Abstract
Over the past decade, amniotic fluid-derived stem cells have emerged as a novel, experimental approach for the treatment of a wide variety of congenital anomalies diagnosed either in utero or postnatally. There are a number of unique properties of amniotic fluid stem cells that have allowed it to become a major research focus. These include the relative ease of accessing amniotic fluid cells in a minimally invasive fashion by amniocentesis as well as the relatively rich population of progenitor cells obtained from a small aliquot of fluid. Mesenchymal stem cells, c-kit positive stem cells, as well as induced pluripotent stem cells have all been derived from human amniotic fluid in recent years. This article gives a pediatric surgeon’s perspective on amniotic fluid stem cell therapy for the management of congenital anomalies. The current status in the use of amniotic fluid-derived stem cells, particularly as they relate as substrates in tissue engineering-based applications, is described in various animal models. A roadmap for further study and eventual clinical application is also proposed.
Introduction
Congenital anomalies are the end products of aberrant organogenesis in utero. Some of the more common congenital anomalies encountered in neonatal intensive care units include diaphragmatic hernia, gastroschisis, esophageal atresia, spinal bifida and heart defects. Perhaps not surprisingly, these birth defects also represent a major burden in pediatric disease and lead to a significant proportion of infant hospitalization days worldwide.Citation1 Over the past 50 years, the technologies available to treat these children, including mechanical ventilator support, extracorporeal membrane oxygenation and complex surgical reconstruction, can dramatically improve the long-term prognosis in affected children. Nevertheless, the mortality and morbidity for many of these patients can still be quite high, and termination of pregnancy rates of up to 25% are not uncommon in some countries.
In the effort to improve clinical outcomes in neonates who would otherwise have a grim prognosis, perinatal cell-based therapies using amniotic fluid stem cells have been proposed in recent years.Citation2,Citation3 This regenerative medicine treatment strategy requires a multidisciplinary approach involving the expertise of surgeons, maternal-fetal medicine specialists, neonatologists, cell biologists and materials scientists, among others. The hope is that amniotic fluid stem cells may eventually offer a new promise for the smallest and most vulnerable members of our society.
Amniotic Fluid Stem Cells
Thanks in part to ongoing advances in the resolution of fetal ultrasound imaging, the prenatal diagnosis of a significant number of congenital anomalies has now become commonplace in most major obstetrical units in the developed world. The increased awareness of fetal anomalies has enabled families and their physicians an ability to optimize care in ways that were previously impossible. For example, one can now contemplate the utility of fetal intervention for selected disorders such as spina bifida and congenital diaphragmatic hernia (CDH). More importantly, referral to major pediatric centers capable of providing the full complement of neonatal care and expertise can help to optimize outcomes in the immediate newborn period.
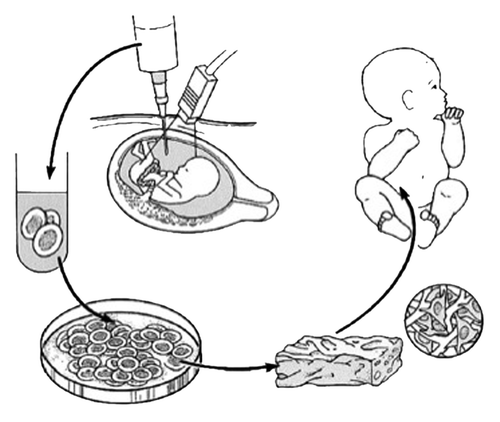
Although the amniotic fluid represents a logical source of cells for regenerative medicine approaches in patients with congenital anomalies, it has been only recently that amniotic fluid derived stem cells were truly contemplated in terms of their potential as therapy ().Citation4 A major advantage of using amniotic fluid as a stem cell source lies in the relative ease of harvesting autologous fetal stem cells. For decades, amniocenteses have been performed for diagnostic purposes with low morbidity. The procedure is already considered to be part of the standard diagnostic workup to rule out major chromosomal and genetic defects in some fetal disorders. After 15 weeks gestation, amniocentesis is safe with a less than 1% rate of fetal loss when performed by experienced personnel under ultrasound guidance. By contrast, harvesting stem cells prenatally from placenta, cord blood, bone marrow and liver is much more difficult and is associated with higher fetal morbidity. In fact, the safety of an amniocentesis has now enabled commercial banking of amniotic fluid in some developed countries. Other advantages of using amniotic fluid when compared with other cell sources are their apparent enhanced plasticity as well as the feasibility of having autologous cell-based therapy available and ready to use either before or at the time of birth.Citation5
Figure 1. Schematic diagram of tissue engineering from amniotic fluid-derived stem cells for the treatment of congenital anomalies. Autologous fetal stem cells are obtained by amniocentesis. The cells are expanded ex vivo in parallel with the remainder of gestation and subsequently placed on biodegradable scaffolds prior to implantation at birth. Reprinted with permission.Citation5
It was once thought that amniotic fluid was composed primarily of fetal urine with terminally differentiated epithelioid cells derived from the fetal skin and amnion. We now know that amniotic fluid contains progenitor cells within a much larger, heterogenous population of somatic cells.Citation6 In all fetuses, our group, among others, has found that human mesenchymal stem cells (MSCs) can be readily isolated in serum-rich media from a 1–2 mL amniotic fluid specimen.Citation6-Citation9 These amniotic fluid derived MSCs (AF-MSCs) have similar features to MSCs more commonly obtained from adult bone marrow and fulfill the minimal criteria for MSCs as outlined elsewhere.Citation10,Citation11 AF-MSCs express class I MHC antigens as well as the full panel of mesenchymal markers (e.g., CD73, CD90 and CD105). They do not express hematopoietic and endothelial markers (e.g., CD31, CD34 and CD45). By definition, AF-MSCs have a restricted differentiation potential specific to the mesodermal lineage, including bone, cartilage, and fat, when cultured under controlled conditions.
