Abstract
Embryonic stem cells (ESC) are self-renewing and can generate all cell types during normal development. Previous studies have begun to explore fates of ESCs and their mesodermal derivatives after injection into explanted intact metanephric kidneys and neonatal kidneys maturing in vivo. Here, we exploited a recently described recombinant organ culture model, mixing fluorescent quantum dot labeled mouse exogenous cells with host metanephric cells. We compared abilities of undifferentiated ESCs with ESC-derived mesodermal or non-mesodermal cells to contribute to tissue compartments within recombinant, chimeric metanephroi. ESC-derived mesodermal cells downregulated Oct4, a marker of undifferentiated cells, and, as assessed by locations of quantum dots, contributed to Wilms’ tumor 1-expressing forming nephrons, synaptopodin-expressing glomeruli, and organic ion-transporting tubular epithelia. Similar results were observed when labeled native metanephric cells were recombined with host cells. In striking contrast, non-mesodermal ESC-derived cells strongly inhibited growth of embryonic kidneys, while undifferentiated ESCs predominantly formed Oct4 expressing colonies between forming nephrons and glomeruli. These findings clarify the conclusion that ESC-derived mesodermal cells have functional nephrogenic potential, supporting the idea that they could potentially replace damaged epithelia in diseased kidneys. On the other hand, undifferentiated ESCs and non-mesodermal precursors derived from ESCs would appear to be less suitable materials for use in kidney cell therapies.
Introduction
Pluripotent embryonic stem cells (ESCs) exist within, and can be isolated from, the inner cell mass of the mouse and human blastocyst.Citation1,Citation2 They normally generate endoderm, mesoderm and ectoderm, from which all cells in the mature organism derive. ESCs express octamer-binding transcription factor 4 (Oct4), a pluripotency marker downregulated during differentiation.Citation3 In vitro, ESCs can undergo limitless self-renewal, particularly when cultured under adherent conditions.Citation1 In suspension culture, however, they spontaneously form aggregates called embryoid bodies (EBs), so-called because their initial development resembles that of inner cell masses.Citation4 For instance, EBs differentiate to form the three embryonic germ layers, including brachyury-expressing (Bra+) nascent mesodermCitation5,Citation6 from which kidneys derive.Citation7 The ability of ESCs to generate any cell typeCitation8 has led to the suggestion that they could be used to replace diseased or injured cells. In preclinical studies, ESC-derived retinal pigmented epithelial cells have treated macular degeneration,Citation9 and the first human Phase I trial using ESC derivatives to treat spinal cord injury began in 2010.Citation10
Previous studies have begun to explore the fates of LacZ or green fluorescent protein (GFP) labeled ESCs or their immediate derivatives after injection into murine metanephric kidneys grown in cultureCitation11-Citation13 and neonatal kidneys maturing in vivo.Citation12 In the former context, although ESCs generated tubule-like structures, the therapeutic potential of ESCs may be limited by their propensity to form tumors.Citation2,Citation14 Others have first partially differentiated ESCs into a mesodermal lineage before injecting them into intact kidneys,Citation11,Citation12 reporting that implanted cells differentiated into proximal tubules (PTs), as assessed by morphology and segment-specific markers. However, although PTs derive from the metanephric mesenchyme (MM)/nephron lineage, labeled cells were hardly ever detected in glomeruli, even though podocytes also derive from this lineage. Furthermore, any potential adverse effect of the exogenous implanted cells on the development of host precursor cells was not specifically investigated in these studies,Citation11-Citation13 nor was the physiological functionality of implanted cells.
Here, we exploited a recently described recombinant kidney organ culture model, mixing labeled mouse ESCs or their immediate derivatives with host metanephric cells. In the resulting recombinant, chimeric organs, we compared abilities of undifferentiated ESCs with ESC-derived mesodermal or non-mesodermal cells to integrate with, and contribute to, nascent kidney structures. Our strategy used four key techniques. First, we exploited an ESC line, Bra-GFP,Citation15 in which GFP is expressed from within the Brachyury locus, enabling mesodermal cells to be selected from ESC-derived EBs using fluorescence activated cell sorting (FACS). Second, to visualize fates of exogenous cells, they were first labeled with fluorescent nanocrystals called quantum dots (QDs), a technique previously used to ascertain fates of mesenchymal stem cells within hearts.Citation16 QDs can label cells rapidly and efficiently without affecting their differentiation potential,Citation16 and an advantage of QDs over traditional fluorescent markers is that they resist photobleaching. We have recently shown that the specific QD technology we are using, as assessed in kidney-derived stem cells over several days observation, affects neither proliferation nor expression of lineage-specific renal markers. Moreover, labeled cells do not excrete QDs, and transfer of QDs to adjacent kidney cells is negligible.Citation17 On the other hand, QDs do not replicate upon cell division, so that not all progeny of initially labeled cells will be found to contain dots.Citation18 Third, these labeled cells were intimately mixed with disaggregated cells from “host” metanephric rudiments, potentially facilitating the integration of exogenous cells into nascent structures.Citation19,Citation20 Last, we tested the ability of labeled cells to participate in organic ion transport.Citation21-Citation24
Results
Gene expression in Bra-GFP+ cells
To investigate whether the nephrogenic potential of mouse ESCs could be enhanced by differentiating them to mesoderm, a Bra-GFP mouse ESC line was used to isolate an enriched population of mesodermal cells.Citation5 As described,Citation15 ESCs were seeded onto non-adherent dishes to generate EBs that gave rise to GFP+ cells after 4 d of culture (results not shown). To confirm that GFP+ cells were mesodermal, GFP+ and GFP- cells were separated by FACS, and reverse transcription polymerase chain reaction (RT-PCR) performed. As expected, GFP+ cells expressed the nascent mesodermal marker, Bra, as well as Forkhead box protein c1 (Foxc1), normally expressed by paraxial mesodermCitation25 and later by the nephrogenic cord,Citation26 and the intermediate mesodermal marker, Odd-skipped-related 1 (Osr1), essential for metanephrogenesis.Citation27,Citation28 The GFP- population appeared to express lower levels of these genes but showed more prominent expression of the ectodermal marker, Paired box protein 6 (Pax6) ().
