannot contract, embryos stop elongation midway (so-called pat mutants). Understanding the basis of the Pat phenotype requires a brief description of the animal’s anatomy (): the key is that muscles are anchored to the outer cuticle through basal and apical hemidesmosome-like junctions (CeHDs) in the epidermis, which are connected by intermediate filaments (IFs). This cellular organization makes a stiff entity that transmits body muscle contractions to the cuticle exoskeleton, allowing animals to move (). Like vertebrate HDs, CeHDs contain a plakin protein called VAB-10A, which is closely related to Plectin and BPAG1e.Citation23 All HD-like components are necessary to reach or go beyond mid-elongation.Citation23
Figure 2. Contribution of the basal and apical ECM to embryonic elongation. (A) Side representations of wild-type embryos at three stages of elongation (early to late), showing individual epidermal cells (green, yellow, blue). These cells lengthen 4x along the A/P axis, while maintaining contacts with the same neighbors, and reduce their dorso/ventral dimension resulting in a 2.5x reduction of embryonic diameter. The drawings also show the apical ECM (black) and C. elegans hemidesmosome-like junctions (CeHDs, red). CeHDs evolve over time from a dotted and disorganized to a well-organized striped pattern. The aECM starts to form early on; muscles become active slightly before the 2-fold stage. Elongation most likely involves two distinct phases, since the Rho-kinase is dispensable after mid-elongation,Citation36 when muscles start contracting.Citation5 (B) Anatomy of the muscle-epidermis connection showing a cross-section through the embryo (left). The BM found at the muscle-epidermis interface contains Perlecan (UNC-52) which acts to anchor muscle myofilaments through integrins, and the epidermis most likely through myotactin (a large single-pass transmembrane nematode specific protein) which organizes C. elegans hemidesmosome-like (CeHD) junction at the basal side of the cell.Citation23 A distinct set of receptors is found at apical CeHDs (dotted rectangles, right). Muscles are A/P-oriented and anchored to the cuticle through basal and apical CeHDs, which are bridged by intermediate filaments. Some CeHD components were omitted for clarity. GIT-1 anchoring to CeHDs depends on a muscle tensional input; we hypothesize that tension induces a conformational change within some CeHD components (symbolized here by VAB-19 and VAB-10 becoming more squarish, although the identity of the actual CeHD component that would undergo this change is unknown). (C) Three mutant situations with consequences on embryonic elongation. The mechanotransduction pathway was identified through a synthetic lethal screen conducted in a weak vab-10 mutant (with no elongation defect on its own); combining it with a strong pak-1 mutant, which on its own only slows down elongation, induces strong elongation and CeHD organization defects (like in a vab-10 null background, left). In strong pat mutants elongation is blocked at the 2-fold stage, and CeHDs are mildly affected (middle). In a double mutant combination for two putative aECM components (right), embryos elongate but rupture at the end of elongation with herniae and epidermis integrity defects (green cytoplasm in eggshell).
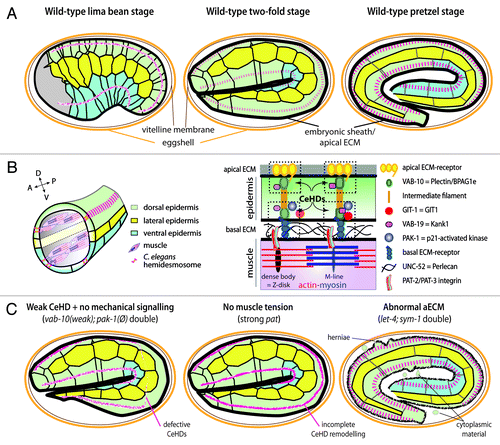
Our laboratory recently characterized a novel mechanotransduction pathway that accounts, to a large extent, for why muscle-deficient embryos cannot elongate.Citation24 This pathway involves a tensional input provided by muscle contractions, which are transmitted through the muscle-epidermis BM to induce a mechanotransduction signaling output in the epidermis. Analysis of this pathway has brought three important lessons. First, it shows that a mechanical signal can act like a more “classical” inductive biochemical signal in devlopment to stimulate epidermal morphogenesis and CeHD remodeling. This pathway accounts to a large extent for how muscle and epidermal cells cooperate to drive embryonic elongation. Second, this pathway involves CeHD components, establishing for the first time that HDs can mediate mechanotransduction like focal adhesions. Third, intermediate filaments represent one molecular target of this mechanotransduction pathway, which enlarges the spectrum of possible cellular mechanotransduction targets.