Studies have shown that AF-MSCs have features that may make them more desirable for cell-based applications when compared with MSCs from other sources.Citation12,Citation13 AF-MSCs seem to have a more primitive phenotype along the spectrum of cellular differentiation when compared with other perinatal MSCs as demonstrated by variable expression of selected markers of pluripotency, including Oct4, SSEA3 and SSEA4.Citation14,Citation15 Moreover, in contrast to bone marrow derived MSCs, the neurogenic potential of AF-MSCs has also been consistently shown by numerous investigators.Citation16,Citation17 AF-MSCs also proliferate more rapidly in culture than postnatal somatic cells and can be easily expanded under good manufacturing practice conditions.Citation5,Citation9,Citation18 Given the relatively small size of the fetus, the importance of this issue should not be underestimated since a large number of cells may be required for therapeutic effect. There is also data to support AF-MSCs as the ideal cell vehicle for high-efficiency gene transduction.Citation19
MSCs are perhaps best known for their potential immunomodulatory role in inflammatory conditions and in disease processes such as graft-vs.-host disease.Citation20 Although the mechanistic role of AF-MSCs in this regard remains poorly described, it has been hypothesized that AF-MSCs may play a particularly important paracrine function in the immune response at the maternal-fetal interface where the fetal chorion meets the maternal decidua basalis. Some, but not all, investigators have suggested that the immunologic properties of amniotic fluid stem cells may also enable allogeneic cell therapy applications, particularly if the cells are delivered in the fetal environment.Citation21
Other amniotic fluid stem cells with different pluripotent characteristics have been explored by several laboratories worldwide. These include hematopoietic progenitors as well as neural cells within amniotic fluid.Citation22,Citation23 Atala and colleagues, among others, have focused much of their research efforts on a specific subset of c-kit (CD117) positive cells found in normal amniotic fluid specimens.Citation24 These cells, which are isolated using immunomagnetic beads during primary culture, constitute less than 1% of the somatic cell population of amniotic fluid and express the same panel of MSCs phenotypic markers as AF-MSCs.Citation25 Nevertheless, c-kit positive amniotic fluid cells seem to have some much broader pluripotency with clonal cell lines demonstrating the ability to differentiate into lineages of all three germ layers.Citation26 For example, these researchers have shown that c-kit positive amniotic fluid stem cells are capable of hepatogenic differentiation. These cells have also been differentiated into endothelium as well as cardiomyocytes when co-cultured with neonatal cardiomyocytes.Citation27 In vivo, these cells have been associated with improved regeneration of damaged smooth muscle in rat cryoinjured bladders.Citation1 Despite these findings, c-kit positive amniotic fluid cells have not been generally embraced as having equivalent pluripotency to human embryonic stem (hES) cells or induced pluripotent stem (iPS) cells. However, a potential advantage of these c-kit positive cells is that they are not known to form teratomas when injected into immunodeficient mice and do not require xenogenic feeder layers or other special substrates to support their long-term growth in culture.
Shortly after the first human iPS cells derived from adult skin fibroblasts were reported, the reliable generation of iPS cells from amniotic fluid (AF-iPS) cells were demonstrated.Citation28-Citation31 The inherently primitive nature of AF-MSCs (i.e., positive for Oct4, SSEA3, SSEA4, etc.) may make amniotic fluid cells particularly ideal for genetic reprogramming into iPS cells for perinatal therapeutic purposes. In some cases, fewer than four Yamanaka reprogramming factors have been shown to be sufficient for successful reprogramming of AF-MSCs into AF-iPS cells.Citation31 Like hES cells, AF-iPS cells have been found to have unlimited expansion potential and are capable to differentiating into tissues derived from all three germ layers.Citation30 In theory, the generation of AF-iPS cells could give each child their own reservoir of stem cells for the rest of their life. These cells would be capable of differentiating into hepatocytes, islet cells, cardiomyocytes, neurons, etc. However, in contrast to hES cells, the delivery of differentiated cells derived from autologous AF-iPS cells in patients with congenital anomalies would be performed with much less ethical controversy and without concern for immunologic rejection in the absence of chronic immunosuppressive therapy.