Figure 1. Chimera formation. (A) RT-PCR confirmed that GFP+ cells prominently expressed mesodermal genes, Bra, Foxc1 and Osr1 whereas the ectodermal marker, Pax6, was more prominent in GFP- cells; NTC, no template control. (B–D) KRCs (blue) from E13.5 metanephroi (B), ESCs (orange) (C) and FACS-sorted Bra+ (green) or Bra- (yellow) cells (D) were dissociated to single cells and labeled with QDs (thus becoming red cells) and mixed with unlabeled KRCs (blue) in a ratio of 1 to 8 prior to re-aggregation.
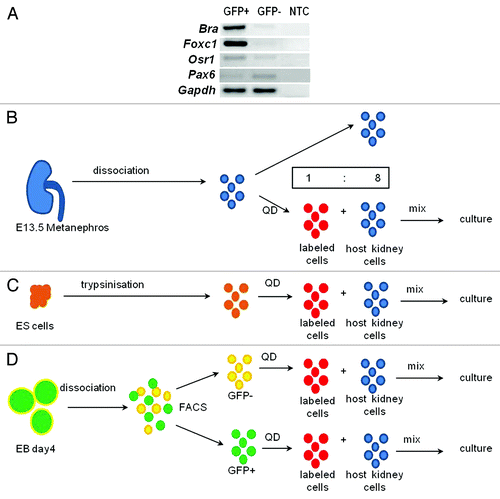
Exogenous cell effects on rudiment growth and differentiation
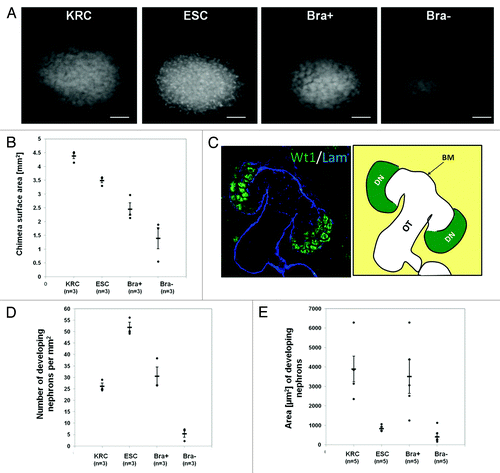
Chimeric organs were generated using host cells from dissociated embryonic day (E) 13.5 mouse kidneys mixed in an 8/1 ratio (total of 18 × 104 cells) with Bra+, Bra-, undifferentiated ESCs, or native kidney rudiment cells (KRCs), the latter representing a positive control (). Mouse metanephroi originate at E10.5 when they comprise a ureteric bud (UB) growing into MM.Citation7 By E13.5, the UB has branched several times, forming a collecting duct tree, and MM has begun to undergo mesenchymal/epithelial transition to generate nephrons. At this time point, these first layers of nascent nephrons comprise vesicles and comma-shaped bodies and have yet to differentiate into either glomerular podocytes, which express both Wilms’ tumor 1 (Wt1) and synaptopodin, or PTs.Citation7 Following 3 d of culture, chimeras made with ESCs or Bra+ cells tended to be smaller, as assessed by explant areas, than those made with KRCs. Furthermore, examining individual explants, it was obvious that some Bra- recombinant organs were very growth-retarded (). We defined “developing nephrons” as being discrete, Wt1+ structures on whole-mount immunostaining (). KRC and Bra+ chimeric metanephroi contained on average approximately 25–30 developing nephrons/mm2, ESC chimeras contained on average around 50/mm2, while no Bra- chimeric organ contained over 8/mm2 (). Average cross-sectional areas of developing nephrons in KRC and Bra+ chimeras were similar, whereas those in both ESC and Bra- chimeras were approximately 3–4 times smaller (). Given that the Bra- chimeric organs appeared both tiny and nephron-deplete, we elected not to analyze them further.
Figure 2. Effect of exogenous cells on chimera development. (A) Photomicrographs of Wt1 immunostained rudiments. (B) Graph showing explant areas. All chimeric rudiment areas are significantly different from the control (KRC chimera) (p < 0.05). (C) Left panel shows a confocal image of a day 3 KRC chimera immunostained for Wt1 (green) and laminin (blue). Schematic in right panel indicates developing nephron structures (DN), basement membrane (BM) and other tubules (OT), which comprise nephron tubules and UB branches. (D and E) Graphs showing numbers of developing nephrons per unit area (D) and areas of these structures (E). In D and E, while ESC and Bra- are significantly different from the KRC control chimeras (p < 0.05), there is no significant difference between Bra+ chimeras and controls (p > 0.05). In B, D and E, each point represents data from a single explant, with mean and standard error shown for each group. Note that the number and size of developing nephrons in KRC and Bra+ chimeras were similar, whereas ESC chimeras had significantly higher numbers of developing nephrons that had a smaller cross-sectional area. When organs were made with Bra- cells, they were generally very small and contained only a few, small nephrons. Scale bars in (A), 500 µm.
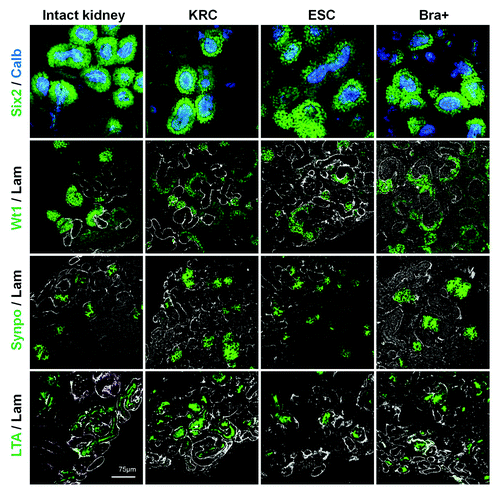
Next, we undertook confocal microscopy of KRC, ESC and Bra+ chimeras probed with a panel of metanephric cell and tubule markers (). In all three varieties of chimeric organ, after 3 d culture, we detected calbindin-28-expressing UB branch tips capped by cells expressing sine oculis homeobox homolog 2 (Six2), a transcription factor marking induced, condensing MM beginning to form nephrons.Citation29,Citation30 A range of structures expressed Wt1, including nephron vesicles and also crescents of podocyte precursors in S-shaped bodies.Citation31 After 5 d culture of KRC, ESC and Bra+ recombinant organs, we detected cell clusters expressing synaptopodin, representing podocytes,Citation32 and tubules binding Lotus tetragonolobus agglutinin (LTA) on their luminal/apical zones, as do PTs in vivo.Citation33 These patterns resembled those in cultured intact E13.5 kidneys ().
