Abstract
During embryogenesis, the development of the respiratory tract is closely associated with the formation of an extensive neuronal network. While the topic of respiratory innervation is not new, and similar articles were published previously, recent studies using animal models and genetic tools are breathing new life into the field. In this review, we focus on signaling mechanisms that underlie innervation of the embryonic respiratory tract.
Introduction
The mammalian respiratory tract consists of the trachea and lung. It arises from ventral foregut endoderm.Citation1 After progenitor specification, the lung primordia bifurcate ventral-laterally to form two primary lung buds. These buds continue to invade the surrounding mesenchyme, elongate and branch to ultimately form a tree-like structure of epithelium tubules and alveoli. Along with lung bud formation, the trachea forms ventrally and separates from the primitive esophagus that is formed in the dorsal side of the foregut. Meanwhile, the mesenchyme forms from lateral plate mesoderm and gives rise to other cell types in the respiratory tract, such as airway smooth muscle (ASM), trachea cartilage, lymphatics, and blood vessels.Citation1 In mice, the trachea and lung form around E9, and the respiratory tract continues to develop after birth before reaching maturity around 3–4 weeks postnatally.Citation1-Citation3
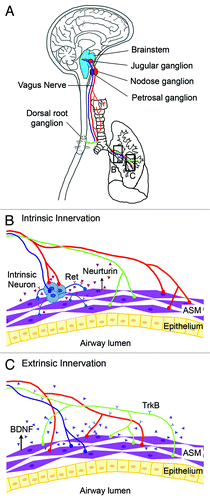
During respiratory tree development, a complex neuronal network forms.Citation4 This network includes axons from extrinsic neurons, whose cell bodies are located outside of the respiratory tract, and intrinsic neurons whose cell bodies reside in the trachea and major bronchi and cluster to form ganglia ().Citation5,Citation6 Previous studies identified the location of extrinsic neuronal cell bodies by using a combination of retrograde/anterograde labeling techniques, immunohistology, and microscopy.Citation7-Citation12 Extrinsic neurons within the dorsal and ventral respiratory nuclei in the medulla oblongata and within the jugular and nodose ganglia supply parasympathetic efferents and most of the sensory afferents, respectively ().Citation6,Citation13-Citation15 These axons travel along the vagus nerve to innervate ASM and neuroendocrine bodies (NEBs) in the lung epithelium ().Citation14,Citation16 Some efferents also connect to intrinsic neurons that provide post-ganglionic parasympathetic input to the trachea and bronchi ().Citation6 In addition, sensory afferents from the dorsal root ganglia connects with thoracic ganglia to supply sympathetic innervation to the blood vessels and submucosal gland ().Citation6 This complex neural network functions to control breathing, smooth muscle tone, and mucous secretion, and to trigger reflexes such as cough.Citation13-Citation15
Figure 1. Schematic diagram showing extrinsic nerves and intrinsic innervation of the respiratory tract. (A) Extrinsic neurons have their cell bodies in the jugular, nodose, and petrosal ganglia within the brainstem. These extrinsic neurons extend their axons via the vagus nerve (in red and blue) and provide sensory and parasympathetic respiratory innervation respectively. In addition, sensory neurons located in the dorsal root ganglion also provide extrinsic innervation (in green) to the respiratory tract. (B) Neural crest-derived intrinsic neurons (in blue) cluster within the trachea and main bronchi. Intrinsic neurons express the Ret receptor. The survival, proliferation, and/or differentiation of intrinsic neurons within the respiratory tract is dependent on the GDNF family ligands that include GDNF and neurturin. (C) ASM in the embryonic lung expresses BDNF. BDNF serves as a target-derived neurotrophic signal for extrinsic innervation by TrkB+ extrinsic nerves. The lung is largely devoid of intrinsic neurons.
Although lung innervation has been described by previous studies in humans, primates, rodents, and several other animals, signals that regulate respiratory neurogenesis are not fully understood.Citation17-Citation20 Here, we review current knowledge on mechanisms of respiratory neurogenesis during embryonic development. Most studies on neurogenic signals were performed in mice, where ASM and NEBs are the major targets of innervation. This review is designed to highlight key findings in the development of respiratory tract innervation rather than a comprehensive overview of every study published in this field. We apologize to authors whose contribution is not acknowledged.
