Abstract
Metabolomics is one of the relative newcomers of the omics techniques and is likely the one most closely related to actual real-time disease pathophysiology. Hence, it has the power to yield not only specific biomarkers but also insight into the pathophysiology of disease. Despite this power, metabolomics as applied to kidney disease is still in its early adolescence and has not yet reached the mature stage of clinical application, i.e., specific biomarker and therapeutic target discovery. On the other hand, the insight gained from hints into what makes these diseases tick, as is evident from the metabolomics pathways which have been found to be altered in kidney cancer, are now beginning to bear fruit in leading to potential therapeutic targets. It is quite likely that, with greater numbers of clinical materials and with more investigators jumping into the field, metabolomics may well change the course of kidney disease research.
Introduction
The inventory of useful biomarkers for kidney disease, despite many person-years of investigation in the field, remains inadequate. From the original serum markers of renal function, BUN and creatinine, to the more recent (and vastly improved) acute kidney injury (AKI) markers such as neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), IL-18, cystatin C, and N-acetyl-β-d-glucosaminidase (NAG) (reviewed inCitation1), there is a paucity of specific markers for specific kidney diseases. Rather than separately investigating each of a number of potential marker proteins or small molecules, from any number of independent laboratories (each with a low likelihood for success), it is now possible to take an omics approach and to evaluate whole biomarker “space.” Of the omics disciplines used at present, the newest and probably the best applicable for this job is metabolomics. For reasons that will be discussed below, metabolomics is ideally suited for biomarker, as well as for therapeutic target, discovery; the fact that kidney researchers have that special fluid, urine, which is in intimate association with our favorite organ and contains many of its metabolites, increases the utility of metabolomics in kidney disease research.
Why Metabolomics?
Loosely defined, metabolomics is “the non-targeted measurement of all of the low-molecular-weight compounds, including both endogenous and exogenous, which appear in a particular solid tissue or biofluid in a living organism.” Of course of the 8,000 known metabolites, any given experiment will only measure a fraction of these due to the problems of low abundance and ambiguous structural determination. While some authors restrict this set to endogenous metabolites, it is clear that the handling of exogenous materials (such as medications and body flora) is as much a critical component of an organism’s physiology or pathophysiology as its normal endogenous metabolic output. Metabolomics can be further divided into global (or non-targeted as described above) or targeted analysis, the former being an entirely blind approach to evaluation of metabolites, and the latter utilizing known metabolites (once the involved pathway(s) are elucidated). Even more stratification can be done to utilize “fast-lane” (i.e., pattern identification of chromatograph peaks without metabolite identification) and “slow-lane” (chemical identification of all significant peaks) analyses.Citation2
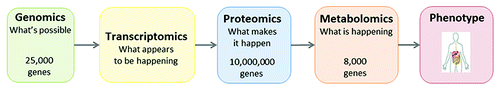
Why is metabolomics likely superior to other omics for clinical translation? While transcriptomics and genomics measure the blueprint for the organism’s metabolism, and proteomics measures the means by which these orders are performed, metabolomics represents the real-time and most distal processes occurring in the living organism. For example, not all genes are transcribed and those that are can undergo post-translational modification such that the levels of the genes may have no relevance to their conjugate protein levels. Furthermore, many proteins, especially those involved in intermediary metabolism, are enzymes and here too the levels can be irrelevant to activity; in addition, there are far fewer metabolites than genes and proteins which makes the metabolomics-associated statistical analysis easier (). In nephrology, it so happens that we have been regularly using metabolomics since the middle ages: whenever medieval physician tasted urine for sweetness (mellitus means “sweetened with honey”), they were using targeted metabolomics for glucose.Citation3 To this day, we use targeted metabolomics whenever we use a urinary dipstick at the bedside or clinical laboratory. More recently, yet prior to its current appellation, metabolomics-type evaluations of both plasma and urine have been used for diagnosing inborn errors of metabolism.Citation4-Citation7
Figure 1. Metabolites are most closely related to phenotype. Metabolomics is most closely related to phenotype. In addition, there are many fewer extant metabolites than genes, proteins, and transcripts which considerably simplify data acquisition and analysis.