Therapy for Spina Bifida
Spina bifida is a neural tube defect with an estimated incidence of approximately 1 in 1,500 live births. Palliative interventions, including early postnatal closure, extensive physical therapy and serial ventriculoperitoneal shunting, remain the mainstays of treatment. In its most severe form, known as myelomeningocele (MMC), the natural sequelae in affected children include hydrocephalus, bowel and bladder incontinence and distal lower motor dysfunction.
In early 2011, in utero closure of the MMC defect during the second trimester was shown to be beneficial in selected cases of MMC in the context of a randomized trial performed at three highly specialized centers.Citation32 The rationale for fetal closure has been to alter the flow of cerebrospinal fluid that contributes to the Arnold-Chiari malformation and to prevent further damage of the exposed spinal cord caused by the acidic amniotic fluid. Unfortunately, the procedure has been associated with a number of drawbacks, including the use of large incisions through the maternal abdominal wall and uterus. The fetus is also at increased risk for preterm labor and its associated complications. Finally, even after successful prenatal closure of spina bifida defects during mid-gestation, the trial found that most MMC patients still cannot ambulate independently. This is probably not very surprising given that fetal surgery does little to address the spinal cord damage that has already occurred during the first half of pregnancy.
Regenerative medicine strategies to facilitate bona fide regeneration of the spinal cord would represent a remarkable advance for these children. In this regard, several investigators have suggested the potential role of different stem cell-based therapies in several animal models of fetal MMC.Citation33,Citation34 For example, Fauza and colleagues employed murine neural stem cells in a sheep model with evidence of donor cell engraftment and improved ambulation at birth.Citation35 In an effort to create an animal model with greater clinical relevance, Fauza and colleagues recently presented preliminary data on the intra-amniotic delivery of neural stem cells isolated from amniotic fluid in a syngeneic rat MMC model (data unpublished). Unfortunately, this research is in its very early stages, and the effectiveness of these stem cell-based strategies on actual spinal cord regeneration remains to be determined. However, if these studies do eventually show evidence of enhanced neurologic function, one might envision a treatment strategy in the future that would include deriving autologous stem cells following an amniocentesis with subsequent delivery of those cells directly to the MMC spinal cord at the time of prenatal or postnatal spinal cord closure.
Therapy for Congenital Heart Disease
Congenital heart disease represents another common birth defect managed in most major pediatric referral intensive care units. In cases of end-stage organ failure, the only viable option for survival in these children remains cardiac transplantation, a potentially risky operation with long-term morbidities associated with chronic immunosuppression, infection and cancer. The differentiation of stem cells from autologous amniotic fluid into functional cardiomyocytes could circumvent the scarcity of heart donors. Although preliminary data suggests that some amniotic fluid stem cells do have cardiomyogenic differentiation potential, these cells are more likely to play a role as paracrine mediators of tissue regeneration.Citation36,Citation37 Several studies in the adult literature have shown that bone marrow MSCs facilitate remodeling, thereby improving myocardial function in animal models of acute myocardiac infarction.Citation38 Similar effects on the native myocardium have more recently been suggested by stem cells isolated from amniotic fluid.Citation39 In one such model, c-kit positive amniotic fluid stem cells were found to be cardioprotective in a rat model of ischemic heart disease.Citation40
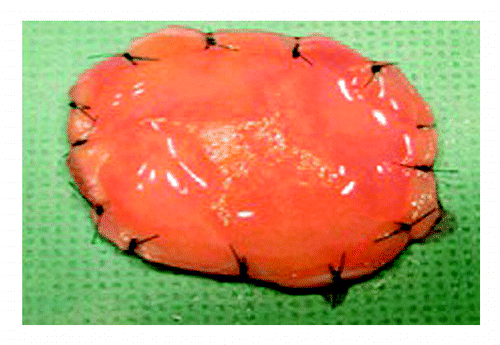
In some infants, surgical reconstruction of the heart and great vessels is currently performed using an off-the-shelf, acellular prosthetic implant. Unfortunately, these implants typically fail to grow with the infant into adulthood, resulting in the need for multiple operations during childhood to optimize heart function. Bone marrow-based tissue engineered vascular conduits have been used clinically with favorable results but have yet to become widespread.Citation41 To date, the greatest experience with amniotic fluid based-stem cell technologies has been with the fabrication of tissue-engineered heart valves.Citation42,Citation43 In one such study, Hoestrup and colleagues successfully created functional heart valves using CD133-positive and CD133-negative human amniotic fluid cells (). The CD133-negative population appears to closely resemble AF-MSCs based on phenotypic expression of CD44 and CD105. The CD133-positive population was used to create a layer of endothelium to minimize thrombogenicity. A cellularized, tissue engineered heart valve may obviate the need for long-term anticoagulation medications. Furthermore, such a valve might have the ability to grow and remodel over time, thereby avoiding revisional operations in the future.