Intrinsic Neurogenesis Within the Respiratory Tract
Earlier observations in the airways of humans and other species show that intrinsic neurons express neural crest cell markers, suggesting their neural crest origin.Citation21,Citation22 Follow-up studies using engraftment of avian neural tissues and lineage labeling in mouse embryos definitively prove that intrinsic neurons in the respiratory tract are exclusively derived from vagal neural crest cells.Citation23,Citation24 These neural crest cells generate both neurons and glial cells that cluster to form ganglia, mostly found in the dorsal trachea and upper respiratory tract ().Citation23,Citation24 As the size of the airway tapers off along the proximal-distal axis of the respiratory tree, there are fewer intrinsic neurons.Citation23,Citation24 In mice, a small number of intrinsic neurons are located in the secondary and tertiary bronchi with little to none in the distal lung ().
In addition to intrinsic neurons in the respiratory tract, vagal neural crest cells also give rise to enteric neurons in the gastrointestinal tract.Citation25,Citation26 These two groups of neural crest cells likely migrate together initially. Upon separation of the trachea from the esophagus at E10.5 in mouse embryos, neural crest cells that migrate into the space between the esophagus and the trachea begin to take different paths.Citation23,Citation24
Innervation of the gastrointestinal tract by enteric neural crest cells is well characterized compared with the respiratory innervation by intrinsic neurons. Expressed in the enteric wall, glial cell derived neurotrophic factor (GDNF) is an essential chemo-attractant for enteric neural crest cells.Citation27-Citation30 GDNF belongs to a family that also includes neurturin, artemin, and persephin. Each family member binds to unique GDNF family co-receptors 1, 2, 3 and 4 (GFRα1–4), respectively.Citation31,Citation32 When the GDNF family ligand binds to the GFRα co-receptor, the common tyrosine kinase receptor RET is recruited for downstream signaling.Citation31,Citation32 Enteric neural crest cells predominantly express GFRα1.Citation23,Citation30 Consistently, genetic disruption of GDNF or RET diminishes the migration of enteric neural crest cells and subsequent formation of the enteric nervous system.Citation27-Citation30,Citation33
In contrast, GDNF is not expressed in the trachea, and GDNF deficiency has no effect on the respiratory intrinsic neurons.Citation23,Citation24 This suggests that respiratory neural crest cells depend on different chemo-attractants for migration. Further characterization of other GDNF family member and receptor expression shows that neurturin is expressed in the respiratory tract, and the respiratory neural crest cells express both GFRα1 and GFRα2.Citation23 However, the neurturin knockout mouse embryos have normal airway intrinsic innervation.Citation23 In addition, loss of RET function, which demolishes the signaling activity of all GDNF family members, does not affect the number of respiratory intrinsic neurons at E14.5.Citation24 These findings indicate that the respiratory neural crest cells are independent of the GDNF family for migration into the respiratory tract. However, Ret deficiency leads to a 50% reduction in the size of the ganglia at E18.5.Citation23 Thus, GDNF family signaling, through the Ret receptor, likely plays a role in the survival, proliferation and/or differentiation of these neural crest cells after they reach the respiratory tract. Signals for the migration of neural crest cells in the respiratory tract have yet to be identified.
In addition to the difference in essential migratory signals between the respiratory and gastrointestinal neural crest cells, cells that migrate into these two organs also differ in their progenitor status. Recently, using avian embryos, the Burns lab has shown that after graft into the vagal neural tube, neural crest cells that have already reached the gut can still migrate into the lung and the gut.Citation34 However, neural crest cells collected from the lung fail to migrate after graft.Citation34 These findings suggest that respiratory neural crest cells are committed once they reach their destination whereas gastrointestinal neural crest cells maintain their migratory potentials even after they migrate into the gut.
ASM Innervation by Extrinsic Neurons
While intrinsic neurons likely provide parasympathetic innervation to the trachea and main bronchi, a large body of evidence indicates that intrinsic neurons do not contribute significantly to lung innervation (). First, most intrinsic neurons are located in the trachea and main bronchi.Citation20,Citation23,Citation24 Only a small number of intrinsic neurons are found in the secondary and tertiary bronchi with little to none in the distal lung.Citation23,Citation24 Second, intrinsic neurons have short axons, suggesting that they function locally.Citation35 Third, although RET mutant embryos have a reduction in the size of resident ganglia at E18.5, they have no defects in lung innervation.Citation23 Finally, vagotomy results in an almost complete loss of innervation of airway targets, such as NEBs.Citation36 Collectively, these findings indicate that the lung is innervated predominantly by extrinsic neurons. However, the role of intrinsic lung innervation cannot be fully elucidated until functional data are obtained from animal models selectively deficient in intrinsic neurons.