So, measuring levels of metabolites in a given biofluid or tissue is a direct measure of the metabolism, and hence the life-sustaining or disease-promoting process, which are occurring in real-time. Thus, the systematic identification of the changes in as many metabolites as possible that can be measured is a goldmine of information which can lead to considerable insight into pathogenesis of disease.
Techniques Of Metabolomics Analysis
Analytical technologies
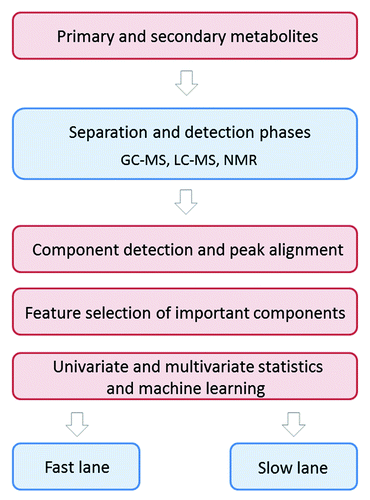
The analytical technologies used in metabolomics are quite complex (way beyond the scope of this talk) but are based on a simple premise: one needs to employ a chemical separation phase followed by a detection phase. We have called the non-identified metabolite model (if you are just asking “Is the patient healthy or sick?”) the “fast lane” analysis; this model contains only a separation phase. To evaluate actual causation and the important biochemical pathways involved in the disease, one has to perform structural determination of the metabolites which requires both separation phase and detection phase (if you are asking “Why is the patient sick?”), and this we have named the “slow lane” modelCitation2 ().
Figure 2. Comprehensive small molecule biomarker detection in urine. Algorithm describing the metabolomic approaches for biomarker identification using complementary analytical techniques covering the whole metabolome or small molecule space. GC-MS: gas chromatography–mass spectrometry, LC-MS: liquid chromatography–mass spectrometry, NMR: nuclear magnetic resonance
The separation phase is generally performed by gas chromatography (GC), high-performance liquid chromatography (HPLC), or capillary electrophoresis (CE). Compared with GC, HPLC has lower chromatographic resolution, but it does have the advantage that a much wider range of analytes can potentially be measured.Citation8 The detection phase is usually accomplished using mass spectrometry (MS) or nuclear magnetic resonance (NMR), each technique with its specific advantages. The main advantages of NMR over MS are high analytical reproducibility and simplicity of sample preparation as well as the derivation of more structural information,Citation9 whereas its principal disadvantage is that it is relatively insensitive.Citation10 Coupling MS with liquid chromatography (LC) or GC enables the measurement of hundreds of individual metabolites in a single sample.Citation10
Statistical methods
The statistical methods used in metabolomics are complex and beyond the scope of what I can speak about today; please seeCitation11 for a review. Briefly, metabolite profiles generally undergo some preprocessing prior to more advanced statistical modeling, to account for spectral artifacts, and to render profiles from different samples more comparable. The first step of processing of mass spectrometry data typically consists of baseline correction, filtering or decomposition, and signal extraction (peak detection and quantification),Citation12 and the final step is normalization. Sample concentrations are often adjusted by using information from the biological context including creatinine concentration or osmolality at the time of sampling. Another way for normalization is to determine a samplewise bias factor from the data themselves.Citation13 It is not uncommon to attempt to find affected pathways from metabolic profiles when searching for drug targets, and for biomarkers studies, it can be extremely rare to identify a single metabolite that will prove clinically viable, such that multiplexing is often required. The statistical techniques for the latter technique are manifold and beyond the scope of this paper.
Applications Of Metabolomics To Kidney Disease
While metabolomics has to date not yielded any clinically useful biomarkers in nephrology, there have been several metabolomics studies in various kidney diseases (). In this section, we discuss metabolomic findings in those diseases with special emphasis on autosomal dominant polycystic kidney disease (ADPKD) and renal cell carcinoma (RCC), the diseases which we have been studying in the laboratory.