Table 1. Markers used to identify renal cell populations
Figure 3. Segment-specific markers in intact and recombinant chimeric metanephroi. The expression patterns of Six2 (nuclear signal, green), calbindin-28 (cytoplasmic signal, blue), Wt1 (nuclear signal, green) and synaptopodin (cytoplasmic signal, green) as well as apical LTA binding (green) in intact metanephric kidneys (left hand set of four panels) and in chimeric rudiments generated by mixing host cells with either KRC, ESC or Bra+ exogenous cells. Laminin staining is shown in white. Rudiments in the top two panels were analyzed after 3 d culture, whereas those in the bottom two panels were analyzed after 5 d culture. Scale bars, 75 µm.
Oct4 expression
Having established that the addition of ESCs and Bra+ cells was compatible with chimeric metanephric maturation, we determined the detailed fates of exogenous cells. We found that both ESCs and freshly sorted Bra+ cells expressed Oct4 transcripts (). Upon immunostaining, Oct4 was undetectable in intact (non-recombinant) metanephroi (data not shown), and Oct4 transcripts were undetectable in just-dissociated native KRCs (). Oct4+ cells were plentiful in both ESC and Bra+ chimeras on the day each recombinant organ was created (“day 0” in ). Subsequently, in ESC chimeric organs, prominent Oct4+ colonies were present for up to 8 d, the limit of the observation period.
Figure 4. Expression of Oct4. (A) RT-PCR for Oct4 in undifferentiated ESCs, Bra+ (GFP+) cells and KRCs. Note that undifferentiated ESC and Bra+ cells express Oct4 just before being introduced into the chimera, whereas KRCs do not; NTC, no template control. (B) Oct4 (green nuclei) and laminin (white BMs) immunostaining within chimeras containing ESCs or Bra+ cells. Chimeric organs at day 0 were fixed 2–3 h following re-aggregation. Note plentiful large Oct4+ colonies in ESC chimeras. In contrast, there is a paucity of Oct4+ cells in the Bra+ chimeras. Scale bars, 60 μm
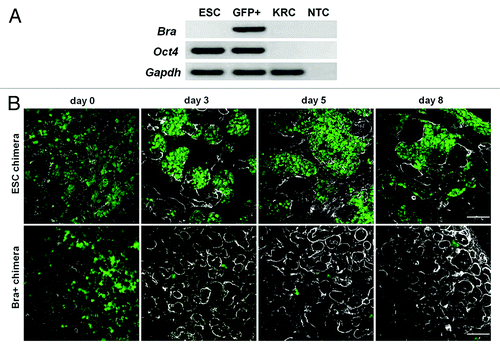
In marked contrast, Bra+ recombinant organs displayed only sparse, tiny Oct4+ colonies on day 3, and hardly any Oct4+ positive cells were detected at day 8, suggesting that cells derived from Bra+ exogenous cells had switched off Oct4 expression and begun to differentiate.
Locations of QDs in explants
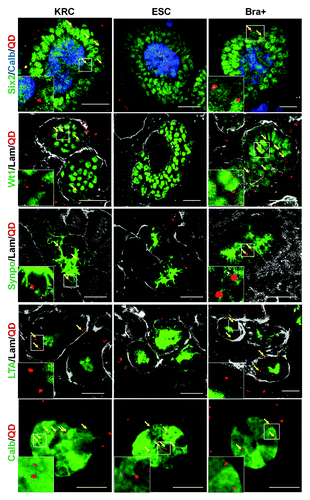
We measured the extent to which exogenous cells (or their progeny) appeared in different zones of chimeric organs, as assessed by detection of QD+ cells. Following 3 d culture, rudiments were immunostained for Wt1 and laminin. For these analyses, we defined three zones: (1) “developing nephrons” contained Wt1+ cells surrounded by laminin+ BM, (2) “other tubules” contained Wt1- cells surrounded by BM (note that, in ESC recombinants, these would comprise both normal kidney tubules and Oct4+ aggregates, as described above) and (3) “stroma” where cells neither expressed Wt1 nor were surrounded by BM (). In KRC, ESC and Bra+ recombined organs, we detected QD+ cells in each of the three zones. The numbers of QD+ cells within developing nephrons per section area (0.84 mm2) were similar in chimeric organs made with Bra+ or KRCs, whereas QDs were rarely detected in this compartment in ESC recombinant organs (). The proportion of Wt1+QD+ cells within the developing nephrons of Bra+ chimeras averaged 12–13%, and was not significantly different from that of KRC chimeras. In contrast, Wt1+QD+ cells comprised only 4% of cells within the developing nephrons of ESC chimeras, indicating that in comparison to KRCs and Bra+ cells, ESCs have a limited ability to integrate into these structures (). With respect to “other tubules,” numbers of QD+ cells per section area were similar in the KRC, ESC and Bra+ groups (). With the same panel of markers used in , we ascertained more precisely which kidney cell types contained QDs. At 3–5 d of culture, in both KRC and Bra+ chimeric organs, subsets of cells expressing either Six2, Wt1, synaptopodin or calbindin-28 proteins contained QDs, and the fluorescent marker was also found in some tubule cells binding LTA at their apical surfaces, marking them as PT epithelia. In ESC chimeric organs, QDs were found in calbindin-28+ cells, but were only rarely detected in LTA-binding PTs and synaptopodin+ structures ().
Figure 5. QD+ cell quantification. (A) Graph presenting the extent of integration of labeled cells into developing nephrons and other tubules per unit area (0.84 mm2). Numbers of QD+ cells in KRC and Bra+ chimeras (n = 3 organs for each) within developing nephrons was not significantly different, but the numbers in ESC chimeras was significantly less (p < 0.05). (B) Percentages of QD+ cells contributing to developing nephrons. Note that significantly fewer labeled cells contributed to developing nephrons (p < 0.05) in ESC chimeras vs. the other groups (n = 3 rudiments analyzed for each group, and for each rudiment six nephrons were analyzed).
