Abstract
The current prevalence and severity of heart defects requiring functional replacement of cardiac tissue pose a serious clinical challenge. Biologic scaffolds are an attractive tissue engineering approach to cardiac repair because they avoid sensitization associated with homograft materials and theoretically possess the potential for growth in similar patterns as surrounding native tissue. Both urinary bladder matrix (UBM) and cardiac ECM (C-ECM) have been previously investigated as scaffolds for cardiac repair with modest success, but have not been compared directly. In other tissue locations, bone marrow derived cells have been shown to play a role in the remodeling process, but this has not been investigated for UBM in the cardiac location, and has never been studied for C-ECM. The objectives of the present study were to compare the effectiveness of an organ-specific C-ECM patch with a commonly used ECM scaffold for myocardial tissue repair of the right ventricle outflow tract (RVOT), and to examine the role of bone marrow derived cells in the remodeling response. A chimeric rat model in which all bone marrow cells express green fluorescent protein (GFP) was generated and used to show the ability of ECM scaffolds derived from the heart and bladder to support cardiac function and cellular growth in the RVOT. The results from this study suggest that urinary bladder matrix may provide a more appropriate substrate for myocardial repair than cardiac derived matrices, as shown by differences in the remodeling responses following implantation, as well as the presence of site appropriate cells and the formation of immature, myocardial tissue.
Introduction
Congenital heart defects (CHDs) are the most common form of birth defect, accounting for greater than 29% of all birth defect related deaths in the United States annually.Citation1 It is estimated that over 1 million Americans are currently suffering from or were born with a form of CHD.Citation2 Additionally, over 5 in 1,000 infants will require surgical cardiologic intervention within the first year of life.Citation3 Many congenital malformations are resolved spontaneously or with minimal surgical intervention. However, some CHDs require complex surgical procedures and the implantation of reconstructive materials to resolve abnormal heart function. There are currently a number of commercially available products that have the ability to patch defects without offering the capability of restoring function to the damaged myocardiumCitation4,Citation5 Traditionally used cardiac patch materials include cryopreserved homograft tissue, bovine pericardium, and synthetic materials—all of which are commonly associated with multiple failure modes, including rejection, stenosis, aneurysm, thrombosis, and calcificiation.Citation6-Citation9 An “off the shelf” material capable of repairing damaged myocardial tissue and restoring native function would therefore be ideal in a clinical setting and would provide benefits to currently used materials.
Naturally derived extracellular matrix (ECM) scaffolds have been shown to serve as adequate patch materials in a variety of body systems in both preclinical and clinical trials.Citation10-Citation14 ECM scaffolds have gained significant attention recently due to their inherent biocompatibility and ability to degrade and remodel toward site-appropriate tissue in a variety of organ systems. Among the most widely investigated ECM patch materials for myocardial repair include small intestinal submucosa (SIS), urinary bladder matrix (UBM), decellularized pulmonary artery, and cardiac-derived ECM products. Currently, CorMatrix SIS is one of the only clinically available products for myocardial repair, having been successfully used in dozens of pediatric patients to correct a variety of CHD malformations.Citation15 The material has been able to repair both cardiac and vascular related defects with no evidence of calcification at 2 years after implantation. UBM scaffolds have not yet become available in a clinical setting, but have been investigated as a cardiac patch material in several preclinical applications.Citation16-Citation20 UBM scaffolds have shown the ability to rapidly and completely degrade in a cardiac environment. Preliminary studies have also shown the ability of UBM scaffolds to support the deposition of small areas of cardiac specific cells as well as minor indications of mechanical support and electrical communication with surrounding native cardiac tissue.