Table 1. Kidney diseases to which metabolomics have been applied
ADPKD
ADPKD is the leading hereditary cause of end-stage renal disease in the US. Its diagnosis is generally made by imaging techniques mainly because there are no extant biomarkers. This is unfortunate, because early diagnosis of ADPKD in at-risk patients might lead to interventions (some in the pipeline) that can slow its progression. Our laboratory has utilized the Jck cystic mouse model (which is no longer the ideal model for ADPKD) in a metabolic investigation of potential urinary biomarkers of cystic kidney diseaseCitation14; we showed that the cystic mouse can be discriminated from its wild-type counterpart by urine analysis alone. At day 26 of life, before there is serological evidence of kidney dysfunction, affected mice are distinguishable by urine metabolomic analysis; this finding persists through 45 d until 64 d, at which time body weight differences confound the results. Several biologically relevant metabolic pathways which are altered very early in this disease were identified and could be studied further. The most highly represented altered pathways include both purine and galactose metabolism pathways. Furthermore, several specific candidate biomarkers, including allantoic acid and adenosine, which are augmented in the urine of young cystic mice were determined. These markers and pathway components, once validated in homologous mouse models and extended to human disease, may prove useful as a noninvasive means of diagnosing cystic kidney diseases and to suggest novel therapeutic approaches.
RCC
RCC is often incidentally diagnosed by nephrologists by imaging procedures while assessing a patient for AKI or hematuria. Since under these circumstances, the tumor is often metastatic. Since progression free survival of patients treated with currently available therapeutics for metastatic RCC is only one to two years, it is essential to find a biomarker to detect this disease at its early stage.Citation15 We performed a pilot “fast track” study using several complementary separation techniques (which cover most of the metabolome) and showed that urine analysis can separate patients with early-stage RCC from control patients who do not have RCC.Citation2 An extension of that study demonstrated significant changes in quinolinate, 4-hydroxybenzoate, and gentisate (at a false discovery rate of 0.26),Citation16 an interesting finding because these metabolites are involved in common pathways of specific amino acid and energetic metabolism, consistent with high tumor protein breakdown and utilization, and the Warburg effect.Citation17 Furthermore, grade dependent analysis of urine metabolites showed differential urinary concentrations of several acylcarnitines as a function of both cancer status and kidney cancer grade, with most acylcarnitines being increased in the urine of cancer patients and in those patients with high cancer grades,Citation18 suggesting involvement of fatty acid oxidation in RCC energy metabolism. In addition to biomarker studies, a human RCC to mouse xenograft metabolomics study of urine, blood, and malignant tissues resulted in identification of several novel therapeutic targets by showing that there exists alteration of metabolites in tryptophan, peroxisome proliferator-activated receptor α (PPARα), and fatty acid oxidation pathwaysCitation17; these targets are currently being validated in our laboratory with initially promising results.
Metabolomics studies in other kidney diseases
One of the earliest metabolomics studies to be undertaken in kidney disease found urinary glucose, lactate, amino acids, citric acid cycle intermediates as potential biomarkers for AKI.Citation19 This study was followed by two studies that found glycolysis and citric acid cycle intermediates.Citation20 and that found urinary amino acids and polyaminesCitation21 as potential biomarkers for AKI However, none of these studies has resulted in new specific markers of this disease.
One of the most common forms of chronic kidney disease (CKD) worldwide is diabetic nephropathy, so naturally the technique of metabolomics has been applied to evaluate potential metabolite markers of this disease. Potential biomarkers for this disease have been reported by multiple studies.Citation22-Citation24 Several energy pathway related metabolites including fatty acids and citrate cycle intermediates for diabetes mellitus nephropathyCitation22,Citation23 and urinary acetate, lactate, allantoin and trimethylamine for diabetes insipidusCitation25 have been introduced as potential biomarkers.
While the biochemical basis of the uremic syndrome has been studied extensively, it is still unclear which of the compounds that are retained in the blood as a result of kidney failure actually cause the uremic syndrome and its complications; however it is becoming increasingly clear that the signs of symptoms of uremia will entail a suite of altered metabolites, rather than a single metabolite or small number of such. Metabolomics is an ideal technique for studying this syndrome, as it is quite probable that most, if not all, of the uremic toxins are actually fairly small (< 1 kD) metabolites that are well within the scope of global metabolomics. One study detected uremic toxins, indoxyl sulfate, phenyl sulfate, hippuric acid, and p‐cresyl sulfateCitation26 and the other study found 52 metabolites increased as estimated glomerular filtration rate (eGFR) declined.Citation27 However, both studies did not validate specificity and pathophysiology, much less causality, of these metabolites.