Figure 2. Gross appearance of a tissue-engineered heart valve seeded with amniotic fluid-derived stem cells. Reprinted with permission.Citation42
Therapy for Diaphragmatic Hernia
The diaphragm, a structure that is largely composed of skeletal muscle, separates the chest from the abdominal cavity. In CDH, the diaphragm fails to correctly form on one side, resulting in herniation of the abdominal viscera into the ipsilateral hemithorax. The defect is caused by a failure of fusion of four mesenchymal derivatives during early embryogenesis. A well-known complication of CDH repair using synthetic, acellular prosthetics is recurrent herniation, a problem that eventually occurs in up to 50% of survivors.Citation44 This re-herniation is thought to be secondary to the inability of the prosthesis to cover the resultant diaphragmatic defect as child grows during the first several years of life. Therefore, it has been argued that a fully cellularized patch with the ability to grow and remodel over time represents the ideal prosthesis in this patient population. Since most cases of CDH are now diagnosed prenatally, there remains considerable interest in harvesting amniotic fluid cells prenatally in preparation for implantation of a better, long-term diaphragmatic prosthesis at birth.
Fauza and colleagues have extensively explored this concept using sheep amniotic fluid-derived fibroblasts resuspended in collagen and seeded onto decellularized dermis ().Citation45-Citation47 The incidence of recurrent herniation was markedly reduced in juvenile lambs using a patch composed of amniotic fluid-derived fibroblasts (termed diaphragmatic tendon) when compared with an acellular patch. Long-term survival of the transplanted donor cells was suggested by positive staining for green fluoroscence protein. Other studies have explored the feasibility of using skeletal muscle as the preferred cell type for a diaphragmatic prosthesis.Citation46 Whether skeletal muscle cells might provide a better construct for diaphramatic reconstruction when compared with fibroblasts remains to be determined since neural innervation of the neodiaphragm is likely to be severely compromised regardless of cell type.
Therapy for Chest Wall Defects
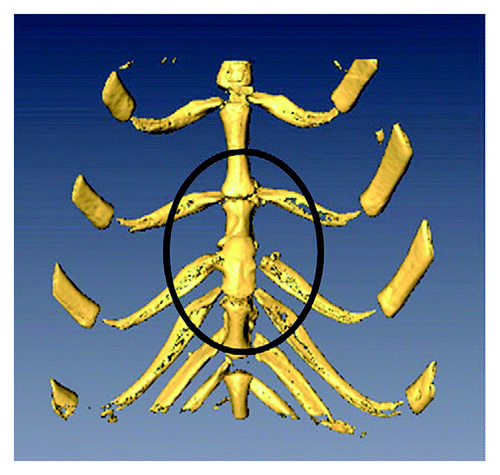
On occasion, pediatric surgeons encounter newborns with chest wall anomalies. The most severe of these defects, termed ectopia cordis, presents with protrusion of the heart through a split sternum. Reconstruction of these defects remains a challenging endeavor and is associated with high mortality and morbidity rates. Providing adequate coverage of the chest wall has been particularly difficult because of the lack of rigid, autologous tissue that can grow and remodel over time. For this reason, a tissue-engineered implant composed of osteocytes derived from AF-MSCs on nanofiber scaffolds has been explored as an alternative method for major chest wall reconstruction in experimental models. In one such model, tissue-engineered bone from AF-MSCs was created in rabbits.Citation48 These osteogenic constructs were subsequently used to repair a large sternal defect in an autologous fashion with evidence of engraftment and bony mineralization over time ().Citation49 More recently, this same principle was successfully applied in a rabbit model of craniofacial repair.Citation50
Figure 4. Three-dimensional micro-CT scan of a repaired full thickness sternal defect (contained within the black oval) using an amniotic fluid mesenchymal stem cell-based osseous construct. Reprinted with permission.Citation49
Therapy for Tracheal Anomalies
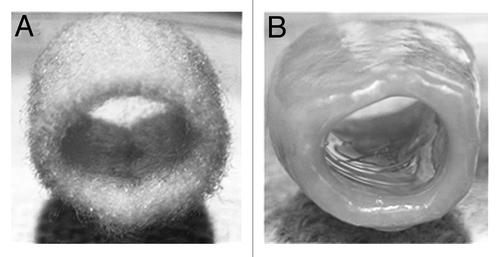
Severe obstructive malformations of the trachea, including tracheal agenesis and atresia, are well-described but rare entities in pediatric surgery. Despite the ability to diagnose many of these problems in utero, the condition is almost always lethal. Methods of definitive tracheal reconstruction are currently limited by a paucity of available autologous tissue that can mimic the structural and mechanical properties of native airway cartilage. Chondrocytes derived from AF-MSCs could serve as a viable alternative for perinatal tracheal repair ().Citation51 This therapeutic concept has been successfully demonstrated in a fetal sheep model of tracheal repair.Citation4 In this study, tissue-engineered cartilage derived from amniotic fluid enabled newborn lambs to have a functional airway for up to 10 d after birth. Unfortunately, these tracheal constructs were not derived from autologous amniotic fluid and were therefore prone to immunologic rejection. Research on the role of different biodegradable scaffolds with more favorable biomechanical properties are needed to help bring this technology toward clinical application. Of note, the successful implantation of a tissue-engineered airway structure derived from autologous bone marrow MSCs has already reported in an adult.Citation52
Figure 5. Gross appearance of a three-dimensional polyglycolic acid-based tubular scaffold before (A) and after (B) seeding with amniotic fluid-derived mesenchymal stem cells under chondrogenic conditions after 15 weeks in culture. Reprinted with permission.Citation51
Therapy for Inflammatory Conditions of Prematurity
Although not considered to be congenital anomalies per se, conditions associated with premature infants born four or more weeks early often result in profound organ-specific inflammatory and/or infectious conditions shortly after birth. These diseases represent yet another intriguing avenue for amniotic fluid stem cell-based therapy. The most common disorders in premature infants include bronchopulmonary dysplasia (BPD), a major cause of respiratory mortality and morbidity in neonatal care units, as well as necrotizing enterocolitis, an intestinal infection that strikes up to 10% of premature infants. In necrotizing enterocolitis, significant portions of the small and large intestine can be lost, resulting in profound sepsis and ultimately death in up to 40% of infants. The possible immunomodulatory role of MSCs in these disorders is just beginning to be explored.Citation53,Citation54 For example, in a hyperoxia animal model of BPD, bone marrow-derived MSCs have recently been shown to be protective against neonatal lung injury.Citation54 The proposed mechanisms for the observed salutary effects may involve pro-angiogenic factors as well as the activation of endogenous resident progenitor cells.Citation55 Whether AF-MSCs can be equally efficacious in preserving normal perinatal organ function in these models remains to be determined.