During embryogenesis, the outgrowth of axons into the distal lung is closely associated with the formation of ASM. Studies in the fetal porcine and human lung show that the developing tubules are covered in a layer of ASM and ensheathed in a newly formed neuronal network.Citation19,Citation20,Citation37 Two large nerve trunks run the length of the bronchial tree. They give rise to a network of bundles, with fine fibers covering up to the growing tips of the airways.Citation19,Citation20 This close temporal and spatial relationship between ASM formation and axon outgrowth suggest an ASM-derived trophic mechanism for innervation.
The extrinsic neurons depend on the nerve growth factor (NGF) family for ASM innervation. The NGF family includes NGF, brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT3), and NT4.Citation38,Citation39 They signal through high-affinity tyrosine kinase Trk receptors with relative selectivity: TrkA for NGF, TrkB for BDNF and NT4, and TrkC for NT3. Both BDNF and NT4 are expressed by embryonic ASM.Citation40 The BDNF knockout embryos have reduced axon branches and shortened axons targeting the ASM without any change in lung morphogenesis or ASM differentiation.Citation40 Thus, BDNF serves as a target-derived neurotrophic factor for ASM innervation by extrinsic neurons during embryogenesis.Citation40 These findings also provide further evidence that intrinsic neurons and extrinsic neurons require distinct neurogenic signals for innervation of the respiratory tract.
To ensure appropriate innervation, BDNF expression needs to be temporally coordinated with ASM differentiation. Notably, BDNF mRNA is expressed as early as E11.5 in the lung mesenchyme prior to its differentiation into ASM.Citation40 To coordinate, a post-transcriptional regulation is at play to repress the translation of BDNF mRNA until ASM is formed. One of the mechanisms of post-transcriptional regulation of BDNF expression is through a microRNA, miR-206. MiR-206 is expressed in lung mesenchyme, and its expression is downregulated upon ASM differentiation. In addition, miR-206 targets BDNF mRNA for degradation.Citation40 Furthermore, the miR-206 knockout mice exhibit premature airway innervation.Citation40 Collectively, these findings support miR-206 as a post-transcriptional regulator for coordinated BDNF protein expression, ASM differentiation, and ASM innervation.
Additionally, NT4, which binds to the same TrkB receptor as BDNF, may play a redundant role in ASM innervation. Consistent with this hypothesis, previous studies showed that mice deficient in both BDNF and NT4 have a diminished number of neurons in the nodose-petrosal ganglion complex, one of the locations where extrinsic neurons reside ().Citation41 In addition, airway innervation was more reduced in the TrkB−/− mice than in the NT4−/− mice.Citation42
NEB Innervation
NEBs are specialized clusters of pulmonary neuroendocrine cells that originate from the airway epithelium in the mammalian lung. Whereas solitary pulmonary neuroendocrine cells are found in the trachea, bronchioles, and terminal airways, NEBs are localized only in the intrapulmonary airways.Citation43 The cytoplasm of NEB contains secretory granules that are loaded with bioactive molecules, such as neuropeptide, monoamines, and purine transmitters.Citation16,Citation36,Citation43 NEBs are usually found at or near the bifurcation sites of the airwayCitation36 and juxtapose invariant Clara cells, a cell population with stem-cell like properties.Citation44,Citation45 Due to these unique structural, chemical, and positional properties, NEBs have been speculated to function as mechanoreceptors and play a role in O2 sensing and regeneration of the distal pulmonary epithelium.Citation46-Citation48 However, precise roles of NEBs remain elusive.
NEBs in the lung epithelium are innervated by a mixture of sensory and cholinergic nerves.Citation36 These sensory afferents originate from the nodose ganglia and dorsal root ganglia, and the cholinergic efferents come from the brain stem and intrinsic ganglia.Citation16,Citation36 NEB innervation by P2X2+ and P2X3+ axons is reduced in NT4−/− mice, indicating that NT4 is required for NEB purinergic innervation.Citation49 Whether NEB innervation regulates the role of NEBs during homeostasis and regeneration of the lung epithelium is unknown.