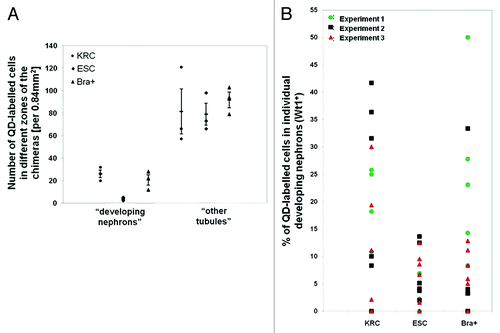
Figure 6. Expression of kidney specific markers by QD+ cells in chimeras. Representative confocal photomicrographs showing the expression of different markers in chimeras made with KRC, ESC and Bra+ cells. Yellow arrows point to QDs within specific cells. Boxed areas outlined in white are presented as enlargements within the main frame of some of the images. Note that QD+ cells are not seen in Six2, Wt1, synaptopodin and LTA (all in green) positive regions in the ESC chimera vs. the presence of QDs in these regions within rudiments made with Bra+ and positive control KRC cells. Basement membrane structures are stained with laminin (white). Scale bar, 20 μm.
ESC-derived mesodermal cells generate functional tubular cells
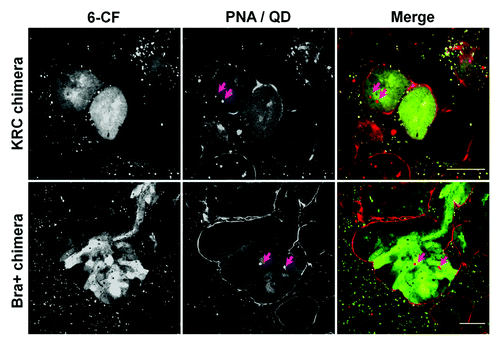
We used an organic anion transporter (OAT) assay to investigate whether rudiment cells manifested OAT-dependent uptake of the organic anion mimic, 6-carboxyfluorescein (6-CF).Citation21-Citation24 In the absence of the OAT inhibitor, probenecid, 6-CF is transported from the interstitium into the cells before being secreted into the tubular lumen of intact rudiments. Peanut agglutinin (PNA)-rhodamine was also used to label outer surfaces of tubules (). QD+ tubule cells in both KRC control and Bra+ chimeras demonstrated active transport of 6-CF (; Fig. S1), whereas those in ESC chimeras did not (data not shown).
Figure 7. Uptake of organic ions by E13.5 intact kidney rudiments. In the absence of the OAT inhibitor, probenecid, vital staining of intact E13.5 kidney rudiments with 6-CF (green), indicates uptake by tubular epithelial cells. Uptake of 6-CF is blocked in the presence of probenecid. Vital staining with PNA (red) was used to identify tubule BMs. The rudiments had been cultured for 5 d prior to staining. Scale bars, 100 μm.
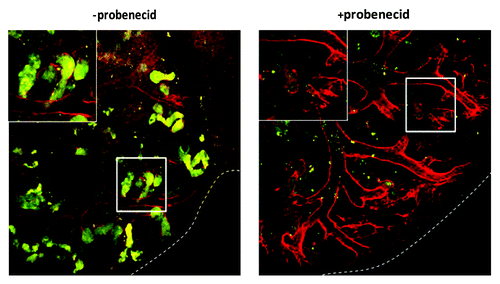
Figure 8. QD+ ESC-derived mesodermal cells take up organic ions. KRC and Bra+ chimeric organs contain QD+ (arrowed) cells which take up 6-CF. For each type of chimeric organ, the following images are shown of the same field: from left to right, the first image shows 6-CF, the second image shows PNA/QDs, and the third frame is a merged, color image (6-CF in green and PNA/QDs in red). Scale bars, 20 μm.
Discussion
By identifying labeled cells in recombinant metanephric kidneys in organ culture, our results clarify the conclusion that ESC-derived mesodermal cells have nephrogenic potential because they were found within glomeruli and transporting tubules, supporting the notion that they may serve as progenitors to replace damaged epithelia in diseased kidneys. On the other hand, undifferentiated ESCs and non-mesodermal precursors derived from ESCs respectively failed to downregulate Oct4 and inhibited kidney growth and nephrogenesis, and would therefore appear to be less suitable materials to use in kidney cell therapies. Our findings are summarized in , where they are compared with previous studies investigating fates of ESC and/or their mesodermal derivatives after injection into developing kidneys.Citation11-Citation13
Table 2. Studies investigating the contribution of ESC and their derivatives to developing renal structures
Recombined metanephroi made with KRC, ESC and Bra+ exogenous cells contained plentiful developing nephrons. Versus both KRC positive control and Bra+ chimeras, those made using undifferentiated ESCs contained a greater number of Wt1+ nephrons per explant area, and these nephrons were smaller in size, suggesting that undifferentiated ESCs somehow alter the normal regulated pattern of nephrogenesis. One possibility is that, following incorporation into kidney rudiments, ESCs secrete factors capable of promoting early nephrogenesis, including WntsCitation34 or fibroblast growth factors,Citation35 or Notch2.Citation36 As mentioned in the Introduction, when ESCs differentiate into more specialized cell types they downregulate Oct4. We found that ESC chimeric rudiments contained prominent Oct4+ colonies located between normal-looking tubules. We also noted a lack of propensity for ESCs to appear in developing Wt1+ nephrons, as assessed by detection of QD+ cells. We speculate that this could be due to the fact that the UB secretes leukemia inhibitory factor (LIF),Citation37 a known inhibitor of mouse ESC differentiation.Citation38 An earlier study reported that mouse ESCs readily generated PT cells following injection into metanephroi ex vivo, as evidenced by the appearance of ESC-derived columnar cells binding LTA.Citation13 However, these chimeric rudiments were not analyzed for Oct4 expression, and it is thus possible that at least some of the LTA+ cells observed were in fact ESC-derived columnar epiblast-like cells binding LTA.Citation39
In the current study, in contrast to recombined rudiments made with undifferentiated ESCs, those made with Bra+ cells contained very few Oct4+ cells from the 3rd day of culture onwards. This is consistent with the report of Vigneau and colleagues, who showed that Bra+ cells do not generate teratomas following injection into neonatal kidneys in vivo, whereas ESCs do.Citation12 Indeed, it is well established that maintained expression of the Oct4 pluripotency marker indicates a tumorigenic potential.Citation40,Citation41 Kim and DresslerCitation11 found that if ESCs were first cultured as EBs in mesoderm-inducing culture conditions, they could generate PTs after injection into explanted kidney rudiments. Likewise, Vigneau and coworkersCitation12 showed that Bra+ cells isolated from mouse ESC-derived EBs could generate PT cells following injection into neonatal mouse kidneys which then matured in vivo. The present study supports the notion that Bra+ cells can integrate into nascent PTs. Furthermore, for the first time, we show that such labeled tubule epithelial cells are physiologically active, as evidenced by their ability to transport organic anions. Notably, in neither previous studyCitation11,Citation12 did ESC-derived mesodermal cells appear to generate glomerular podocytes. This is in contrast with our results, which show that in chimeras made with Bra+ cells, some cells located in clusters expressing synaptopodin contained QDs, suggesting that Bra+ cells are indeed capable of generating podocytes. The reasons for the observed differences could be due to the fact that in the current study we use a disaggregation/re-aggregation methodCitation20 to introduce exogenous cells, whereas in the other studies, cells were introduced by bolus injections.Citation11,Citation12 Another possible advantage of the current strategy is that any inhibitory effect of intact epithelia or basement membranes in restricting the integration of exogenous cells would be minimised, permitting improved cell integration.