Limitations and Future Directions
The field of amniotic fluid stem cells has become one of the most fast-paced and furtile areas of research within the regenerative medicine community.Citation56 Given that the concept of amniotic fluid-based cell therapy was essentially non-existent until approximately a decade ago, it is likely only a matter of time until the successful results that have already been demonstrated in animal models can be safely translated to the bedside.
Nevertheless, several limitations in this fascinating field deserve special mention. One barrier toward progress in amniotic fluid-based cell therapy remains the relative rarity of each of these congenital anomalies which prohibits the ability to adequately study the efficacy of these treatments in a single center controlled trial. Moreover, the preparation of these stem cells, whether they be MSCs, iPS cells or other progenitor cell types, often includes the use of xenogeneic reagents which are currently prohibited from human use or require close regulation by clinical safety boards, such as the Food and Drug Administration in the US. Finally, clinical translation of AF-iPS cell-based technologies has been hampered by the need to efficiently perform iPS cell reprogramming without the use of permanently integrating viruses. In particular, adeno- and lenti-viral reprogramming methods remain highly undesirable for therapy because of their association with insertional mutagenesis and oncogenesis if used in patients. Fortunately, there is now recent evidence to suggest that amniotic fluid cells may be easier to reprogram into iPS cells when compared with neonatal foreskin fibroblasts.Citation30,Citation57 This finding implies that different transgene-free approaches to iPS cell derivation of amniotic fluid cells will be forthcoming in the next several years.
| Abbreviations: | ||
| AF-iPS | = | amniotic fluid-derived induced pluripotent stem |
| AF-MSC | = | amniotic fluid-derived mesenchymal stem cell |
| BPD | = | bronchopulmonary dysplasia |
| CDH | = | congenital diaphragmatic hernia |
| hES | = | human embryonic stem cell |
| iPS | = | induced pluripotent stem |
| MMC | = | myelomeningocele |
| MSC | = | mesenchymal stem cells |
Acknowledgments
The author gratefully acknowledges Dr Dario O. Fauza from Harvard Medical School for his expertise and assistance with some of the color images as well as the Harold Amos Award from the Robert Wood Johnson Foundation (N014453-00) for their generous financial support.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Shaw SW, David AL, De Coppi P. Clinical applications of prenatal and postnatal therapy using stem cells retrieved from amniotic fluid. Curr Opin Obstet Gynecol 2011; 23:109 - 16; http://dx.doi.org/10.1097/GCO.0b013e32834457b1; PMID: 21386681
- Fauza D. Amniotic fluid and placental stem cells. Best Pract Res Clin Obstet Gynaecol 2004; 18:877 - 91; http://dx.doi.org/10.1016/j.bpobgyn.2004.07.001; PMID: 15582544
- Cananzi M, Atala A, De Coppi P. Stem cells derived from amniotic fluid: new potentials in regenerative medicine. Reprod Biomed Online 2009; 18:Suppl 1 17 - 27; http://dx.doi.org/10.1016/S1472-6483(10)60111-3; PMID: 19281660
- Kunisaki SM, Freedman DA, Fauza DO. Fetal tracheal reconstruction with cartilaginous grafts engineered from mesenchymal amniocytes. J Pediatr Surg 2006; 41:675 - 82, discussion 675-82; http://dx.doi.org/10.1016/j.jpedsurg.2005.12.008; PMID: 16567175
- Kaviani A, Guleserian K, Perry TE, Jennings RW, Ziegler MM, Fauza DO. Fetal tissue engineering from amniotic fluid. J Am Coll Surg 2003; 196:592 - 7; http://dx.doi.org/10.1016/S1072-7515(02)01834-3; PMID: 12691937
- In ’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 2003; 102:1548 - 9; http://dx.doi.org/10.1182/blood-2003-04-1291; PMID: 12900350
- In ’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004; 22:1338 - 45; http://dx.doi.org/10.1634/stemcells.2004-0058; PMID: 15579651