In recent efforts to preserve and mimic the natural biochemical and mechanical environment of organs requiring repair, organ-specific scaffolds have recently shown promise in a variety of tissue engineering applications,Citation21-Citation31 including the heart.Citation32-Citation34 Repair of many CHDs requires the reconstruction or augmentation of malformed or underdeveloped vessels. Decellularization of the aorta and pulmonary artery is thought to provide the most appropriate material to facilitate host tissue integration and growth with pediatric patients. However, this approach has been met with limited success in preclinical and clinical studies with primary failure modes of calcification and occlusion.Citation35,Citation36,Citation37 In the case of myocardial tissue repair, an organ-specific cardiac ECM is thought to provide the most appropriate platform for a cardiac patch material. Singelyn et al. have recently developed an injectable form of C-ECM with the ability to support cardiac specific cells within the matrix after injection.Citation32,Citation38 The matrix is able to self-assemble and support neovascularization in highly localized areas within the myocardial tissue. However, a significant drawback of this approach is the inability to repair a full-thickness defect in the myocardium. It has recently been shown that an intact porcine heart can be fully decellularized to generate C-ECM patches.Citation39,Citation40 C-ECM patches generated in this manner have shown the ability to support cardiac function as well as the infiltration of small areas of cardiac specific cells.Citation33
UBM and C-ECM have each been investigated individually as scaffolds for myocardial repair, but have shown limited success.Citation16,Citation17,Citation19,Citation20,Citation41 To date, a direct comparison of the scaffolds has not been performed in a cardiac location, due to variations in the animal and surgical models used to investigate each scaffold. However, recent studies have developed an effective surgical model to evaluate materials for repair of full-thickness defects in rat hearts.Citation33,Citation42 Previous studies have been able to evaluate functional and histological outcomes within repaired hearts, but did not investigate the origin of repopulating cells at the site of repair. In the present study, UBM and C-ECM patches were directly compared for repair of a full thickness defect created in the right ventricle outflow tract (RVOT) of rats. As a secondary objective, the role that bone marrow derived cells play in the remodeling of an ECM scaffold in a cardiac location will be investigated through the use of a chimera rat model in which the bone marrow cells express green fluorescent protein (GFP).
Results
Bone marrow transplantation and surgical outcomes
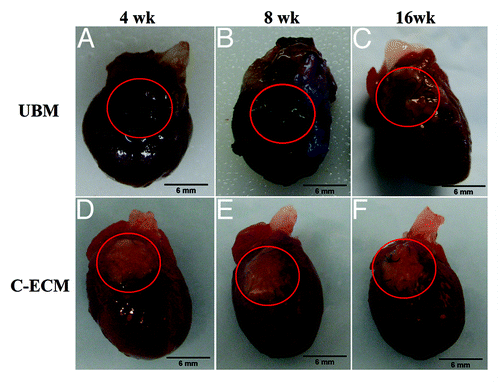
Prior to RVOT surgeries, a chimera population was created from wild type recipient rats and GFP+ marrow donors. The observed survival rate of bone marrow transplantation was approximately 66%, with complete engraftment achieved between 30–60d after transplant. Currently accepted rates of chimerism are approximately 95% of the white blood cell population expressing the GFP protein. Blood smear analysis revealed a large GFP+ cell population within peripheral blood samples, and this was verified through flow cytometry analysis. The animals used in this study expressed an average GFP + white blood cell population of 94.9%, confirming the creation of the chimera rats. Both UBM and C-ECM patches measured 6mm in diameter and were implanted with the luminal side of the scaffold on the blood contacting surface. () UBM patches measured approximately 0.25mm in thickness and C-ECM patches measured approximately 0.25–0.4mm in thickness. Intra-operative and post-operative mortality associated with the surgical procedure in both UBM and C-ECM groups was approximately 25%. The patches replaced approximately 25% of the RV wall in both groups and suture lines indicated the original placement of the scaffold up to the 16 week time point. ()
Figure 2. Macroscopic images of the patched area of rat hearts at 4, 8, and 16 weeks after implantation. (A) UBM patches were incorporated into the native tissue by 4 weeks after surgery. (A–C) The original white color of the patches were not evident at any time point. (D–F) C-ECM patches retained a whitish appearance and preserved the native thickness of the ventricle wall through the end point of the study.
