Abstract
Stem cells reside in stem cells niches, which maintain the balance of self-renewal and differentiation of stem cells. In stem cell niches, cell-cell, cell-extracellular matrix interactions and diffusible signals are important elements. However, another pivotal element is that localized and diffusible signals are all organized as three-dimensional (3-D) structures, which is easily neglected by in vitro cell biology research. Under 3-D culture conditions, the morphology of cells exhibited differently from cultured in traditional two-dimensional (2-D) conditions. Under 3-D culture conditions, the self-renewal and pluripotency of neural stem cells (NSCs) and bone marrow mesenchymal stem cells (BMSCs) were enhanced compared with culturing under 2-D conditions. 3-D cultures could change the transcriptional profile of NSCs compared with 2-D cultures. We hypothesized that 3-D cultures could reprogram mature cells such as fibroblasts to an immature state, like the pluripotent stem cells. The primary results indicated that several ES marker genes were upregulated by 3-D cultures. Though further experiments are needed, this work may provide a method of reprogramming mature cells without gene modifications.
Stem cells, a family of multipotential cells, can differentiate into diverse specialized cell types to build up a tissue or an organ. At the same time, stem cells maintain their multipotential stemness by symmetric divisions. In general, the stemness of stem cells was maintained by stem cell niches. The important principles and common elements of stem cell niches include cell-cell, cell-extracellular matrix (ECM) interactions and diffusible signaling factors,Citation1 which are organized in a three-dimensional (3-D) structure. For decades, several diffusible or localized signaling factors have been identified and characterized. However, under the traditional two-dimensional (2-D) ex situ culture conditions, the responses of stem cells to these factors cannot emulate the in vivo cell patterning or developmental potential.Citation2,Citation3 The stem cell niches decided the fate of stem cells in a proper spatiotemporal manner.Citation4 Thus, the 3-D growing structure could be one of important parameters regulating homeostasis of differentiation and self-renewal of stem cells. Compared with the traditional ex situ 2-D culture, the cell morphology was different from its shape in situ. Cultured on 2-D substrates fibroblasts exhibited stress fibers or wound repair response.Citation5 An in vitro 3-D culture system could provide 3-D substrates similar to in vivo environments. Cells cultured in 3-D matrices would undergo a more complex physical environment and strikingly different geometry than cells culture in 2-D.Citation6 Our previous work showed that neural stem cells (NSCs) cultured in collagen scaffolds aggregated with each other, however, on 2-D dishes, NSCs spread their processes all over the dish.Citation7 Similar phenomenon was observed in the 3-D culture of bone marrow mesenchymal stem cells (BMSCs).Citation8 The interactions between cells and scaffolds were observed by scanning electron microscope (SEM).Citation7,Citation8
The self-renewal and differentiation behaviors of stem cells may depend on the growth environments. Our work have also shown the effects of 3-D cultures on the stemness of stem cells.Citation7,Citation8 The data indicated that 3-D cultures could keep the stemness of stem cells better than 2-D cultures. NSCs cultured in 3-D collagen scaffolds differentiated lower than cultured in 2-D. The second colony formation ability of NSCs in 3-D was much higher than those in 2-D. These results showed that in the differentiation medium, there were more NSCs maintaining self-renewal statements in 3-D collagen scaffolds than those in 2-D cultures.Citation7 Another experiment focused on the 3-D culture of BMSCs. In the cell cycle analysis, the percentage of BMSCs in 3-D collagen scaffolds staying in S phase was significantly higher than those in 2-D cultures. At the same time, more colonies were formed by 3-D cultured BMSCs, and the mRNA of stemness marker genes (Rex1, Oct4, Sox2 and Nanog) were detected higher expressed by 3-D cultured BMSCs.Citation8 Upon differentiation induction, the osteogenic and adipogenic efficiencies of 3-D cultured BMSCs were much higher than those of 2-D cultured cells. From these results, the 3-D culture in the collagen scaffolds was found to enhance the stemness of NSCs and BMSCs compared with 2-D cultures. Similar results were found in the embryonic stem (ES) cells under 3-D cultivation. Cultured in collagen-grafted polyethersulfone electrospun nanofibrous scaffolds, mouse ES cells maintained their pluripotency in a feeder-free manner.Citation9 And a polymer scaffolds comprising of chitosan and alginate kept the self-renewal of human ES cells without the support of feeder or conditioned medium.Citation10
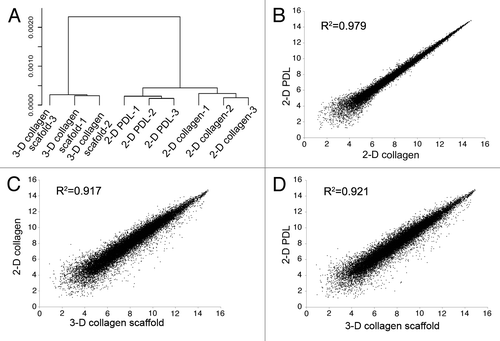
Further investigation was done by comparing the global mRNA profiling of 2-D and 3-D cultured NSCs to provide a general difference between 2-D and 3-D culture conditions. The transcriptional pattern of NSCs between cultured on 2-D poly-d-lysine (PDL)- and collagen- coating conditions was much more similar compared with the similarity between 3-D and 2-D culture conditions (). The scatter plot of microarray data also indicated that the correlation between the two 2-D culture samples are higher than that between 3-D and 2-D cultured cells (). The results indicated that genes changed by culturing in 3-D collagen scaffolds was much greater than genes culturing in 2-D conditions changed only by collagen coating, for the cell-cell and cell-matrix interactions in 3-D cultures were distinct from those in 2-D cultures.