Systemic lupus erythematosus (SLE or lupus) is a chronic autoimmune disease, and kidney involvement with SLE, a.k.a. lupus nephritis (LN), is a frequent and severe complication of SLE that results in significant patient morbidity and mortality. A NMR metabolic profiling study identified urinary metabolites that discriminated between proliferative and pure membranous LN as defined by the ISN/RPS classification, and between LN and primary focal segmental glomerulosclerosis (FSGS).Citation28 This study showed that class V LN patients had normal urinary hippurate levels compared with FSGS patients, who completely lacked urinary hippurate, indicating differences in urinary metabolites between LN and FSGS. Nevertheless, it does not appear that metabolomics will be replacing the all-important kidney biopsy anytime soon for LN.
IgA nephropathy is the most common cause of chronic renal failure among primary glomerulonephritis patients. The ability to diagnose immunoglobulin A nephropathy is hampered by the nonspecificity of its presentation and the lack of any biofluid markers (other than hematuria). In a quest to change this landscape, a serum metabolomics study showed higher levels of phenylalanine, myo-Inositol, lactate, L6 lipids, L5 lipids, and L3 lipids, as well as lower levels of β-glucose, α-glucose, valine, tyrosine, phosphocholine, lysine, isoleucine, glycerolphosphocholine, glycine, glutamine, glutamate, alanine, acetate, 3-hydroxybutyrate, and 1-methylhistidine in IgA nephropathy low and high risk patients compared with the healthy controls.Citation29 However, there was no distinct difference between low and high risk patients.
Application of Metabolomics to the Clinic
In the practice of metabolomics, one can far too easily get bogged down in the technical details and statistical minutiae and miss the beauty and power as well as the abundant potential clinical application of this endeavor. Due to the fact that metabolomics measures the actual processes occurring in the body, which includes the somatic reaction to the presence of the disease, it can be employed to uncover both markers of disease (i.e., individual or multiplexed metabolites evaluated without the knowledge of their role in the disease) and potential therapeutic targets (i.e., pathways imputed from chemically identified altered metabolites).
Discovery and validation of biomarkers
The process of biomarker discovery using metabolomics begins with a very well designed experimental plan which most importantly must include appropriate controls for the disease of interest. For example, in the case of biomarkers, one would need to decide whether to utilize controls which have a different kind of cancer vs. all non-cancer controls; these considerations will stratify biomarkers into those for patients with a cancer type-specific biomarker in contrast to a marker for any cancer in general. In addition, if patient materials are obtained from, for example, a nephrology clinic, it is optimal if the controls without the disease of interest are taken from the same clinic population as opposed to “healthy” people in general. As in all biomarker studies, the likelihood of finding a true marker for the general population is exceedingly low; it is more likely that biofluid markers will be most useful for an at-risk population. In the case of kidney disease, for example, a biomarker for IgA nephropathy will be much more useful for that subgroup of patients with hematuria; and in the case of cancer, a marker of kidney cancer will be more useful in elderly African-American men or for evaluating for recurrence in those who have already had the disease.
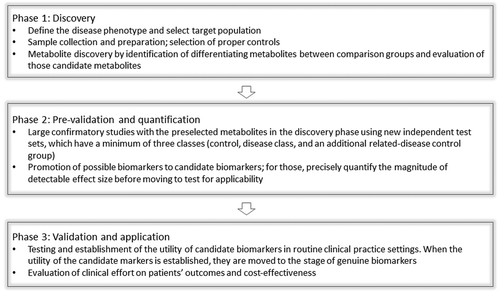
Regardless of the disease and analytical method employed in biomarker discovery, statistical validation is of critical importance. A prediction model needs to be created, whose performance is often described in terms of sensitivity and specificity. This classification entails an internal evaluation at the very least by cross-validation (randomly split the available data into a training set for model construction and a test set(s) for assessing predictive performance) to make sure the model is not over fitted. More optimally, the prediction models should be validated using independent validation data sets with a minimum of three classes: healthy control, disease group, and an additional related-disease control group as described above. Furthermore, for the differentiating metabolites to be clinically applicable as diagnostic biomarkers, such metabolites need to be tested for the predictive ability on samples which are blinded to the investigators (). A full statistical discussion as applied to metabolomics analysis can be found in.Citation11
From biomarkers to therapeutic targets
A unique strength of metabolomics, perhaps considerably more so than other omics, is that this technique allows for “going backwards” by using biomarkers to intuit therapeutic targets. This is done in the surprisingly logical fashion of identifying altered metabolic pathways from the significantly changed metabolites using commercially available software, and from this data finding targets in the form of enzymes or other druggable mediators which allow mediate the pathway of interest and allow it to progress. For example, if the citric cycle is highly represented in a disease as manifested by several metabolites within it (and which ideally appear in an accessible biofluid) changing in the same general direction, then the enzymes which comprise this pathway like fumarase, isocitrate dehydrogenase (IDH), etc., (and which are responsible for generating the metabolites of interest) can be targeted if inhibitors exist or can be synthesized. We have pioneered this strategy and have had remarkable early success in kidney cancer, starting the simple premise that changes which occur concurrently in the tissue, serum, and urine metabolomes reflect a highly activated pathway in the cancer itself.Citation17