To describe this process in further detail, genetic, cellular and biochemical studies suggest that muscles locally stretch or compress the epidermis when they contract along the A/P axis, as both tissues are tightly coupled through their shared perlecan BM.Citation24 Indeed, perlecan itself is important for CeHD remodelingCitation6,Citation24 and myotactin, its presumptive receptor in the epidermis, is essential for mechanotransduction.Citation24 The first molecular event downstream of muscle mechanical stimuli corresponds to the maintenance of a Git1 (GRK-interacting protein 1) homolog to HD-like junctions (named GIT-1); in the absence of the muscle input it diffuses in the cytoplasm ().Citation24 When maintained to HD-like junctions, our data suggest that GIT-1 activates the homologs of the guanine nucleotide exchange factor β-PIX, of the small GTPase Rac and the p21-activated kinase PAK1, to ultimately mediate intermediate filament phosphorylation.Citation24 These events contribute to HD-like junction maturation, which eventually become organized perpendicular to the direction of muscle contraction and stimulate embryonic elongation. Both CeHD remodeling and elongation are defective when a component of the mechanotransduction process is lacking in the background of a weak VAB-10/Plakin mutant, or in muscle mutants (). GIT-1 is unlikely to represent the epidermal mechanosensor; instead, based on the talin paradigm,Citation21 some HD component might undergo a conformational change under tension to create a binding pocket for GIT-1.
The mechanotransduction phenomenon described above might not represent the only situation where muscles stimulate epidermal cells. An unexpected finding came out from gene expression profiling of larvae mutant for unc-95 which encodes a muscle-specific LIM-domain protein that is required, though not essential, for myofilament assembly.Citation25 It revealed that several transcripts encoding cuticle-associated proteins are upregulated, leading to small cuticle defects.Citation26 This observation was confirmed by real-time PCR of other mild myofilament assembly mutants,Citation26 raising the possibility that muscle contractions induce a mechanotransduction pathway that modulates gene expression. Whether the signaling process that we uncoveredCitation24 relays the changes in transcription when muscles cannot contract normally has not yet been tested.
What could be the lessons of these findings for vertebrate species? First, tension might also stimulate HD remodeling in tissues such as the skin or the intestine. Second, and more generally, in other situations where contractile cells are juxtaposed to epithelial cells, the contraction of the former could promote the morphogenesis and/or the repair of the latter. Such situations are numerous, since many organs involve an epithelial layer and a smooth muscle or a myoepithelial layer. It will be interesting to find out whether a Git1/β-PIX/Rac/PAK1 signaling pathway also operates to relate tensional inputs in such cases. In C. elegans at least two important questions remain: what is the identity of the primary CeHD mechanosensor, and do BM modifications (such as those induced by SPON-1 and PXN-2 incorporation) contribute to a late aspect of this mechanotransduction process?
The Apical Extracellular Matrix
Recent work in different species has established that epithelial cells secrete a specialized extracellular matrix at their apical surface (aECM). This aECM influences the morphogenesis of both tubes and sheets and act by modulating junction and/or cytoskeleton reorganization.Citation27 For instance the Drosophila Zona Pellucida (ZP) domain-containing Piopio and Dumpy help stabilize the transition from intercellular to autocellular junction in small tracheal capillaries.Citation28 Two other Drosophila ZP-domain proteins, Dusky and Miniature, are essential when the initially hexagonal wing disk cells flatten to adopt a different shape with a starry contour.Citation29 In C. elegans, a family of ZP-domain proteins called the cuticlins contribute to shaping a specialized cuticular ridge (the alae) present only in young hatchlings, dauer larvae and adults.Citation30 Below, I discuss the potential contribution of two protein families to the embryonic aECM.
Extracellular leucine-rich repeats only proteins (eLRRons)
It has long been known that C. elegans embryos are surrounded by a vitelline membrane, and a tightly apposed aECM layer called the embryonic sheath that is replaced by the collagen-based exoskeletal cuticle at the end of the elongation process ().Citation31 Embryonic sheath removal by trypsin treatment results in deformed embryos exhibiting elongation defects.Citation31 Such phenotypes can be indicative of epithelial integrity problems, poor maintenance of the internal pressure and/or abnormal cytoskeletal organization. Until recently, embryonic sheath composition was unknown, precluding any further understanding of its function.