- Tsai MS, Lee JL, Chang YJ, Hwang SM. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod 2004; 19:1450 - 6; http://dx.doi.org/10.1093/humrep/deh279; PMID: 15105397
- Kunisaki SM, Armant M, Kao GS, Stevenson K, Kim H, Fauza DO. Tissue engineering from human mesenchymal amniocytes: a prelude to clinical trials. J Pediatr Surg 2007; 42:974 - 9, discussion 979-80; http://dx.doi.org/10.1016/j.jpedsurg.2007.01.031; PMID: 17560205
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284:143 - 7; http://dx.doi.org/10.1126/science.284.5411.143; PMID: 10102814
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8:315 - 7; http://dx.doi.org/10.1080/14653240600855905; PMID: 16923606
- Roubelakis MG, Pappa KI, Bitsika V, Zagoura D, Vlahou A, Papadaki HA, et al. Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: comparison to bone marrow mesenchymal stem cells. Stem Cells Dev 2007; 16:931 - 52; http://dx.doi.org/10.1089/scd.2007.0036; PMID: 18047393
- Kim J, Lee Y, Kim H, Hwang KJ, Kwon HC, Kim SK, et al. Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif 2007; 40:75 - 90; http://dx.doi.org/10.1111/j.1365-2184.2007.00414.x; PMID: 17227297
- Prusa AR, Marton E, Rosner M, Bernaschek G, Hengstschläger M. Oct-4-expressing cells in human amniotic fluid: a new source for stem cell research?. Hum Reprod 2003; 18:1489 - 93; http://dx.doi.org/10.1093/humrep/deg279; PMID: 12832377
- Pappa KI, Anagnou NP. Novel sources of fetal stem cells: where do they fit on the developmental continuum?. Regen Med 2009; 4:423 - 33; http://dx.doi.org/10.2217/rme.09.12; PMID: 19438317
- Tsai MS, Hwang SM, Tsai YL, Cheng FC, Lee JL, Chang YJ. Clonal amniotic fluid-derived stem cells express characteristics of both mesenchymal and neural stem cells. Biol Reprod 2006; 74:545 - 51; http://dx.doi.org/10.1095/biolreprod.105.046029; PMID: 16306422
- Jezierski A, Gruslin A, Tremblay R, Ly D, Smith C, Turksen K, et al. Probing stemness and neural commitment in human amniotic fluid cells. Stem Cell Rev 2010; 6:199 - 214; http://dx.doi.org/10.1007/s12015-010-9116-7; PMID: 20221716
- Miranda-Sayago JM, Ferńndez-Arcas N, Benito C, Reyes-Engel A, Carrera J, Alonso A. Lifespan of human amniotic fluid-derived multipotent mesenchymal stromal cells. Cytotherapy 2011; 13:572 - 81; http://dx.doi.org/10.3109/14653249.2010.547466; PMID: 21208022
- Grisafi D, Piccoli M, Pozzobon M, Ditadi A, Zaramella P, Chiandetti L, et al. High transduction efficiency of human amniotic fluid stem cells mediated by adenovirus vectors. Stem Cells Dev 2008; 17:953 - 62; http://dx.doi.org/10.1089/scd.2007.0188; PMID: 18564037
- Götherström C, Ringdén O, Tammik C, Zetterberg E, Westgren M, Le Blanc K. Immunologic properties of human fetal mesenchymal stem cells. Am J Obstet Gynecol 2004; 190:239 - 45; http://dx.doi.org/10.1016/j.ajog.2003.07.022; PMID: 14749666
- Moorefield EC, McKee EE, Solchaga L, Orlando G, Yoo JJ, Walker S, et al. Cloned, CD117 selected human amniotic fluid stem cells are capable of modulating the immune response. PLoS One 2011; 6:e26535; http://dx.doi.org/10.1371/journal.pone.0026535; PMID: 22046303
- Ditadi A, de Coppi P, Picone O, Gautreau L, Smati R, Six E, et al. Human and murine amniotic fluid c-Kit+Lin- cells display hematopoietic activity. Blood 2009; 113:3953 - 60; http://dx.doi.org/10.1182/blood-2008-10-182105; PMID: 19221036
- Prusa AR, Marton E, Rosner M, Bettelheim D, Lubec G, Pollack A, et al. Neurogenic cells in human amniotic fluid. Am J Obstet Gynecol 2004; 191:309 - 14; http://dx.doi.org/10.1016/j.ajog.2003.12.014; PMID: 15295384
- De Coppi P, Bartsch G Jr., Siddiqui MM, Xu T, Santos CC, Perin L, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 2007; 25:100 - 6; http://dx.doi.org/10.1038/nbt1274; PMID: 17206138
- Arnhold S, Glüer S, Hartmann K, Raabe O, Addicks K, Wenisch S, et al. Amniotic-Fluid Stem Cells: Growth Dynamics and Differentiation Potential after a CD-117-Based Selection Procedure. Stem Cells Int 2011; 2011:715341; http://dx.doi.org/10.4061/2011/715341; PMID: 21437196