A potential concern for all such studies is that it might be possible for labeled exogenous cells to fuse with host tubules, leading to false positives.Citation42 However, using an elegant genetic strategy in which reporter R26R-EYFP ESCs were injected into Ksp-Cre host metanephroi, Kim and DresslerCitation11 found no evidence for fusing of exogenous cells with host tubules. A second concern with respect to using QDs as a label is that the fluorescent dots might theoretically be released from labeled exogenous cells and taken up by host cells. However, we have recently shown that the extent of QD transfer in rudiment chimeras is negligible.Citation17 Moreover, we noted that the percentage of QD+ cells within Wt1+ developing nephrons in KRC and Bra+ chimeras was several-fold greater than that found in chimeric rudiments made from undifferentiated ESCs. Similarly, synaptopodin+ glomeruli containing subsets of QD+ cells were detected in rudiments made with KRC and Bra+ cells but not with those made with ESCs. Collectively, these results support the conclusions that the differentiation/integration behavior of exogenous cells is cell-type specific and that our results cannot be explained simply by invoking an unspecific transfer of QDs between exogenous and host cells. It is worth noting that because QDs do not replicate along with the cells, the proliferation of QD+ cells is associated with attenuation of the QD signal. Thus, the percentage of QD+ cells within developing nephrons in KRC and Bra+ chimeras probably represents a conservative estimate of integrated stem cells and/or their progeny.
A striking finding in our study was that Bra- cells had a negative effect on metanephros development, resulting in very small recombinant organs which contained only sparse Wt1+ nephrons. Using the same chimeric rudiment culture assay, we have recently shown that mouse and human bone marrow-derived mesenchymal stem cells (MSCs) were unable to integrate into developing renal structures, and similar to Bra- cells, had a negative effect on rudiment development.Citation43 Conditioned medium derived from the MSCs had a similar negative effect,Citation43 suggesting that secreted molecules were likely to be responsible. A previous study has shown that human bone marrow-derived CD34+ cells, which consist mainly of hematopoietic stem cells, were also unable to generate renal structures in both kidney rudiments and damaged adult kidneys, and instead contributed to hematopoietic lineages, and to a lesser extent, endothelial lineages.Citation44 In the current study, the mechanisms whereby the Bra- cells inhibit kidney rudiment development have not been explored, but it is noteworthy that compared with the Bra+ mesodermal cells, the Bra- population expressed higher levels of the ectodermal marker, Pax6, whose encoded protein directly upregulates transforming growth factor b2,Citation45 which codes for a known inhibitor of metanephrogenesis.Citation46 Vigneau et al.Citation12 did not report any obvious negative effect of Bra- cells on kidney development in culture, although this was not formally quantified by measuring explant growth and nephrogenesis.
Materials and Methods
Cell culture
The mouse Bra-GFP ESC lineCitation15,Citation47 was subcultured every 2–3 d on tissue culture dishes (Nunc) in medium comprising high glucose DMEM (Sigma) supplemented with 10% ESC-grade FCS (PAA Laboratories), 2 mM L-glutamine, 1% penicillin/streptomycin, 1.5 × 10−4 M monothioglycerol (MTG) (all from Sigma) and 1,000 U ml−1 LIF (Millipore). For differentiation as EBs, cells were seeded on non-adherent dishes (Sarsted) at 3 × 104 cells ml−1 in differentiating medium comprising 15% FCS IMDM supplemented with 2 mM L-glutamine, 4.5 × 10−4 M MTG, 1× ITS (Sigma), 0.5 M ascorbic acid, 1% penicillin/streptomycin (Sigma) in the absence of LIF. EBs at day 4 of development were disaggregated using 1× trypsin/EDTA (Sigma). For FACS, cells were used at a final density of 106 cells ml−1. Sorting was performed with a FACS Vantage SE (BD Biosciences) and the autofluorescence level of the cells was set using wild-type mouse E14 ESCs. FACS data were analyzed with Cell Quest PRO software (BD Biosciences).