Figure 1. Transcriptional profiles analysis between NSCs cultured under 2-D and 3-D conditions. A. Unsupervised clustering analysis of the NSCs cultured in 3-D collagen scaffolds, 2-D PDL-coating dishes and 2-D collagen-coating dishes based on microarray expression data. B-D, scatter plot of microarray data comparing NSCs cultured in 3-D and 2-D culture conditions.
The induced pluripotent stem (iPS) cells research is one of hotspots in current biology. Generally, somatic or differentiated cells, such as fibroblasts, could be reprogrammed to a pluripotent state by overexpressing a combination of several transcriptional factors. These transcriptional factors mainly function in maintaining pluripotency in early embryos and ES cells.Citation11-Citation14 Studies on 3-D culture showed that 3-D cultures could maintain the stemness of NSCs, BMSCs and ES cells compared with 2-D cultures, which indicated that 3-D cultures may serve as a factor maintaining pluripotency of stem cells similar to those reprogramming factors. Thus, we are hypothesizing that 3-D culture could function in the reprogramming process. To test the hypothesis, we examined mouse embryonic fibroblasts (MEFs) cultured on 3-D scaffolds.
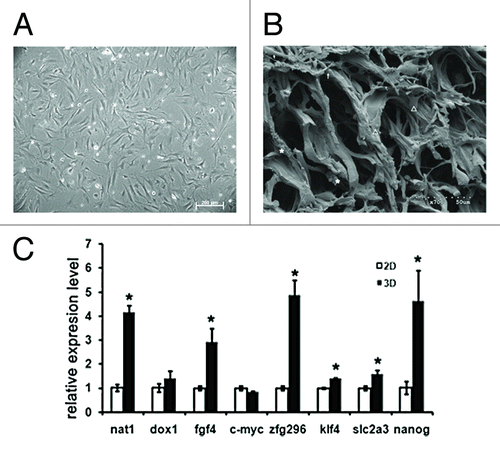
We prepared a 3-D porous collagen scaffold as described previously.Citation15 Briefly, the collagen solution of 0.5M acetic acid was neutralized by 4 M NaOH, then dialyzed in deionized water for 5 d and lyophilized. Scaffolds of appropriate size were cut and crosslinked. Finally, the collagen scaffolds with an average pore diameter of 80μm were obtained. MEFs were isolated from E13.5 mouse embryos and seeded on 2-D culture dishes and in 3-D collagen scaffolds. The phase-contrast image showed that MEFs cultured on 2-D dishes exhibited sheet-like polygonal morphologies (), whereas cultured in 3-D collagen scaffolds, MEFs displayed diversity morphologies with round, shuttle shape-like or spread-out appearances (). The interactions between cells and 3-D matrix were observed. Would the 3-D culture condition reprogram MEFs to iPS-like stem cells? After 12-d cultured on 2-D culture dishes or in 3-D collagen scaffolds, MEFs were collected and the qPCR analysis of ES marker genes was performed.Citation16 As shown in , several ES marker genes mRNA levels have been upregulated in MEFs under 3-D culture conditions. Nanog and Fgf4 are important to stem cell pluripotency, and Slc2a3, the direct target of Oct4 geneCitation17 was found upregulated although the Oct4 protein upregulation was not detected. The ES-special gene Nat1Citation18 was also increased. At the same time iPS factor Klf4Citation15 and Zfgp296 which was important to improve iPS efficiencyCitation19 were both upregulated by the 3-D culture (). The results above suggested a reprogramming tendency of MEFs by the 3-D culture. The cells may be heterogeneous similar to the process of the iPS cells induction. It is likely that only a portion of cells could be induced to reprogram under 3-D cultivations. These results have indicated that 3-D culture not only maintained the stemness of stem cells, but also provided a cell reprogramming method that avoided any gene modification of cells. Further investigation should be performed to understand the mechanism and to improve the reprogramming efficiency.
Figure 2. Morphology and pluripotency induction of MEFs by 3-D culture. A, The phase-contrast image of MEFs cultured on 2-D culture dish. B, The image of scanning electronic microscopy of MEFs cultured in 3-D collagen scaffolds. Diversity morphologies of cells in 3-D were observed with round (indicated by asterisk), shuttle shape-like (indicated by arrow) or spread-out appearances (indicated by triangle). C, qPCR analysis of ES marker genes in MEFs cultured under 3-D and 2-D conditions for 12 d. *p < 0.001.