C-ECM scaffolds
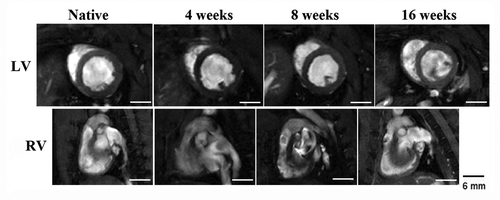
The C-ECM patches were incorporated into the native tissue by 4 weeks and the patches retained a whitish appearance through the 16 week time point. () The thickness of scaffolds was not statistically different than native RV wall thickness (0.93 ± 0.07 mm) at all time points with wall thickness values of 1.26 ± 0.14, 0.98 ± 0.1, and 1.07 ± 0.11 mm at 4, 8, and 16 weeks, respectively. Cardiac ejection fraction of hearts were significantly reduced at 4 weeks after reconstruction (p < 0.05), however, a return toward normal ventricular ejection and RV shortening values by 16 weeks was observed. () No significant differences of LV end diastolic volume were observed following reconstruction. There was no RV outflow tract obstruction observed due to either patch material. ()
Table 1. Left ventricular ejection fraction, right ventricle shortening fraction, and LV end diastolic volume of reconstructed hearts (n = 5 for each material at each time point)
Figure 3. C-ECM patched hearts showed no geometric changes in the RV and LV when compared with native hearts that had not undergone surgery. The LV of reconstructed hearts maintains its native circular shape, indicating minimal pressure changes within the RV after patch implantation. Scale indicates 6 mm.
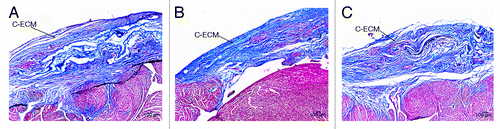
Histological examination of C-ECM reconstructed hearts showed that the patches had been incorporated into the native tissue by 4 weeks with cellular presence evident within the scaffold. Masson’s Trichrome staining showed that the presence of collagen remained at the site of patch implantation throughout the study. () The scaffold was easily observed at all time points and the reconstructed area of the RVOT remained highly collagenous. The section of the ventricular wall repaired by the C-ECM scaffolds appeared to have a similar thickness as the surrounding native wall at all time points with minimal dilation detected, although a thickening of the surrounding native myocardial wall was observed.
Figure 4. Histological examination of C-ECM patches using Masson’s Trichrome. The patches are incorporated into the native tissue by 4 weeks (A). The scaffold was also easily observed at 8 weeks (B) and 16 weeks (C) with a similar thickness as the surrounding ventricular wall and little evidence of remodeling. Scale indicates 100 µm.
Immunofluorescent examination of cardiac specific markers within the C-ECM patched hearts showed cellular presence in patches by 8 weeks after reconstruction, with a large population of GFP + cells evident in the patch. () However, the cells had not completely penetrated the thickness of the scaffold and were localized to the luminal half of the patches. C-ECM patches expressed small areas of α-actinin, commonly evident in the sarcomere structure of cardiomyocytes. However, staining was intermittent and did not show normal striated cells, suggesting that cells within the patch were immature and possessed little contractility. Positive staining within the red channel of was primarily autofluorescence from the C-ECM patch, and was included to illustrate the boundaries of the remaining material with respect to cell infiltration. The C-ECM patches showed positive staining for α-smooth muscle actin (α-SMA) in intermittent areas of the tissue and were not associated with GFP expressing cells. () Cells that had accumulated along the endocardial surface were confirmed as endothelial cells through von Willebrand factor staining. () Connexin 43 staining was also performed to identify gap junctions and electrical connections between cardiomyocytes, however, there was no observable staining within the patched area, suggesting that the cardiomyocytes present within the scaffolds were immature. (Data not shown) There was also little indication of fibrotic tissue development of the patches at all time points. Few differences were observed in cell staining within C-ECM patches between 8 week and 16 week time points.
Figure 5. Immunofluorescent examination of (A) C-ECM and (B) UBM patches for α-actinin at 8 weeks after reconstruction. A distinct presence of GFP (+) cells (green) was observed within the C-ECM patches with minimal staining for α-actinin (α-actinin, red; draq 5, blue). (B) A distinct presence of GFP (+) cells (green) as well as GFP (-) cells (blue) was observed throughout the thickness of the UBM patches with intermittent staining for α-actinin. Scale indicates 100 µm.