As far as kidney disease is concerned, a similar approach can indeed be taken as long as the particular renal disease is characterized by a known or discovered metabolic abnormality, which of course includes the vast majority of kidney disease. This is evident for example, because the finding that kidney disease is associated with hypertension and/or accelerated atherosclerosis suggests that there is an altered metabolic process underlying this process. In addition, diseases such as lupus and diabetes can be associated with the aptly named “metabolic syndrome” which is a suite of metabolic changes leading to complications of these diseases.
Validation of therapeutic targets
Given the very high bar of any metabolite or suite of metabolites to reach in order to become a useful biomarker (witness the sad dearth of available such markers in cancer despite much funding and investigation), the full power of metabolomics will more likely be realized by the utility of this technique to identify new targets. Validating such targets should take place both at the bench and in vivo, as well as (ultimately) at the bedside.
Bench validation it is generally quite straightforward and is initially performed in tissue culture. This process entails the purchase or synthesis of the metabolite(s) of interest and then simply evaluating whether it has the observed effect in cell or tissue culture. For example, if a candidate metabolite is found to be increased in a proliferative disease of interest, then one would expect that, if it represents a true target, it would increase the proliferation of appropriate cells in culture.Citation16
As an independent means of validation, one can use an in vivo approach with xenograft or allograft mice, for cancer, or appropriate renal disease animal models. Once a specific pathway found is found from the metabolomic data set to be activated, then small molecule, orphan drug, or approved drug inhibitors can be identified and administered to the animal to determine whether it attenuates the disease. Even if the identified altered metabolic pathways do not meet the strictest statistical criteria for significance, it is reasonable to test inhibitors of such pathway, if available, in animal models.
Summary
The technique of metabolomics has enormous potential in clinical medicine, especially in nephrology, due to the fact that it measures the distal-most changes in the biochemical basis of organismal biology. The field of metabolomics as applied to kidney disease has resulted in a plethora of published studies leading to new and potentially clinical useful metabolite markers. At this stage of research, however, most of these metabolites are without appropriate validation. With further investigation, the metabolomics studies of kidney disease have the capacity to direct further research into renal disorders which are in serious need of biomarkers and insight into their pathogenesis: only when this happens will the full power of metabolomics in kidney disease research be unleashed.
Jeffrey Miner, PH.D., Professor of Medicine, Washington University School of Medicine
In the jck mouse data you showed, for the first two time points there were differences in the metabolites, and then all of the sudden at the third time point, when you would think the cysts would be getting bigger, there were no more differences.
Robert H. Weiss, MD, Professor of Medicine, University of California Davis
Right, but look at the renal function. As was evidenced by the high BUN of 70,these guys had end stage renal disease, so since we are looking at urine, it is likely that renal filtration of metabolites was compromised in these animals and nothing was coming out into the urine.
Moe Mahjoub, Ph.D., Assistant Professor of Medicine, Washington University School of Medicine
I don’t know if you touched on this but did you see any overlap in signature, from the RCC’s to the JCK to PKD? Are there things that are shared among those, can you just tell whether the kidney is messed up in general using certain things?
Robert H. Weiss, M.D
You know I am sure there are metabolites that would overlap between human RCC and the mouse jck PKD model, but we have actually have not looked at that yet .The reason for this is that I think the JCK is so different from human RCC, but you are correct that it would be interesting to compare all of our metabolomics studies. We are in the process of setting up a human ADPKD metabolomics biomarker study with Arlene Chapman at Emory University, in which we are looking at urine and blood to see if some of the altered metabolites and pathways are similar to the changes in cancer. I am quite sure that we will find an intersection here because these two diseases use a lot of the same biochemical pathways, such as proliferative and apoptotic pathways.