Recombinant organ culture
CD1 mouse (Charles River) metanephroi were isolated and transferred onto isopore (1.2 μm) membrane filters (Millipore), placed on a metal grid and grown in kidney culture medium containing 10% FCS MEME (Sigma) supplemented with 2 mM L-glutamine and 1% penicillin/streptomycin (Sigma). Chimeric rudiments were generated as described,Citation20 except that E13.5 instead of E11.5 rudiments were used. Metanephroi were trypsinised in 1× trypsin/EDTA solution for 5–7 min at 37°C generating single cells. The trypsin reaction was stopped using 10% FCS-DMEM medium and cells were centrifuged at 1400 × g for 1 min. The supernatant was discarded and pelleted cells resuspended in kidney culture medium. These embryonic kidney-derived cells, obtained by disaggregating E13.5 mouse kidney rudiments to give a single cell suspension, are a heterogeneous population of kidney rudiment cells (KRCs). Our rationale for using KRCs as our positive control is that we know if we allow these cells to re-aggregate, they are capable of generating nascent nephrons and ureteric buds. We expect that the heterogeneous population of KRCs would initially include MM, UB cells, stromal cells and endothelial cells, but that after a few days of in vitro culture, the endothelial cells would die, as previously shown.Citation48 Bra+ and Bra- cells sorted from day 4 EBs, mouse ESC, and KRCs were labeled with QDs (Invitrogen, Qtracker® Cell Labeling Kit, Q25021MP). In brief, QDs (10 nM) in 200 µl complete kidney culture medium were applied to 1 × 106 cells in suspension and incubated for 60 min at 37°C and 5% CO2, then washed 4x with complete growth medium and used for chimera formation. We have recently shown that over 90% of cells are labeled with QDs following this procedure.Citation17 Pellets comprising a 1:8 ratio of exogenous:KRCs (2 × 104 exogenous cells:16 × 104 KRCs) were transferred onto isopore membrane filters (Millipore) on metal grids, and cultured for up to 8 d. For the first 24 h of incubation 5 μM Y27632 Rho kinase inhibitor (Chemicon Int.) was applied. Samples were fixed in cold methanol for subsequent immunostaining.
Quantitative analysis of developing nephrons in chimeric metanephroi
The number of Wt1+ developing nephrons was counted in six randomly selected fields of view of day 3 KRC, ESC, Bra+ and Bra- rudiment chimeras (n = 3 of each). The surface area of the different types of explants (n = 3 of each) and cross-sectional area of individual Wt1+ developing nephrons (n = 5 for each rudiment type) was determined using the equation for calculating the surface area of an ellipse (S = πr1r2).
Lectin- and immuno-staining
Following methanol fixation, samples were incubated for 2 h at room temperature in the dark with 10 μg ml−1 LTA (Vector Laboratories). Samples were blocked for 1 h in goat serum, before overnight incubation at 4°C with the primary antibodies at concentrations shown in . Alexa fluor secondary antibodies (Invitrogen) were incubated overnight at 4°C and imaged using a confocal microscope (Leica, AOBS SP2).
Table 3. List of antibodies used in the study
Quantification of QD-labeled cells in metanephroi
Numbers of QD+ cells in developing nephrons, other tubules and stroma were determined following immunostaining of day 3 KRC, ESC and Bra+ rudiment chimeras (n = 3 of each) for Wt1 and laminin. For each rudiment, the total number of QD+ cells within six random high power confocal fields (representing a total area of 0.84 mm2) was determined. To determine the percentages of QD+ cells within developing nephrons, 6 randomly selected nephrons (defined as Wt1+ cells bounded by laminin+ basement membrane) were examined from day 3 KRC, ESC and Bra+ chimeras (n = 3 of each type).
OAT assay
A modification of the method described by Sweet et al.Citation23 and Rosines et al.Citation49 was used. Chimeras grown for 5 d were washed twice in PBS for 5 min, then transferred into PBS containing 1 μM 6-CF (Sigma) and 20 μg ml−1 PNA-rhodamine (PNArh, Vector Laboratories) prior to incubation at 25°C for 1h in the dark. Control samples were incubated in OAT blocking solution, comprising 2 mM probenecid (Sigma) in PBS, 1 μM 6-CF and PNArh. Following incubation, samples were washed twice in ice-cold PBS and then incubated with 6-CF for 1h, followed by incubation in 8 mM probenecid in PBS for 15 min to prevent the secretion of 6-CF by tubular cells. After blocking, samples were transferred onto a glass coverslip and mounted with 80% (v/v) glycerol. The samples were immediately imaged by confocal microscopy.
RT-PCR
Total RNA was extracted from cells using Trizol (Invitrogen) and reverse transcribed using random hexamers (ThermoScientific) and Superscript III (Invitrogen). Cycling parameters were as follows: 1cycle of 95°C/5 min, 33 cycles of 95°C/6 sec, 58°C/30 sec, 72°C/30 sec, and 1 cycle of 72°C/5 min. Primers used are presented in .
Table 4. List of primers used in the study
Statistical analyses
Quantitative data are shown as a mean ± standard error. Sets were compared using Student’s t-test with p < 0.05 considered as significant.
Additional material
Download Zip (194.7 KB)Acknowledgments
This work was supported by the EC 6th Framework program grants “MolFun” and “KIDSTEM,” the EC 7th Framework program reintegration grant “KidRegen” (awarded to Aleksandra Rak-Raszewska) and Alder Hey Children’s Kidney Fund. A.S.W. acknowledges support from the Manchester Biomedical Research Centre. We thank: Georges Lacaud (Paterson Institute) for the gift of the Bra-GFP ESC line; Jamie Davies and Mathieu Unbekandt (University of Edinburgh) for sharing the protocol of disaggregation and re-aggregation of kidney rudiments; Monica Barclay (University of Liverpool) for help with FACS.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/organogenesis/article/22597
References
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981; 292:154 - 6; http://dx.doi.org/10.1038/292154a0; PMID: 7242681
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998; 282:1145 - 7; http://dx.doi.org/10.1126/science.282.5391.1145; PMID: 9804556
- Ovitt CE, Schöler HR. The molecular biology of Oct-4 in the early mouse embryo. Mol Hum Reprod 1998; 4:1021 - 31; http://dx.doi.org/10.1093/molehr/4.11.1021; PMID: 9835353
- Robertson E, ed. Embryo-derived stem cell lines. In teratocarcinomas and embryonic stem cells; a protocal approach. IRL Press Limited; Oxford, 1987.