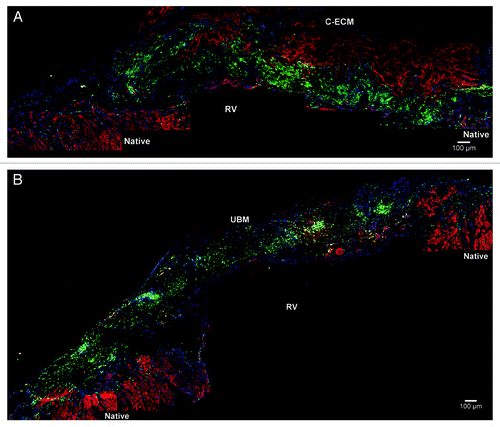
Figure 6. Positive staining was confirmed for α-smooth muscle actin (red) in both (A) C-ECM and (B) UBM scaffolds (GFP, green; draq5, blue) Scale indicates 100 µm.
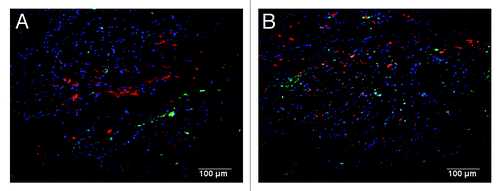
Figure 7. A continuous endothelial lining along the endocardial surface was observed in (A) C-ECM and (B) UBM scaffolds as evidenced by von Willebrand factor (green; draq5, blue). Scale indicates 100 µm.
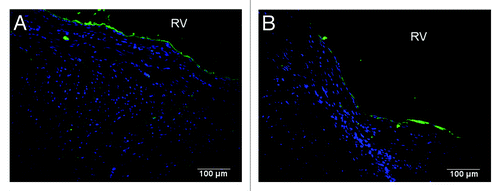
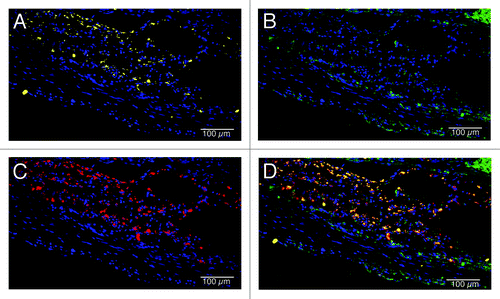
C-ECM scaffolds were analyzed for macrophage response at the 4 week time point to determine the acute response to the patches. () Macrophages had penetrated the inner half of the patches and expressed a mix of M1 and M2 cells. There were distinct spatial differences in the macrophage response at the interface with native tissue, which consisted of primarily an M1 type macrophage response, and the endocardial surface of the material, which consisted of a predominantly M2 type macrophage response.
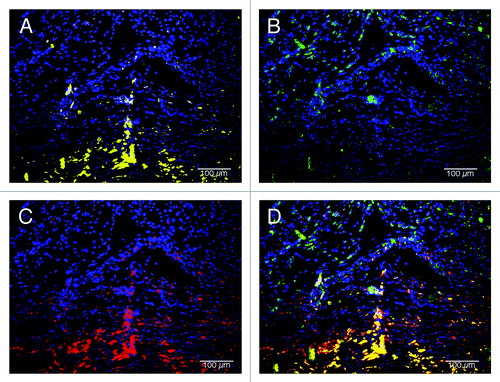
Figure 8. Macrophage phenotype analysis of C-ECM patches at 4 weeks after surgery. Macrophages had completely penetrated the patches and expressed a mix of M1 and M2 cells. (A) M1 macrophages (CD86, yellow; draq5, blue), (B) M2 macrophages (CD206, green; draq5, blue), (C) pan-macrophage (CD68, red; draq5, blue), (D) combined image. Scale indicates 100 µm.
UBM scaffolds
The UBM patches were completely incorporated into the native tissue by 4 weeks after RVOT repair surgery and the original white color of the patch was not evident upon explant at any of the time points of the study, which was a preliminary indication that the patch supported host cell infiltration and revascularization. () The thickness of UBM scaffolds was not statistically different than native RV wall thickness (0.93 ± 0.07 mm) at 4 and 16 weeks after surgery with wall thickness values of 1.02 ± 0.13 and 1.06 ± 0.16 mm, respectively. However, at 8 weeks after surgery, the RV wall thickness (0.35 ± 0.12 mm) was significantly thinner than native values. Cardiac ejection fraction was reduced at 4 weeks after implantation, however, by 16 weeks a complete return toward native ventricular ejection values was observed. () Likewise, the RV shortening fraction was compromised at 4 weeks, and by the end of the study it had also returned to native values. No geometric changes were observed in the LV, as observed from end diastolic volume, or RV of hearts through 16 weeks, suggesting that no RV outflow tract obstruction was seen by patch implantation. ()
Figure 9. UBM patched hearts showed no geometric changes in the RV and LV when compared with native hearts that had not undergone surgery. The LV of reconstructed hearts maintains its native circular shape through the end of the study, indicating minimal pressure changes within the RV after patch implantation. Scale indicates 6 mm.