Marc Hammerman, MD Chromalloy Professor of Renal Diseases in Medicine Washington University School of Medicine
I find it very interesting that you referred to PKD as a non-metastasizing tumor. What do you think PKD might have in common with other ‘non-metastasizing tumors’ and why doesn’t PKD metastasize?
Robert H. Weiss, M.D
I think you need to go to Kansas down the street. Dr. Grantham at the University of Kansas wrote an article about that 20 y ago.Citation31 He brought that up a long time ago before we knew what we know now. And now we think that PKD is a 2 hit model similar to cancer where you get loss of heterozygosity. That helps explain why there are discrete cysts along the tubule, because prior to that concept, the real question was why is the whole kidney is not one giant cyst? As it turns out, each cyst is a monoclonal proliferation of epithelial cells due to a second mutation. So you have the germ line mutation and wherever you get that second hit in a polycystin is where you get that cyst growth. So that is why I tell patients, don’t be an airline pilot if you have PKD and it is theoretically a good idea to take antioxidants like vitamin C, to try to avoid excessive mutagens and reactive oxygen species. . I’m a nephrologist who does cancer research, and we got interested in PKD research mainly because of its similarity to cancer; this turned out to be a good idea.
Vikas R. Dharnidharka, MD, M, P.H., Associate Professor of Pediatrics, Director, Pediatric Nephrology Washington University School of Medicine
My question might be perhaps even a little more basic but when you talk about the protein family having 10 million units, then you are condensing down to 8000 metabolites, and those are a very small molecular weight. So are the higher molecular weight compounds not of significance?
Robert H. Weiss, M.D
The higher molecular weight compounds would first of all probably not be filtered into the urine, but second, you are probably talking about proteomics rather than metabolomics. We consider di- or tri-peptides as metabolites but larger such molecules are true proteins and would be studied by the field of proteomics. Thus, these compounds would indeed have significance but aren’t evaluated by metabolomics techniques. .
| Abbreviations: | ||
| AKI | = | acute kidney injury |
| NGAL | = | neutrophil gelatinase-associated lipocalin |
| KIM-1 | = | kidney injury molecule-1 |
| NAG | = | N-acetyl-β-d-glucosaminidase |
| GC | = | gas chromatography |
| HPLC | = | high-performance liquid chromatography |
| CE | = | capillary electrophoresis |
| MS | = | mass spectrometry |
| NMR | = | nuclear magnetic resonance |
| LC | = | liquid chromatography |
| ADPKD | = | autosomal dominant polycystic kidney disease, RCC, renal cell carcinoma |
| PPARα | = | peroxisome proliferator-activated receptor alpha |
| CKD | = | chronic kidney disease |
| eGFR | = | estimated glomerular filtration rate |
| SLE | = | systemic lupus erythematosus |
| LN | = | lupus nephritis |
| FSGS | = | focal segmental glomerulosclerosis |
| IDH | = | isocitrate dehydrogenase |
Acknowledgments
This work was supported by NIH grants 5UO1CA86402 (Early Detection Research Network), 1R01CA135401–01A1, and 1R01DK082690–01A1 (to R.H.W.), and the Medical Service of the US Department of Veterans’ Affairs (R.H.W.).
Note
Edited transcripts of research conferences sponsored by Organogenesis and the Washington University George M. O’Brien Center for Kidney Disease Research (P30 DK079333) are published in Organogenesis. These conferences cover organogenesis in all multicellular organisms including research into tissue engineering, artificial organs and organ substitutes and are participated in by faculty at Washington University School of Medicine, St. Louis Missouri USA.