Concluding Remarks
Despite recent progress on the mechanisms of respiratory innervation, future studies are required to fully understand these processes. For example, additional signals that control NEB innervation remain to be identified. In addition, mechanisms underlying ASM innervation in postnatal life need to be further investigated. Mounting evidence indicates that altered innervation is involved in the pathogenesis of respiratory diseases.Citation50-Citation52 Identification of the signals required for disease-related neural plasticity will likely provide groundwork for identification of new therapeutic targets.
| Abbreviations: | ||
| ASM | = | airway smooth muscle |
| BDNF | = | brain derived neurotrophic factor |
| GDNF | = | glial cell-derived neurotrophic factor |
| NEBs | = | neuroendocrine bodies |
| NGF | = | nerve growth factor |
| NT | = | neurotrophin |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Cardoso WV, Lü J. Regulation of early lung morphogenesis: questions, facts and controversies. Development 2006; 133:1611 - 24; http://dx.doi.org/10.1242/dev.02310; PMID: 16613830
- Ten Have-Opbroek AA. The development of the lung in mammals: an analysis of concepts and findings. Am J Anat 1981; 162:201 - 19; http://dx.doi.org/10.1002/aja.1001620303; PMID: 7032272
- Burri PH. Structural aspects of prenatal and postnatal development and growth of the lung. In: McDonald JA, ed. Lung Growth and Development. New York: Marcel Dekker, 1997:1-35.
- Dey R, Hung KS. Development of innervation in the lung. In: McDonald JA, ed. Lung Growth and Development. New York: Marcel Dekker, 1997:244-265.
- Hirsch EF, Kaiser GC. The Innervation of the lung. Springfield, IL: Thomas, 1969.
- Belvisi MG. Overview of the innervation of the lung. Curr Opin Pharmacol 2002; 2:211 - 5; http://dx.doi.org/10.1016/S1471-4892(02)00145-5; PMID: 12020459
- Bystrzycka EK. Afferent projections to the dorsal and ventral respiratory nuclei in the medulla oblongata of the cat studied by the horseradish peroxidase technique. Brain Res 1980; 185:59 - 66; http://dx.doi.org/10.1016/0006-8993(80)90670-8; PMID: 7353180
- Connelly CA, Ellenberger HH, Feldman JL. Are there serotonergic projections from raphe and retrotrapezoid nuclei to the ventral respiratory group in the rat?. Neurosci Lett 1989; 105:34 - 40; http://dx.doi.org/10.1016/0304-3940(89)90007-4; PMID: 2485883
- Holtman JR Jr., Marion LJ, Speck DF. Origin of serotonin-containing projections to the ventral respiratory group in the rat. Neuroscience 1990; 37:541 - 52; http://dx.doi.org/10.1016/0306-4522(90)90422-Z; PMID: 2133358
- Núñez-Abades PA, Portillo F, Pásaro R. Characterisation of afferent projections to the nucleus ambiguus of the rat by means of fluorescent double labelling. J Anat 1990; 172:1 - 15; PMID: 2272895
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 1991; 254:726 - 9; http://dx.doi.org/10.1126/science.1683005; PMID: 1683005
- Haxhiu MA, Jansen ASP, Cherniack NS, Loewy AD. CNS innervation of airway-related parasympathetic preganglionic neurons: a transneuronal labeling study using pseudorabies virus. Brain Res 1993; 618:115 - 34; http://dx.doi.org/10.1016/0006-8993(93)90435-P; PMID: 8402166
- Carr MJ, Undem BJ. Bronchopulmonary afferent nerves. Respirology 2003; 8:291 - 301; http://dx.doi.org/10.1046/j.1440-1843.2003.00473.x; PMID: 14528878
- Canning BJ. Reflex regulation of airway smooth muscle tone. J Appl Physiol 2006; 101:971 - 85; http://dx.doi.org/10.1152/japplphysiol.00313.2006; PMID: 16728519
- Myers AC. Electrophysiology of airway nerves. Curr Protoc Pharmacol 2007; Chapter 11:10; PMID: 21948159
- Adriaensen D, Timmermans JP, Brouns I, Berthoud HR, Neuhuber WL, Scheuermann DW. Pulmonary intraepithelial vagal nodose afferent nerve terminals are confined to neuroepithelial bodies: an anterograde tracing and confocal microscopy study in adult rats. Cell Tissue Res 1998; 293:395 - 405; http://dx.doi.org/10.1007/s004410051131; PMID: 9716729