- Gekas J, Walther G, Skuk D, Bujold E, Harvey I, Bertrand OF. In vitro and in vivo study of human amniotic fluid-derived stem cell differentiation into myogenic lineage. Clin Exp Med 2010; 10:1 - 6; http://dx.doi.org/10.1007/s10238-009-0060-2; PMID: 19730985
- Guan X, Delo DM, Atala A, Soker S. In vitro cardiomyogenic potential of human amniotic fluid stem cells. J Tissue Eng Regen Med 2011; 5:220 - 8; http://dx.doi.org/10.1002/term.308; PMID: 20687122
- Ye L, Chang JC, Lin C, Sun X, Yu J, Kan YW. Induced pluripotent stem cells offer new approach to therapy in thalassemia and sickle cell anemia and option in prenatal diagnosis in genetic diseases. Proc Natl Acad Sci U S A 2009; 106:9826 - 30; http://dx.doi.org/10.1073/pnas.0904689106; PMID: 19482945
- Li C, Zhou J, Shi G, Ma Y, Yang Y, Gu J, et al. Pluripotency can be rapidly and efficiently induced in human amniotic fluid-derived cells. Hum Mol Genet 2009; 18:4340 - 9; http://dx.doi.org/10.1093/hmg/ddp386; PMID: 19679563
- Galende E, Karakikes I, Edelmann L, Desnick RJ, Kerenyi T, Khoueiry G, et al. Amniotic fluid cells are more efficiently reprogrammed to pluripotency than adult cells. Cell Reprogram 2010; 12:117 - 25; http://dx.doi.org/10.1089/cell.2009.0077; PMID: 20677926
- Anchan RM, Quaas P, Gerami-Naini B, Bartake H, Griffin A, Zhou Y, et al. Amniocytes can serve a dual function as a source of iPS cells and feeder layers. Hum Mol Genet 2011; 20:962 - 74; http://dx.doi.org/10.1093/hmg/ddq542; PMID: 21156717
- Adzick NS, Thom EA, Spong CY, Brock JW 3rd, Burrows PK, Johnson MP, et al, MOMS Investigators. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 2011; 364:993 - 1004; http://dx.doi.org/10.1056/NEJMoa1014379; PMID: 21306277
- Dhaulakhandi DB, Rohilla S, Rattan KN. Neural tube defects: review of experimental evidence on stem cell therapy and newer treatment options. Fetal Diagn Ther 2010; 28:72 - 8; http://dx.doi.org/10.1159/000318201; PMID: 20689263
- Li H, Gao F, Ma L, Jiang J, Miao J, Jiang M, et al. Therapeutic potential of in utero mesenchymal stem cell (MSCs) transplantation in rat fetuses with spina bifida aperta. J Cell Mol Med 2012; 16:1606 - 17; http://dx.doi.org/10.1111/j.1582-4934.2011.01470.x; PMID: 22004004
- Fauza DO, Jennings RW, Teng YD, Snyder EY. Neural stem cell delivery to the spinal cord in an ovine model of fetal surgery for spina bifida. Surgery 2008; 144:367 - 73; http://dx.doi.org/10.1016/j.surg.2008.05.009; PMID: 18707035
- Sartore S, Lenzi M, Angelini A, Chiavegato A, Gasparotto L, De Coppi P, et al. Amniotic mesenchymal cells autotransplanted in a porcine model of cardiac ischemia do not differentiate to cardiogenic phenotypes. Eur J Cardiothorac Surg 2005; 28:677 - 84; http://dx.doi.org/10.1016/j.ejcts.2005.07.019; PMID: 16188450
- Yeh YC, Lee WY, Yu CL, Hwang SM, Chung MF, Hsu LW, et al. Cardiac repair with injectable cell sheet fragments of human amniotic fluid stem cells in an immune-suppressed rat model. Biomaterials 2010; 31:6444 - 53; http://dx.doi.org/10.1016/j.biomaterials.2010.04.069; PMID: 20621766
- Sassoli C, Pini A, Mazzanti B, Quercioli F, Nistri S, Saccardi R, et al. Mesenchymal stromal cells affect cardiomyocyte growth through juxtacrine Notch-1/Jagged-1 signaling and paracrine mechanisms: clues for cardiac regeneration. J Mol Cell Cardiol 2011; 51:399 - 408; http://dx.doi.org/10.1016/j.yjmcc.2011.06.004; PMID: 21712044
- Bollini S, Pozzobon M, Nobles M, Riegler J, Dong X, Piccoli M, et al. In vitro and in vivo cardiomyogenic differentiation of amniotic fluid stem cells. Stem Cell Rev 2011; 7:364 - 80; http://dx.doi.org/10.1007/s12015-010-9200-z; PMID: 21120638
- Bollini S, Cheung KK, Riegler J, Dong X, Smart N, Ghionzoli M, et al. Amniotic fluid stem cells are cardioprotective following acute myocardial infarction. Stem Cells Dev 2011; 20:1985 - 94; http://dx.doi.org/10.1089/scd.2010.0424; PMID: 21534857
- Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C, et al. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg 2010; 139:431 - 6, 436, e1-2; http://dx.doi.org/10.1016/j.jtcvs.2009.09.057; PMID: 20106404