Now, Sundaram and colleagues identified three related apically located proteins bearing leucine-rich repeats (LET-4, EGG-6 and SYM-1) with potential characteristics of embryonic sheath components.Citation32 Consistent with this idea, the distance between the vitelline membrane and the sheath is increased in let-4 mutants and there is cytoplasmic material in the eggshell of both egg-6 and let-4 mutants. However, a definitive association of EGG-6/LET-4/SYM-1 with the embryonic sheath would require confirmation by immuno-electron microscopy. The combined loss of LET-4 and SYM-1 causes embryos to rupture towards the end of the elongation process, which often occurs in areas where the embryo is deformed and could reflect either local adherens junction weakening or poor epidermis integrity.Citation32 Likewise, single egg-6 or let-4 fail to maintain the junction between two cells of the excretory system, which form unicellular tubes.Citation32 LET-4 and EGG-6 bear transmembrane and cytoplasmic domains, which at least for LET-4 appear dispensable for function,Citation32 whereas SYM-1 has no transmembrane domain.Citation33 The authors suggest that in the epidermis as well as in the excretory system the junctional defects are likely to be indirect, since they occur at a late stage and are not accompanied by the type of polarity defects observed in mutants affecting junction assembly.Citation32 Instead, the presence of cytoplasmic material in the eggshell of let-4 mutants could mean that these proteins help maintain epidermis plasma membrane integrity and/or hydrostatic pressure. While let-4;sym-1 double mutants cause herniae, like enzymatic removal of the embryonic sheath, their elongation phenotype appears less severe and also completely different from the complete elongation collapse observed after loss of the cuticle collagen SQT-3.Citation31 Thus, assuming that eLRRon proteins are indeed embryonic sheath components, they are unlikely to be the only ones.
Hedgehog-related proteins
The Hedgehog signaling pathway has undergone considerable divergence in C. elegans, to the extent that it is considered to be missing, despite the conservation of several key proteins of the pathway.Citation34 In particular, the C. elegans genome encodes over 60 divergent proteins bearing homology to the protease domain of Hedgehog but distinct N-terminal signaling domains without any similarity to Hedgehog, and 22 Patched-related proteins.Citation34 A few of these Hh-related proteins have been characterized. The WRT-5 protein is secreted apically by the pharyngeal tube and epidermis,Citation35 although its exact localization is not as well-defined as for the eLLRon proteins described above. Embryos homozygous mutant for a wrt-5 deletion have abnormal adherens junctions and pleiotropic morphogenetic defects,Citation35 some of which are reminiscent of those resulting from enzymatic removal of the embryonic sheath. Interestingly, QUA-1, another Hh-related protein, is essential for cuticle molting.Citation35 Hence, an intriguing possibility could be that WRT-5 contributes in some way to the C. elegans aECM and extraembryonic sheath, although a signaling role for WRT-5 remains equally possible at this point.
In conclusion, research in C. elegans has contributed in different ways to the identification and functional characterization of ECM components and their receptors. C. elegans provides a simple and integrated system to further dissect the mechanisms of BM invasion and BM implication in mechanotransduction.
| Abbreviations: | ||
| ECM | = | extracellular matrix |
| aECM | = | apical ECM |
| BM | = | basement membrane |
| HD | = | hemidesmosome |
| CeHD | = | C. elegans hemidesmosome |
| IF | = | intermediate filaments |
Acknowledgments
I thank Gabriella Pasti and Christelle Gally for critical reading of this manuscript. Work in my laboratory is supported by the Agence Nationale pour la Recherche and the Association pour la Recherche contre le Cancer for funding, as well as the CNRS, the INSERM and Université de Strabsourg for institutional funding.