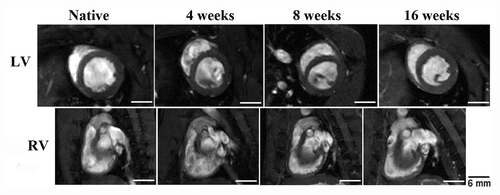
Histological examination of UBM patches showed that by 4 weeks after implantation, there was complete penetration of infiltrating cells. Cellular presence was observed within UBM patches at all time points. () In Masson’s Trichrome staining of the hearts, the morphologic presence of the scaffold was observed at 4 weeks after reconstruction. By 8 weeks, the section of the ventricular wall repaired by the UBM scaffolds showed scattered evidence that the scaffold was still present. There also appeared to be some replacement of the scaffold with small quantities of muscular tissue. By 16 weeks after reconstruction there was an observable return to native ventricular wall thickness and the scaffold could not be observed morphologically.
Figure 10. Histological examination of UBM patches using Masson’s Trichrome at 4 (A), 8 (B), and 16 (C) weeks after implantation. Cell presence is evident at all time points. (A–C) The patches had decreased in thickness at 8 weeks, but were similar to surrounding native tissue at 4 and 16 weeks. By 16 weeks, the UBM patches appeared to be completely degraded and replaced with native tissue. (C) Scale indicates 100 µm.

Immunofluorescent examination of the UBM patched hearts showed a uniform cellular distribution throughout the thickness of the patch material by 8 weeks after reconstruction, with a large population of GFP+ cells evident in the patch. () Cells within the UBM patches also expressed α-SMA in small areas of tissue throughout the patch thickness. () There was no indication of fibrotic tissue development on the patches. Cells within the remodeling UBM scaffolds had developed a continuous monolayer along the endocardial surface of the RV by 8 weeks, and no changes were observed at 16 weeks after surgery. Cells accumulating along the endocardial surface were confirmed as endothelial cells through von Willebrand factor staining. () Connexin 43 staining showed no observable gap junctions within the patched area, suggesting that cardiomyocytes present within the UBM patches were immature at this time point. (Data not shown) However, further analysis of α-actinin staining at 16 weeks within UBM reconstructed hearts showed the presence of cardiomyocytes expressing organized striated sarcomere structure (). GFP+ cells were present within UBM patches at 16 weeks after surgery, and were located in close proximity to cardiomyocytes, although no co-staining was evident. At the 4 week time point, UBM patches elicited a mixed M1/M2 acute macrophage response. () CD206+ (M2) macrophages were primarily localized to the area of the ventricular wall nearest the endocardial wall, while CD86+ (M1) cells were located within the inner portion of the patches, although both populations of cells were observed throughout the thickness of the patches.
Figure 11. α-actinin staining of UBM patches at 16 weeks after surgery. (A) The patched area shows evidence of organized sarcomere structure (red) within cardiomyocytes. (B)GFP + cells (green) can be observed within the patch, but are not associated with actinin staining. (C and D) Insets from multiple areas throughout the patch show striated actinin structure within cardiomyocytes (draq5-blue). Scale indicates 500 µm and 25 μm within insets.
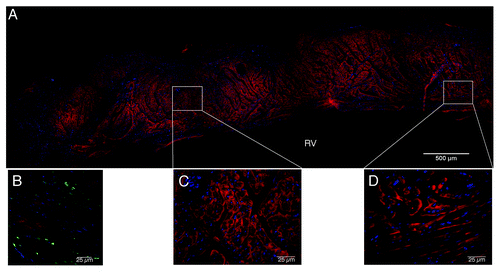
Figure 12. Macrophage phenotype analysis of UBM patches at 4 weeks after surgery. A mixed M1/M2 response was observed in the patches. Macrophages were primarily localized to the inner half of the patches, with a concentration near the interface with native tissue. (A) M1 macrophages (CD86, yellow; draq5, blue), (B) M2 macrophages (CD206, green; draq5, blue), (C) pan-macrophage (CD68, red; draq5, blue), (D) combined image. Scale indicates 100 µm.