- Schmidt D, Achermann J, Odermatt B, Breymann C, Mol A, Genoni M, et al. Prenatally fabricated autologous human living heart valves based on amniotic fluid derived progenitor cells as single cell source. Circulation 2007; 116:Suppl I64 - 70; http://dx.doi.org/10.1161/CIRCULATIONAHA.106.681494; PMID: 17846327
- Weber B, Emmert MY, Behr L, Schoenauer R, Brokopp C, Drögemüller C, et al. Prenatally engineered autologous amniotic fluid stem cell-based heart valves in the fetal circulation. Biomaterials 2012; 33:4031 - 43; http://dx.doi.org/10.1016/j.biomaterials.2011.11.087; PMID: 22421386
- Moss RL, Chen CM, Harrison MR. Prosthetic patch durability in congenital diaphragmatic hernia: a long-term follow-up study. J Pediatr Surg 2001; 36:152 - 4; http://dx.doi.org/10.1053/jpsu.2001.20037; PMID: 11150455
- Fuchs JR, Kaviani A, Oh JT, LaVan D, Udagawa T, Jennings RW, et al. Diaphragmatic reconstruction with autologous tendon engineered from mesenchymal amniocytes. J Pediatr Surg 2004; 39:834 - 8, discussion 834-8; http://dx.doi.org/10.1016/j.jpedsurg.2004.02.014; PMID: 15185207
- Kunisaki SM, Fuchs JR, Kaviani A, Oh JT, LaVan DA, Vacanti JP, et al. Diaphragmatic repair through fetal tissue engineering: a comparison between mesenchymal amniocyte- and myoblast-based constructs. J Pediatr Surg 2006; 41:34 - 9, discussion 34-9; http://dx.doi.org/10.1016/j.jpedsurg.2005.10.011; PMID: 16410104
- Turner CG, Klein JD, Steigman SA, Armant M, Nicksa GA, Zurakowski D, et al. Preclinical regulatory validation of an engineered diaphragmatic tendon made with amniotic mesenchymal stem cells. J Pediatr Surg 2011; 46:57 - 61; http://dx.doi.org/10.1016/j.jpedsurg.2010.09.063; PMID: 21238640
- Steigman SA, Ahmed A, Shanti RM, Tuan RS, Valim C, Fauza DO. Sternal repair with bone grafts engineered from amniotic mesenchymal stem cells. J Pediatr Surg 2009; 44:1120 - 6, discussion 1126; http://dx.doi.org/10.1016/j.jpedsurg.2009.02.038; PMID: 19524727
- Klein JD, Turner CG, Ahmed A, Steigman SA, Zurakowski D, Fauza DO. Chest wall repair with engineered fetal bone grafts: an efficacy analysis in an autologous leporine model. J Pediatr Surg 2010; 45:1354 - 60; http://dx.doi.org/10.1016/j.jpedsurg.2010.02.116; PMID: 20620344
- Turner CG, Klein JD, Gray FL, Ahmed A, Zurakowski D, Fauza DO. Craniofacial repair with fetal bone grafts engineered from amniotic mesenchymal stem cells. J Surg Res 2012; 178:785 - 90; http://dx.doi.org/10.1016/j.jss.2012.05.017; PMID: 22656041
- Kunisaki SM, Jennings RW, Fauza DO. Fetal cartilage engineering from amniotic mesenchymal progenitor cells. Stem Cells Dev 2006; 15:245 - 53; http://dx.doi.org/10.1089/scd.2006.15.245; PMID: 16646670
- Jungebluth P, Alici E, Baiguera S, Le Blanc K, Blomberg P, Bozóky B, et al. Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: a proof-of-concept study. Lancet 2011; 378:1997 - 2004; http://dx.doi.org/10.1016/S0140-6736(11)61715-7; PMID: 22119609
- Zani A, Cananzi M, Eaton S, Pierro A, De Coppi P. Stem cells as a potential treatment of necrotizing enterocolitis. J Pediatr Surg 2009; 44:659 - 60; http://dx.doi.org/10.1016/j.jpedsurg.2008.12.012; PMID: 19302881
- van Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med 2009; 180:1131 - 42; http://dx.doi.org/10.1164/rccm.200902-0179OC; PMID: 19713449
- Tropea KA, Leder E, Aslam M, Lau AN, Raiser DM, Lee JH, et al. Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2012; 302:L829 - 37; http://dx.doi.org/10.1152/ajplung.00347.2011; PMID: 22328358
- Klemmt PA, Vafaizadeh V, Groner B. The potential of amniotic fluid stem cells for cellular therapy and tissue engineering. Expert Opin Biol Ther 2011; 11:1297 - 314; http://dx.doi.org/10.1517/14712598.2011.587800; PMID: 21623704
- Liu T, Zou G, Gao Y, Zhao X, Wang H, Huang Q, et al. High Efficiency of Reprogramming CD34(+) Cells Derived from Human Amniotic Fluid into Induced Pluripotent Stem Cells with Oct4. Stem Cells Dev 2012; 21:2322 - 32; http://dx.doi.org/10.1089/scd.2011.0715; PMID: 22264161