References
- Vazin T, Schaffer DV. Engineering strategies to emulate the stem cell niche. Trends Biotechnol 2010; 28:117 - 24; http://dx.doi.org/10.1016/j.tibtech.2009.11.008; PMID: 20042248
- Joseph NM, Morrison SJ. Toward an understanding of the physiological function of Mammalian stem cells. Dev Cell 2005; 9:173 - 83; http://dx.doi.org/10.1016/j.devcel.2005.07.001; PMID: 16054025
- Gabay L, Lowell S, Rubin LL, Anderson DJ. Deregulation of dorsoventral patterning by FGF confers trilineage differentiation capacity on CNS stem cells in vitro. Neuron 2003; 40:485 - 99; http://dx.doi.org/10.1016/S0896-6273(03)00637-8; PMID: 14642274
- Moore KA, Lemischka IR. Stem cells and their niches. Science 2006; 311:1880 - 5; http://dx.doi.org/10.1126/science.1110542; PMID: 16574858
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 2002; 3:349 - 63; http://dx.doi.org/10.1038/nrm809; PMID: 11988769
- Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol 2003; 13:264 - 9; http://dx.doi.org/10.1016/S0962-8924(03)00057-6; PMID: 12742170
- Han J, Xiao Z, Chen L, Chen B, Li X, Han S, et al. Maintenance of the self-renewal properties of neural progenitor cells cultured in three-dimensional collagen scaffolds by the REDD1-mTOR signal pathway. Biomaterials 2013; 34:1921 - 8; http://dx.doi.org/10.1016/j.biomaterials.2012.11.063; PMID: 23246064
- Han S, Zhao Y, Xiao Z, Han J, Chen B, Chen L, et al. The three-dimensional collagen scaffold improves the stemness of rat bone marrow mesenchymal stem cells. J Genet Genomics 2012; 39:633 - 41; http://dx.doi.org/10.1016/j.jgg.2012.08.006; PMID: 23273767
- Hashemi SM, Soudi S, Shabani I, Naderi M, Soleimani M. The promotion of stemness and pluripotency following feeder-free culture of embryonic stem cells on collagen-grafted 3-dimensional nanofibrous scaffold. Biomaterials 2011; 32:7363 - 74; http://dx.doi.org/10.1016/j.biomaterials.2011.06.048; PMID: 21762983
- Li Z, Leung M, Hopper R, Ellenbogen R, Zhang M. Feeder-free self-renewal of human embryonic stem cells in 3D porous natural polymer scaffolds. Biomaterials 2010; 31:404 - 12; http://dx.doi.org/10.1016/j.biomaterials.2009.09.070; PMID: 19819007
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 2000; 24:372 - 6; http://dx.doi.org/10.1038/74199; PMID: 10742100
- Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev 2003; 17:126 - 40; http://dx.doi.org/10.1101/gad.224503; PMID: 12514105
- Cartwright P, McLean C, Sheppard A, Rivett D, Jones K, Dalton S. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development 2005; 132:885 - 96; http://dx.doi.org/10.1242/dev.01670; PMID: 15673569
- Li Y, McClintick J, Zhong L, Edenberg HJ, Yoder MC, Chan RJ. Murine embryonic stem cell differentiation is promoted by SOCS-3 and inhibited by the zinc finger transcription factor Klf4. Blood 2005; 105:635 - 7; http://dx.doi.org/10.1182/blood-2004-07-2681; PMID: 15358627
- Chen L, Xiao Z, Meng Y, Zhao Y, Han J, Su G, et al. The enhancement of cancer stem cell properties of MCF-7 cells in 3D collagen scaffolds for modeling of cancer and anti-cancer drugs. Biomaterials 2012; 33:1437 - 44; http://dx.doi.org/10.1016/j.biomaterials.2011.10.056; PMID: 22078807
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126:663 - 76; http://dx.doi.org/10.1016/j.cell.2006.07.024; PMID: 16904174
- Saijoh Y, Fujii H, Meno C, Sato M, Hirota Y, Nagamatsu S, et al. Identification of putative downstream genes of Oct-3, a pluripotent cell-specific transcription factor. Genes Cells 1996; 1:239 - 52; PMID: 9140067
- Yamanaka S, Zhang XY, Maeda M, Miura K, Wang S, Farese RV Jr., et al. Essential role of NAT1/p97/DAP5 in embryonic differentiation and the retinoic acid pathway. EMBO J 2000; 19:5533 - 41; http://dx.doi.org/10.1093/emboj/19.20.5533; PMID: 11032820
- Fischedick G, Klein DC, Wu G, Esch D, Höing S, Han DW, et al. Zfp296 is a novel, pluripotent-specific reprogramming factor. PLoS One 2012; 7:e34645; http://dx.doi.org/10.1371/journal.pone.0034645; PMID: 22485183