Discussion
The present study directly compared the ability of UBM and C-ECM patches to repair a critically sized, full thickness defect created in the right ventricular outflow tract of chimera rats. Both scaffolds were able to preserve cardiac performance throughout the study, and both patches were able to support cell infiltration for up to 16 weeks after reconstruction. Neither scaffold showed signs of fibrotic encapsulation, and both scaffolds expressed a continuous endothelial lining along the endocardial wall of the RV at the site of repair. However, UBM patches were rapidly degraded and remodeled, with the formation of new host tissue in the reconstructed area by 16 weeks. Additionally, cells were able to rapidly infiltrate and fully penetrate the thickness of the UBM scaffolds by 8 weeks, with a significant number of cells originating in the bone marrow. Cardiomyocytes with healthy sarcomere patterns were observed throughout UBM, but not C-ECM, patches by the end of the study. The results from the present study suggest that UBM may be a more viable option than organ-specific ECM as a patch for myocardial repair.
It has been previously hypothesized that organ-specific ECM scaffolds are a more attractive option than heterotopically derived scaffolds in many tissue repair applications. The ECM from organ-specific origins is deposited by cells that are desired to repopulate the scaffold, and, for more geometrically complex organs, the structure of the scaffold can be preserved through perfusion decellularization.Citation34,Citation43 This hypothesis is supported by studies that have shown that liver ECM preserves the phenotype of liver specific cells better than heterotopic ECM, and studies with decellularized lung that show the ECM can promote site-appropriate differentiation of mouse embryonic stem cells.Citation28,Citation44 However, there are other examples that show that heterotopic ECM from the urinary bladder and small intestine can promote the formation of site appropriate tissue, sometimes with greater efficacy than the organ-specific ECM. For example, vacuum pressed UBM was equivalent to hydrated decellularized tracheal matrix for patch tracheoplasty, and was superior to a lyophilized form of the same.Citation45 UBM has also been shown to promote site-appropriate remodeling in the esophagus, thoracic wall, and body wall.Citation46-Citation48 Both UBM and C-ECM have been evaluated independently for myocardial repair, but variations in the model have made comparisons difficult.
Following implantation, ECM scaffolds can promote one of three distinct host remodeling responses, specifically encapsulation, integration, or remodeling.Citation48 The encapsulation and integration groups tend to include dermal products that are denser and require more aggressive decellularization protocols. The remodeling group includes SIS and UBM, which undergo a shorter decellularization process that does not use detergents, and are derived from organs that experience more rapid turnover. In addition to the commercially available products, recent efforts have sought to develop organ-specific scaffolds, including C-ECM, which are hypothesized to be preferred scaffold sources since the ECM was deposited by organ specific cells and the morphology matches that of the repair site. However, the process to produce these scaffolds tends to be more similar to the processes used for commercial scaffolds that promote encapsulation or integration responses rather than a constructive remodeling response. In the present study, UBM patches could be observed throughout the remodeling process at 4 and 8 weeks after implantation, but had been degraded and replaced by host tissue by 16 weeks. In contrast, C-ECM patches showed little evidence of remodeling. After implantation, C-ECM patches were integrated into the host myocardium were visible through the end of the study.
In recent years, there has been an increasing clinical demand for a biomaterial that has the ability to restore function to damaged myocardium. The emergence of the regenerative medicine field has provided a number of biologic based materials that have shown the ability to repair and integrate into native tissue, as well as materials that are able to remodel and restore function to damaged tissue. In a cardiac location, previous studies have investigated heterotopically derived ECM scaffolds for repair and have been met with moderate success.Citation16,Citation17,Citation19 These studies were able to show local contractility of patches and the presence of small areas of site specific cardiac cells. However, the primary result of these studies was ultimately the integration of ECM patches into the native tissue with collagenous tissue present at the endpoint of the study. The results of the present study suggest that C-ECM becomes integrated into the adjacent myocardium, while UBM promoted remodeling of immature cardiac tissue.