- Rivera-Pérez JA, Magnuson T. Primitive streak formation in mice is preceded by localized activation of Brachyury and Wnt3. Dev Biol 2005; 288:363 - 71; http://dx.doi.org/10.1016/j.ydbio.2005.09.012; PMID: 16289026
- Fujiwara H, Hayashi Y, Sanzen N, Kobayashi R, Weber CN, Emoto T, et al. Regulation of mesodermal differentiation of mouse embryonic stem cells by basement membranes. J Biol Chem 2007; 282:29701 - 11; http://dx.doi.org/10.1074/jbc.M611452200; PMID: 17690109
- Vize P, Woolf A, Bard J. The kidney, from normal development to congenital disease. Academic Press; Amsterdam-Tokyo, 2003.
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A 1993; 90:8424 - 8; http://dx.doi.org/10.1073/pnas.90.18.8424; PMID: 8378314
- Lu B, Malcuit C, Wang S, Girman S, Francis P, Lemieux L, et al. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells 2009; 27:2126 - 35; http://dx.doi.org/10.1002/stem.149; PMID: 19521979
- Thomas KE, Moon LDF. Will stem cell therapies be safe and effective for treating spinal cord injuries?. Br Med Bull 2011; 98:127 - 42; http://dx.doi.org/10.1093/bmb/ldr013; PMID: 21586446
- Kim D, Dressler GR. Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J Am Soc Nephrol 2005; 16:3527 - 34; http://dx.doi.org/10.1681/ASN.2005050544; PMID: 16267156
- Vigneau C, Polgar K, Striker G, Elliott J, Hyink D, Weber O, et al. Mouse embryonic stem cell-derived embryoid bodies generate progenitors that integrate long term into renal proximal tubules in vivo. J Am Soc Nephrol 2007; 18:1709 - 20; http://dx.doi.org/10.1681/ASN.2006101078; PMID: 17475814
- Steenhard BM, Isom KS, Cazcarro P, Dunmore JH, Godwin AR, St John PL, et al. Integration of embryonic stem cells in metanephric kidney organ culture. J Am Soc Nephrol 2005; 16:1623 - 31; http://dx.doi.org/10.1681/ASN.2004070584; PMID: 15872079
- Yamamoto M, Cui L, Johkura K, Asanuma K, Okouchi Y, Ogiwara N, et al. Branching ducts similar to mesonephric ducts or ureteric buds in teratomas originating from mouse embryonic stem cells. Am J Physiol Renal Physiol 2006; 290:F52 - 60; http://dx.doi.org/10.1152/ajprenal.00001.2004; PMID: 16106040
- Fehling HJ, Lacaud G, Kubo A, Kennedy M, Robertson S, Keller G, et al. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development 2003; 130:4217 - 27; http://dx.doi.org/10.1242/dev.00589; PMID: 12874139
- Rosen AB, Kelly DJ, Schuldt AJ, Lu J, Potapova IA, Doronin SV, et al. Finding fluorescent needles in the cardiac haystack: tracking human mesenchymal stem cells labeled with quantum dots for quantitative in vivo three-dimensional fluorescence analysis. Stem Cells 2007; 25:2128 - 38; http://dx.doi.org/10.1634/stemcells.2006-0722; PMID: 17495112
- Rak-Raszewska A, Marcello M, Kenny S, Edgar D, Sée V, Murray P. Quantum dots do not affect the behaviour of mouse embryonic stem cells and kidney stem cells and are suitable for short-term tracking. PLoS One 2012; 7:e32650; http://dx.doi.org/10.1371/journal.pone.0032650; PMID: 22403689
- Lin S, Xie X, Patel MR, Yang YH, Li Z, Cao F, et al. Quantum dot imaging for embryonic stem cells. BMC Biotechnol 2007; 7:67; http://dx.doi.org/10.1186/1472-6750-7-67; PMID: 17925032
- Siegel N, Rosner M, Unbekandt M, Fuchs C, Slabina N, Dolznig H, et al. Contribution of human amniotic fluid stem cells to renal tissue formation depends on mTOR. Hum Mol Genet 2010; 19:3320 - 31; http://dx.doi.org/10.1093/hmg/ddq236; PMID: 20542987
- Unbekandt M, Davies JA. Dissociation of embryonic kidneys followed by reaggregation allows the formation of renal tissues. Kidney Int 2010; 77:407 - 16; http://dx.doi.org/10.1038/ki.2009.482; PMID: 20016472
- Sweet DH, Bush KT, Nigam SK. The organic anion transporter family: from physiology to ontogeny and the clinic. Am J Physiol Renal Physiol 2001; 281:F197 - 205; PMID: 11457711
- Sweet DH, Chan LM, Walden R, Yang XP, Miller DS, Pritchard JB. Organic anion transporter 3 (Slc22a8) is a dicarboxylate exchanger indirectly coupled to the Na+ gradient. Am J Physiol Renal Physiol 2003; 284:F763 - 9; PMID: 12488248
- Sweet DH, Eraly SA, Vaughn DA, Bush KT, Nigam SK. Organic anion and cation transporter expression and function during embryonic kidney development and in organ culture models. Kidney Int 2006; 69:837 - 45; http://dx.doi.org/10.1038/sj.ki.5000170; PMID: 16518343
- El-Sheikh AA, Masereeuw R, Russel FGM. Mechanisms of renal anionic drug transport. Eur J Pharmacol 2008; 585:245 - 55; http://dx.doi.org/10.1016/j.ejphar.2008.02.085; PMID: 18417112
- Wilm B, James RG, Schultheiss TM, Hogan BL. The forkhead genes, Foxc1 and Foxc2, regulate paraxial versus intermediate mesoderm cell fate. Dev Biol 2004; 271:176 - 89; http://dx.doi.org/10.1016/j.ydbio.2004.03.034; PMID: 15196959
- Kume T, Deng K, Hogan BL. Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development 2000; 127:1387 - 95; PMID: 10704385
- Mugford JW, Sipilä P, McMahon JA, McMahon AP. Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev Biol 2008; 324:88 - 98; http://dx.doi.org/10.1016/j.ydbio.2008.09.010; PMID: 18835385