References
- Siew ED, Ware LB, Ikizler TA. Biological markers of acute kidney injury. J Am Soc Nephrol 2011; 22:810 - 20; http://dx.doi.org/10.1681/ASN.2010080796; PMID: 21493774
- Kind T, Tolstikov V, Fiehn O, Weiss RH. A comprehensive urinary metabolomic approach for identifying kidney cancerr. Anal Biochem 2007; 363:185 - 95; http://dx.doi.org/10.1016/j.ab.2007.01.028; PMID: 17316536
- Nicholson JK, Lindon JC. Systems biology: Metabonomics. Nature 2008; 455:1054 - 6; http://dx.doi.org/10.1038/4551054a; PMID: 18948945
- Jellum E, Stokke O, Eldjarn L. Application of gas chromatography, mass spectrometry, and computer methods in clinical biochemistry. Anal Chem 1973; 46:1099 - 106; PMID: 4201587
- Shoemaker JD, Elliott WH. Automated screening of urine samples for carbohydrates, organic and amino acids after treatment with urease. J Chromatogr 1991; 562:125 - 38; http://dx.doi.org/10.1016/0378-4347(91)80571-S; PMID: 2026685
- Kuhara T, Shinka T, Inoue Y, Ohse M, Zhen-wei X, Yoshida I, et al. Pilot study of gas chromatographic-mass spectrometric screening of newborn urine for inborn errors of metabolism after treatment with urease. J Chromatogr B Biomed Sci Appl 1999; 731:141 - 7; http://dx.doi.org/10.1016/S0378-4347(99)00205-4; PMID: 10492000
- Kuhara T. Gas chromatographic-mass spectrometric urinary metabolome analysis to study mutations of inborn errors of metabolism. Mass Spectrom Rev 2005; 24:814 - 27; http://dx.doi.org/10.1002/mas.20038; PMID: 15376278
- Gika HG, Theodoridis GA, Wingate JE, Wilson ID. Within-day reproducibility of an HPLC-MS-based method for metabonomic analysis: application to human urine. J Proteome Res 2007; 6:3291 - 303; http://dx.doi.org/10.1021/pr070183p; PMID: 17625818
- Reo NV. NMR-based metabolomics. Drug Chem Toxicol 2002; 25:375 - 82; http://dx.doi.org/10.1081/DCT-120014789; PMID: 12378948
- Veenstra TD. Metabolomics: the final frontier?. Genome Med 2012; 4:40; http://dx.doi.org/10.1186/gm339; PMID: 22546050
- Weiss RH, Kim K. Metabolomics in the study of kidney diseases. Nat Rev Nephrol 2012; 8:22 - 33; http://dx.doi.org/10.1038/nrneph.2011.152; PMID: 22025087
- Griffiths WJ, Koal T, Wang Y, Kohl M, Enot DP, Deigner HP. Targeted metabolomics for biomarker discovery. Angew Chem Int Ed Engl 2010; 49:5426 - 45; http://dx.doi.org/10.1002/anie.200905579; PMID: 20629054
- Vivó-Truyols G, Torres-Lapasió JR, van Nederkassel AM, Vander Heyden Y, Massart DL. Automatic program for peak detection and deconvolution of multi-overlapped chromatographic signals part I: peak detection. J Chromatogr A 2005; 1096:133 - 45; http://dx.doi.org/10.1016/j.chroma.2005.03.092; PMID: 16301076
- Taylor SL, Ganti S, Bukanov NO, Chapman A, Fiehn O, Osier M, et al. A metabolomics approach using juvenile cystic mice to identify urinary biomarkers and altered pathways in polycystic kidney disease. Am J Physiol Renal Physiol 2010; 298:F909 - 22; http://dx.doi.org/10.1152/ajprenal.00722.2009; PMID: 20130118
- Belldegrun AS, Klatte T, Shuch B, LaRochelle JC, Miller DC, Said JW, et al. Cancer-specific survival outcomes among patients treated during the cytokine era of kidney cancer (1989-2005): a benchmark for emerging targeted cancer therapies. Cancer 2008; 113:2457 - 63; http://dx.doi.org/10.1002/cncr.23851; PMID: 18823034
- Kim K, Taylor SL, Ganti S, Guo L, Osier MV, Weiss RH. Urine metabolomic analysis identifies potential biomarkers and pathogenic pathways in kidney cancer. OMICS 2011; 15:293 - 303; http://dx.doi.org/10.1089/omi.2010.0094; PMID: 21348635