- Hung KS. Innervation of rabbit fetal lungs. Am J Anat 1980; 159:73 - 83; http://dx.doi.org/10.1002/aja.1001590107; PMID: 7446443
- Weichselbaum M, Everett AW, Sparrow MP. Mapping the innervation of the bronchial tree in fetal and postnatal pig lung using antibodies to PGP 9.5 and SV2. Am J Respir Cell Mol Biol 1996; 15:703 - 10; http://dx.doi.org/10.1165/ajrcmb.15.6.8969263; PMID: 8969263
- Sparrow MP, Weichselbaum M, McCray PB. Development of the innervation and airway smooth muscle in human fetal lung. Am J Respir Cell Mol Biol 1999; 20:550 - 60; http://dx.doi.org/10.1165/ajrcmb.20.4.3385; PMID: 10100986
- Tollet J, Everett AW, Sparrow MP. Spatial and temporal distribution of nerves, ganglia, and smooth muscle during the early pseudoglandular stage of fetal mouse lung development. Dev Dyn 2001; 221:48 - 60; http://dx.doi.org/10.1002/dvdy.1124; PMID: 11357193
- Burns AJ, Delalande JM. Neural crest cell origin for intrinsic ganglia of the developing chicken lung. Dev Biol 2005; 277:63 - 79; http://dx.doi.org/10.1016/j.ydbio.2004.09.006; PMID: 15572140
- Burns AJ, Thapar N, Barlow AJ. Development of the neural crest-derived intrinsic innervation of the human lung. Am J Respir Cell Mol Biol 2008; 38:269 - 75; http://dx.doi.org/10.1165/rcmb.2007-0246OC; PMID: 17884989
- Langsdorf A, Radzikinas K, Kroten A, Jain S, Ai X. Neural crest cell origin and signals for intrinsic neurogenesis in the mammalian respiratory tract. Am J Respir Cell Mol Biol 2011; 44:293 - 301; http://dx.doi.org/10.1165/rcmb.2009-0462OC; PMID: 20139349
- Freem LJ, Escot S, Tannahill D, Druckenbrod NR, Thapar N, Burns AJ. The intrinsic innervation of the lung is derived from neural crest cells as shown by optical projection tomography in Wnt1-Cre;YFP reporter mice. J Anat 2010; 217:651 - 64; http://dx.doi.org/10.1111/j.1469-7580.2010.01295.x; PMID: 20840354
- Gershon MD, Payette RF, Rothman TP. Development of the enteric nervous system. Fed Proc 1983; 42:1620 - 5; PMID: 6131841
- Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol 1973; 30:31 - 48; PMID: 4729950
- Moore MW, Klein RD, Fariñas I, Sauer H, Armanini M, Phillips H, et al. Renal and neuronal abnormalities in mice lacking GDNF. Nature 1996; 382:76 - 9; http://dx.doi.org/10.1038/382076a0; PMID: 8657308
- Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature 1996; 382:73 - 6; http://dx.doi.org/10.1038/382073a0; PMID: 8657307
- Sánchez MP, Silos-Santiago I, Frisén J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 1996; 382:70 - 3; http://dx.doi.org/10.1038/382070a0; PMID: 8657306
- Young HM, Hearn CJ, Farlie PG, Canty AJ, Thomas PQ, Newgreen DF. GDNF is a chemoattractant for enteric neural cells. Dev Biol 2001; 229:503 - 16; http://dx.doi.org/10.1006/dbio.2000.0100; PMID: 11150245
- Baloh RH, Enomoto H, Johnson EM Jr., Milbrandt J. The GDNF family ligands and receptors - implications for neural development. Curr Opin Neurobiol 2000; 10:103 - 10; http://dx.doi.org/10.1016/S0959-4388(99)00048-3; PMID: 10679429
- Enomoto H. Regulation of neural development by glial cell line-derived neurotrophic factor family ligands. Anat Sci Int 2005; 80:42 - 52; http://dx.doi.org/10.1111/j.1447-073x.2005.00099.x; PMID: 15794130
- Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 1994; 367:380 - 3; http://dx.doi.org/10.1038/367380a0; PMID: 8114940
- Freem LJ, Delalande JM, Campbell AM, Thapar N, Burns AJ. Lack of organ specific commitment of vagal neural crest cell derivatives as shown by back-transplantation of GFP chicken tissues. Int J Dev Biol 2012; 56:245 - 54; http://dx.doi.org/10.1387/ijdb.113438lf; PMID: 22562200