- James RG, Kamei CN, Wang Q, Jiang R, Schultheiss TM. Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development 2006; 133:2995 - 3004; http://dx.doi.org/10.1242/dev.02442; PMID: 16790474
- Self M, Lagutin OV, Bowling B, Hendrix J, Cai Y, Dressler GR, et al. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J 2006; 25:5214 - 28; http://dx.doi.org/10.1038/sj.emboj.7601381; PMID: 17036046
- Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 2008; 3:169 - 81; http://dx.doi.org/10.1016/j.stem.2008.05.020; PMID: 18682239
- Moore AW, McInnes L, Kreidberg J, Hastie ND, Schedl A. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development 1999; 126:1845 - 57; PMID: 10101119
- Shankland SJ, Pippin JW, Reiser J, Mundel P. Podocytes in culture: past, present, and future. Kidney Int 2007; 72:26 - 36; http://dx.doi.org/10.1038/sj.ki.5002291; PMID: 17457377
- Laitinen L, Virtanen I, Saxén L. Changes in the glycosylation pattern during embryonic development of mouse kidney as revealed with lectin conjugates. J Histochem Cytochem 1987; 35:55 - 65; http://dx.doi.org/10.1177/35.1.3794309; PMID: 3794309
- Park JS, Valerius MT, McMahon AP. Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development 2007; 134:2533 - 9; http://dx.doi.org/10.1242/dev.006155; PMID: 17537789
- Qiao J, Uzzo R, Obara-Ishihara T, Degenstein L, Fuchs E, Herzlinger D. FGF-7 modulates ureteric bud growth and nephron number in the developing kidney. Development 1999; 126:547 - 54; PMID: 9876183
- Fujimura S, Jiang Q, Kobayashi C, Nishinakamura R. Notch2 activation in the embryonic kidney depletes nephron progenitors. J Am Soc Nephrol 2010; 21:803 - 10; http://dx.doi.org/10.1681/ASN.2009040353; PMID: 20299358
- Barasch J, Yang J, Ware CB, Taga T, Yoshida K, Erdjument-Bromage H, et al. Mesenchymal to epithelial conversion in rat metanephros is induced by LIF. Cell 1999; 99:377 - 86; http://dx.doi.org/10.1016/S0092-8674(00)81524-X; PMID: 10571180
- Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 1988; 336:684 - 7; http://dx.doi.org/10.1038/336684a0; PMID: 3143916
- Hamada H, Sato M, Murata F, Muramatsu T. Differential expression of lectin receptors in germ layers of the mouse egg cylinder and teratocarcinomas. Exp Cell Res 1983; 144:489 - 95; http://dx.doi.org/10.1016/0014-4827(83)90430-5; PMID: 6840224
- Jones TD, Ulbright TM, Eble JN, Baldridge LA, Cheng L. OCT4 staining in testicular tumors: a sensitive and specific marker for seminoma and embryonal carcinoma. Am J Surg Pathol 2004; 28:935 - 40; http://dx.doi.org/10.1097/00000478-200407000-00014; PMID: 15223965
- Karoubi G, Gugger M, Schmid R, Dutly A. OCT4 expression in human non-small cell lung cancer: implications for therapeutic intervention. Interact Cardiovasc Thorac Surg 2009; 8:393 - 7; http://dx.doi.org/10.1510/icvts.2008.193995; PMID: 19126554
- Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 2002; 416:542 - 5; http://dx.doi.org/10.1038/nature730; PMID: 11932747
- Kuzma-Kuzniarska M, Rak-Raszewska A, Kenny S, Edgar D, Wilm B, Fuente Mora C, et al. Integration potential of mouse and human bone marrow-derived mesenchymal stem cells. Differentiation 2012; 83:128 - 37; http://dx.doi.org/10.1016/j.diff.2011.11.004; PMID: 22364880
- Dekel B, Shezen E, Even-Tov-Friedman S, Katchman H, Margalit R, Nagler A, et al. Transplantation of human hematopoietic stem cells into ischemic and growing kidneys suggests a role in vasculogenesis but not tubulogenesis. Stem Cells 2006; 24:1185 - 93; http://dx.doi.org/10.1634/stemcells.2005-0265; PMID: 16410390
- Wolf LV, Yang Y, Wang J, Xie Q, Braunger B, Tamm ER, et al. Identification of pax6-dependent gene regulatory networks in the mouse lens. PLoS One 2009; 4:e4159; http://dx.doi.org/10.1371/journal.pone.0004159; PMID: 19132093
- Sims-Lucas S, Young RJ, Martinez G, Taylor D, Grimmond SM, Teasdale R, et al. Redirection of renal mesenchyme to stromal and chondrocytic fates in the presence of TGF-beta2. Differentiation 2010; 79:272 - 84; http://dx.doi.org/10.1016/j.diff.2010.01.004; PMID: 20163909
- Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, et al. Development of definitive endoderm from embryonic stem cells in culture. Development 2004; 131:1651 - 62; http://dx.doi.org/10.1242/dev.01044; PMID: 14998924
- Loughna S, Yuan H-T, Woolf AS. Effects of oxygen on vascular patterning in Tie1/LacZ metanephric kidneys in vitro. Biochem Biophys Res Commun 1998; 247:361 - 6; http://dx.doi.org/10.1006/bbrc.1998.8768; PMID: 9642132
- Rosines E, Sampogna RV, Johkura K, Vaughn DA, Choi Y, Sakurai H, et al. Staged in vitro reconstitution and implantation of engineered rat kidney tissue. Proc Natl Acad Sci U S A 2007; 104:20938 - 43; http://dx.doi.org/10.1073/pnas.0710428105; PMID: 18087037
- Sorokin LM, Pausch F, Durbeej M, Ekblom P. Differential expression of five laminin alpha (1-5) chains in developing and adult mouse kidney. Dev Dyn 1997; 210:446 - 62; http://dx.doi.org/10.1002/(SICI)1097-0177(199712)210:4<446::AID-AJA8>3.0.CO;2-G; PMID: 9415429
- Davies J. Control of calbindin-D28K expression in developing mouse kidney. Dev Dyn 1994; 199:45 - 51; http://dx.doi.org/10.1002/aja.1001990105; PMID: 8167378