- Ganti S, Taylor SL, Abu Aboud O, Yang J, Evans C, Osier MV, et al. Kidney tumor biomarkers revealed by simultaneous multiple matrix metabolomics analysis. Cancer Res 2012; 72:3471 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-11-3105; PMID: 22628425
- Ganti S, Taylor SL, Kim K, Hoppel CL, Guo L, Yang J, et al. Urinary acylcarnitines are altered in human kidney cancer. Int J Cancer 2012; 130:2791 - 800; http://dx.doi.org/10.1002/ijc.26274; PMID: 21732340
- Melnick JZ, Baum M, Thompson JR. Aminoglycoside-induced Fanconi’s syndrome. Am J Kidney Dis 1994; 23:118 - 22; PMID: 8285185
- Boudonck KJ, Mitchell MW, Német L, Keresztes L, Nyska A, Shinar D, et al. Discovery of metabolomics biomarkers for early detection of nephrotoxicity. Toxicol Pathol 2009; 37:280 - 92; http://dx.doi.org/10.1177/0192623309332992; PMID: 19380839
- Xu EY, Perlina A, Vu H, Troth SP, Brennan RJ, Aslamkhan AG, et al. Integrated pathway analysis of rat urine metabolic profiles and kidney transcriptomic profiles to elucidate the systems toxicology of model nephrotoxicants. Chem Res Toxicol 2008; 21:1548 - 61; http://dx.doi.org/10.1021/tx800061w; PMID: 18656965
- Suhre K, Meisinger C, Doring A, Altmaier E, Belcredi P, Gieger C, et al. Hrabe de, Angelis M., Wichmann, H. E., Kronenberg, F., Adamski, J., and Illig, T.,Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting. PLoS ONE 2010; 5:e13953; http://dx.doi.org/10.1371/journal.pone.0013953
- Zhao X, Fritsche J, Wang J, Chen J, Rittig K, Schmitt-Kopplin P, et al. Metabonomic fingerprints of fasting plasma and spot urine reveal human pre-diabetic metabolic traits. Metabolomics 2010; 6:362 - 74; http://dx.doi.org/10.1007/s11306-010-0203-1; PMID: 20676218
- Connor SC, Hansen MK, Corner A, Smith RF, Ryan TE. Integration of metabolomics and transcriptomics data to aid biomarker discovery in type 2 diabetes. Mol Biosyst 2010; 6:909 - 21; http://dx.doi.org/10.1039/b914182k; PMID: 20567778
- Mahadevan S, Shah SL, Marrie TJ, Slupsky CM. Analysis of metabolomic data using support vector machines. Anal Chem 2008; 80:7562 - 70; http://dx.doi.org/10.1021/ac800954c; PMID: 18767870
- Kikuchi K, Itoh Y, Tateoka R, Ezawa A, Murakami K, Niwa T. Metabolomic analysis of uremic toxins by liquid chromatography/electrospray ionization-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2010; 878:1662 - 8; http://dx.doi.org/10.1016/j.jchromb.2009.11.040; PMID: 20036201
- Toyohara T, Akiyama Y, Suzuki T, Takeuchi Y, Mishima E, Tanemoto M, et al. Metabolomic profiling of uremic solutes in CKD patients. Hypertens Res 2010; 33:944 - 52; http://dx.doi.org/10.1038/hr.2010.113; PMID: 20613759
- Romick-Rosendale LE, Brunner HI, Bennett MR, Mina R, Nelson S, Petri M, et al. Identification of urinary metabolites that distinguish membranous lupus nephritis from proliferative lupus nephritis and focal segmental glomerulosclerosis. Arthritis Res Ther 2011; 13:R199; http://dx.doi.org/10.1186/ar3530; PMID: 22152586
- Sui W, Li L, Che W, Guimai Z, Chen J, Li W, et al. A proton nuclear magnetic resonance-based metabonomics study of metabolic profiling in immunoglobulin a nephropathy. Clinics (Sao Paulo) 2012; 67:363 - 73; http://dx.doi.org/10.6061/clinics/2012(04)10; PMID: 22522762
- Catchpole G, Platzer A, Weikert C, Kempkensteffen C, Johannsen M, Krause H, et al. Metabolic profiling reveals key metabolic features of renal cell carcinoma. J Cell Mol Med 2011; 15:109 - 18; http://dx.doi.org/10.1111/j.1582-4934.2009.00939.x; PMID: 19845817
- Grantham JJ. Polycystic kidney disease: neoplasia in disguise. Am J Kidney Dis 1990; 15:110 - 6; PMID: 2405652