References
- Brown NH. Extracellular matrix in development: insights from mechanisms conserved between invertebrates and vertebrates. Cold Spring Harb Perspect Biol 2011; 3; In press http://dx.doi.org/10.1101/cshperspect.a005082; PMID: 21917993
- Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 2011; 3; In press http://dx.doi.org/10.1101/cshperspect.a005058; PMID: 21917992
- Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol 2010; 2:a005066; http://dx.doi.org/10.1101/cshperspect.a005066; PMID: 21084386
- Kramer JM. Basement membranes. WormBook 2005; 1 - 15; PMID: 18050423
- Moerman DG, Williams BD. Sarcomere assembly in C. elegans muscle. WormBook 2006; 1 - 16; PMID: 18050483
- Hresko MC, Williams BD, Waterston RH. Assembly of body wall muscle and muscle cell attachment structures in Caenorhabditis elegans. J Cell Biol 1994; 124:491 - 506; http://dx.doi.org/10.1083/jcb.124.4.491; PMID: 8106548
- Rogalski TM, Gilchrist EJ, Mullen GP, Moerman DG. Mutations in the unc-52 gene responsible for body wall muscle defects in adult Caenorhabditis elegans are located in alternatively spliced exons. Genetics 1995; 139:159 - 69; PMID: 7535716
- Rogalski TM, Williams BD, Mullen GP, Moerman DG. Products of the unc-52 gene in Caenorhabditis elegans are homologous to the core protein of the mammalian basement membrane heparan sulfate proteoglycan. Genes Dev 1993; 7:1471 - 84; http://dx.doi.org/10.1101/gad.7.8.1471; PMID: 8393416
- Woo WM, Berry EC, Hudson ML, Swale RE, Goncharov A, Chisholm AD. The C. elegans F-spondin family protein SPON-1 maintains cell adhesion in neural and non-neural tissues. Development 2008; 135:2747 - 56; http://dx.doi.org/10.1242/dev.015289; PMID: 18614580
- Gotenstein JR, Swale RE, Fukuda T, Wu Z, Giurumescu CA, Goncharov A, et al. The C. elegans peroxidasin PXN-2 is essential for embryonic morphogenesis and inhibits adult axon regeneration. Development 2010; 137:3603 - 13; http://dx.doi.org/10.1242/dev.049189; PMID: 20876652
- Zahreddine H, Zhang H, Diogon M, Nagamatsu Y, Labouesse M. CRT-1/calreticulin and the E3 ligase EEL-1/HUWE1 control hemidesmosome maturation in C. elegans development. Curr Biol 2010; 20:322 - 7; http://dx.doi.org/10.1016/j.cub.2009.12.061; PMID: 20153198
- Hresko MC, Schriefer LA, Shrimankar P, Waterston RH. Myotactin, a novel hypodermal protein involved in muscle-cell adhesion in Caenorhabditis elegans. J Cell Biol 1999; 146:659 - 72; http://dx.doi.org/10.1083/jcb.146.3.659; PMID: 10444073
- Hagedorn EJ, Yashiro H, Ziel JW, Ihara S, Wang Z, Sherwood DR. Integrin acts upstream of netrin signaling to regulate formation of the anchor cell’s invasive membrane in C. elegans. Dev Cell 2009; 17:187 - 98; http://dx.doi.org/10.1016/j.devcel.2009.06.006; PMID: 19686680
- Ziel JW, Hagedorn EJ, Audhya A, Sherwood DR. UNC-6 (netrin) orients the invasive membrane of the anchor cell in C. elegans. Nat Cell Biol 2009; 11:183 - 9; http://dx.doi.org/10.1038/ncb1825; PMID: 19098902
- Sherwood DR, Butler JA, Kramer JM, Sternberg PW. FOS-1 promotes basement-membrane removal during anchor-cell invasion in C. elegans. Cell 2005; 121:951 - 62; http://dx.doi.org/10.1016/j.cell.2005.03.031; PMID: 15960981
- Sherwood DR, Sternberg PW. Anchor cell invasion into the vulval epithelium in C. elegans. Dev Cell 2003; 5:21 - 31; http://dx.doi.org/10.1016/S1534-5807(03)00168-0; PMID: 12852849
- Hagedorn EJ, Sherwood DR. Cell invasion through basement membrane: the anchor cell breaches the barrier. Curr Opin Cell Biol 2011; 23:589 - 96; http://dx.doi.org/10.1016/j.ceb.2011.05.002; PMID: 21632231
- Hoffman BD, Grashoff C, Schwartz MA. Dynamic molecular processes mediate cellular mechanotransduction. Nature 2011; 475:316 - 23; http://dx.doi.org/10.1038/nature10316; PMID: 21776077
- Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer 2009; 9:108 - 22; http://dx.doi.org/10.1038/nrc2544; PMID: 19165226
- Moore SW, Roca-Cusachs P, Sheetz MP. Stretchy proteins on stretchy substrates: the important elements of integrin-mediated rigidity sensing. Dev Cell 2010; 19:194 - 206; http://dx.doi.org/10.1016/j.devcel.2010.07.018; PMID: 20708583
- del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science 2009; 323:638 - 41; http://dx.doi.org/10.1126/science.1162912; PMID: 19179532
- Chisholm AD, Hardin J. Epidermal morphogenesis. WormBook 2005; 1 - 22; PMID: 18050408
- Zhang H, Labouesse M. The making of hemidesmosome structures in vivo. Dev Dyn 2010; 239:1465 - 76; PMID: 20205195
- Zhang H, Landmann F, Zahreddine H, Rodriguez D, Koch M, Labouesse M. A tension-induced mechanotransduction pathway promotes epithelial morphogenesis. Nature 2011; 471:99 - 103; http://dx.doi.org/10.1038/nature09765; PMID: 21368832
- Broday L, Kolotuev I, Didier C, Bhoumik A, Podbilewicz B, Ronai Z. The LIM domain protein UNC-95 is required for the assembly of muscle attachment structures and is regulated by the RING finger protein RNF-5 in C. elegans. J Cell Biol 2004; 165:857 - 67; http://dx.doi.org/10.1083/jcb.200401133; PMID: 15210732
- Broday L, Hauser CA, Kolotuev I, Ronai Z. Muscle-epidermis interactions affect exoskeleton patterning in Caenorhabditis elegans. Dev Dyn 2007; 236:3129 - 36; http://dx.doi.org/10.1002/dvdy.21341; PMID: 17937397
- Plaza S, Chanut-Delalande H, Fernandes I, Wassarman PM, Payre F. From A to Z: apical structures and zona pellucida-domain proteins. Trends Cell Biol 2010; 20:524 - 32; http://dx.doi.org/10.1016/j.tcb.2010.06.002; PMID: 20598543
- Jazáwińska A, Ribeiro C, Affolter M. Epithelial tube morphogenesis during Drosophila tracheal development requires Piopio, a luminal ZP protein. Nat Cell Biol 2003; 5:895 - 901; http://dx.doi.org/10.1038/ncb1049; PMID: 12973360
- Roch F, Alonso CR, Akam M. Drosophila miniature and dusky encode ZP proteins required for cytoskeletal reorganisation during wing morphogenesis. J Cell Sci 2003; 116:1199 - 207; http://dx.doi.org/10.1242/jcs.00298; PMID: 12615963
- Page AP, Johnstone IL. The cuticle. WormBook 2007; 1 - 15; PMID: 18050497
- Priess JR, Hirsh DI. Caenorhabditis elegans morphogenesis: the role of the cytoskeleton in elongation of the embryo. Dev Biol 1986; 117:156 - 73; http://dx.doi.org/10.1016/0012-1606(86)90358-1; PMID: 3743895
- Mancuso VP, Parry JM, Storer L, Poggioli C, Nguyen KC, Hall DH, et al. Extracellular leucine-rich repeat proteins are required to organize the apical extracellular matrix and maintain epithelial junction integrity in C. elegans. Development 2012; 139:979 - 90; http://dx.doi.org/10.1242/dev.075135; PMID: 22278925
- Davies AG, Spike CA, Shaw JE, Herman RK. Functional overlap between the mec-8 gene and five sym genes in Caenorhabditis elegans. Genetics 1999; 153:117 - 34; PMID: 10471705
- Bürglin TR, Kuwabara PE. Homologs of the Hh signalling network in C. elegans. WormBook 2006; 1 - 14; PMID: 18050469
- Hao L, Aspöck G, Bürglin TR. The hedgehog-related gene wrt-5 is essential for hypodermal development in Caenorhabditis elegans. Dev Biol 2006; 290:323 - 36; http://dx.doi.org/10.1016/j.ydbio.2005.11.028; PMID: 16413526
- Diogon M, Wissler F, Quintin S, Nagamatsu Y, Sookhareea S, Landmann F, et al. The RhoGAP RGA-2 and LET-502/ROCK achieve a balance of actomyosin-dependent forces in C. elegans epidermis to control morphogenesis. Development 2007; 134:2469 - 79; http://dx.doi.org/10.1242/dev.005074; PMID: 17537791