- Weigand LA, Myers AC. Synaptic and membrane properties of parasympathetic ganglionic neurons innervating mouse trachea and bronchi. Am J Physiol Lung Cell Mol Physiol 2010; 298:L593 - 9; http://dx.doi.org/10.1152/ajplung.00386.2009; PMID: 20118300
- Brouns I, Oztay F, Pintelon I, De Proost I, Lembrechts R, Timmermans JP, et al. Neurochemical pattern of the complex innervation of neuroepithelial bodies in mouse lungs. Histochem Cell Biol 2009; 131:55 - 74; http://dx.doi.org/10.1007/s00418-008-0495-7; PMID: 18762965
- Sparrow MP, Lamb JP. Ontogeny of airway smooth muscle: structure, innervation, myogenesis and function in the fetal lung. Respir Physiol Neurobiol 2003; 137:361 - 72; http://dx.doi.org/10.1016/S1569-9048(03)00159-9; PMID: 14516738
- Ginty DD, Segal RA. Retrograde neurotrophin signaling: Trk-ing along the axon. Curr Opin Neurobiol 2002; 12:268 - 74; http://dx.doi.org/10.1016/S0959-4388(02)00326-4; PMID: 12049932
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 2003; 72:609 - 42; http://dx.doi.org/10.1146/annurev.biochem.72.121801.161629; PMID: 12676795
- Radzikinas K, Aven L, Jiang Z, Tran T, Paez-Cortez J, Boppidi K, et al. A Shh/miR-206/BDNF cascade coordinates innervation and formation of airway smooth muscle. J Neurosci 2011; 31:15407 - 15; http://dx.doi.org/10.1523/JNEUROSCI.2745-11.2011; PMID: 22031887
- Erickson JT, Conover JC, Borday V, Champagnat J, Barbacid M, Yancopoulos G, et al. Mice lacking brain-derived neurotrophic factor exhibit visceral sensory neuron losses distinct from mice lacking NT4 and display a severe developmental deficit in control of breathing. J Neurosci 1996; 16:5361 - 71; PMID: 8757249
- García-Suárez O, Pérez-Pinera P, Laurà R, Germana A, Esteban I, Cabo R, et al. TrkB is necessary for the normal development of the lung. Respir Physiol Neurobiol 2009; 167:281 - 91; http://dx.doi.org/10.1016/j.resp.2009.06.001; PMID: 19523540
- Cutz E. Neuroendocrine cells of the lung. An overview of morphologic characteristics and development. Exp Lung Res 1982; 3:185 - 208; http://dx.doi.org/10.3109/01902148209069653; PMID: 6188605
- Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol 2001; 24:671 - 81; http://dx.doi.org/10.1165/ajrcmb.24.6.4498; PMID: 11415931
- Rock JR, Hogan BL. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu Rev Cell Dev Biol 2011; 27:493 - 512; http://dx.doi.org/10.1146/annurev-cellbio-100109-104040; PMID: 21639799
- Cutz E, Jackson A. Neuroepithelial bodies as airway oxygen sensors. Respir Physiol 1999; 115:201 - 14; http://dx.doi.org/10.1016/S0034-5687(99)00018-3; PMID: 10385034
- De Proost I, Pintelon I, Wilkinson WJ, Goethals S, Brouns I, Van Nassauw L, et al. Purinergic signaling in the pulmonary neuroepithelial body microenvironment unraveled by live cell imaging. FASEB J 2009; 23:1153 - 60; http://dx.doi.org/10.1096/fj.08-109579; PMID: 19050048
- Lembrechts R, Brouns I, Schnorbusch K, Pintelon I, Timmermans JP, Adriaensen D. Neuroepithelial bodies as mechanotransducers in the intrapulmonary airway epithelium: involvement of TRPC5. Am J Respir Cell Mol Biol 2012; 47:315 - 23; http://dx.doi.org/10.1165/rcmb.2012-0068OC; PMID: 22461428
- Oztay F, Brouns I, Pintelon I, Raab M, Neuhuber W, Timmermans JP, et al. Neurotrophin-4 dependency of intraepithelial vagal sensory nerve terminals that selectively contact pulmonary NEBs in mice. Histol Histopathol 2010; 25:975 - 84; PMID: 20552548
- Duarte AG, Myers AC. Cough reflex in lung transplant recipients. Lung 2012; 190:23 - 7; http://dx.doi.org/10.1007/s00408-011-9352-x; PMID: 22139551
- Nassini R, Materazzi S, De Siena G, De Cesaris F, Geppetti P. Transient receptor potential channels as novel drug targets in respiratory diseases. Curr Opin Investig Drugs 2010; 11:535 - 42; PMID: 20419599
- Verhein KC, Fryer AD, Jacoby DB. Neural control of airway inflammation. Curr Allergy Asthma Rep 2009; 9:484 - 90; http://dx.doi.org/10.1007/s11882-009-0071-9; PMID: 19814922