Through MRI examination, differences could be observed between groups in the functional analysis of reconstructed hearts throughout the study. Ejection fraction, an indicator of ventricular health, decreased at 4 weeks, but returned toward normal values by the end of the study. A 4% drop in ejection fraction was the largest difference observed between reconstructed hearts and native values, measured in C-ECM hearts at 4 weeks after reconstruction. This value was the only measurement in the study that was significantly different from native values. Maintenance of cardiac performance may be attributed to the cellular presence within both scaffolds, as there was uniform cell distribution throughout each scaffold by 8 weeks after reconstruction. Additionally, UBM scaffolds had been largely replaced with native tissue by 16 weeks, as evidenced through Masson’s Trichrome and α-actinin staining.
The present study expanded on previous work by Wainwright et al., where a C-ECM scaffold was directly compared with a currently used surgical patch material in the clinic, Dacron™.Citation33 Using a RVOT reconstruction model, Wainwright et al. showed that C-ECM supported localized site-specific cardiac cells and aided in normal cardiac function, while the implantation of Dacron™ patches resulted in fibrous encapsulation through the end point of the study. The results of the present study, however, showed that C-ECM patches were unable to support the infiltration of site-specific cells and a significant decrease in ejection fraction was observed at 4 weeks after surgery. By the end of the study, C-ECM patches had been incorporated into the surrounding myocardium. The major difference between the C-ECM in the two studies was that the C-ECM in the present study was subjected to a longer decellularization process to more effectively remove cellular debris. This potentially adds support to the idea that processing may be a stronger predictor of outcome than tissue origin.Citation49 The present study sought to compare UBM and C-ECM scaffolds as they are currently produced, and did not account for differences in processing techniques between the scaffolds to ensure that each material is fully decellularized through previously optimized protocols. Obvious changes to the mechanical and chemical constituents of the scaffolds are expected if the production methods are altered, and future studies are necessary to examine the effects of controlling for such differences.
The exact cell populations involved in the host response to ECM patch implantation in a cardiac location has not previously been investigated, but it is hypothesized that bone marrow derived cells play a role. Previous mouse studies have suggested that bone marrow cells are recruited to the site of ECM implantation and participate in the remodeling response, although the specific role is not fully understood.Citation50-Citation54 However, the surgical complexity of cardiac repair in mouse models prohibits their use, and larger animal models do not offer the capability to label bone marrow cells and track the origin of resident cells.
In the present study, bone marrow derived cells were among the first to infiltrate both scaffolds through observation of GFP expression. As the scaffolds were repopulated, an increasing number of non-GFP expressing cells could be observed up to 8 weeks after reconstruction. Non-GFP cells appeared to be primarily localized near the periphery of the scaffolds and near the interface with native tissue. By 16 weeks, there was little indication that cells were still migrating from the bone marrow to the site of implantation and a large reduction of GFP expressing cells was observed in both scaffolds. Although the present study did not quantify the exact number of GFP expressing cells, similar expression levels were observed at each time point. In UBM reconstructed hearts, cardiomyocytes could be easily observed throughout the area and were not associated with GFP staining. However, in C-ECM reconstructed hearts, cardiomyocyte presence was absent and GFP expressing cells did not associate with site-specific cell markers.
GFP expression tended to be associated with the presence of macrophages within the scaffold. Recently, macrophage phenotype at early time points (< 1 mo) have been shown to be predictive of downstream encapsulation or site-appropriate tissue remodeling.Citation48,Citation55 Briefly, the persistence of increased pro-inflammatory, M1 type, macrophage populations within implanted ECM scaffold materials has been associated with encapsulation of the material or degradation without downstream formation of site-appropriate tissues. Conversely, increased M2 populations have been associated with a more rapid resolution of the inflammatory response and improved site-appropriate tissue formation. In the present study, a qualitative assessment of macrophage phenotype was performed, showing that both samples elicited a mixed M1/M2 macrophage population. However, the spatial location of these cells within the implanted samples was observed to differ. C-ECM implants were characterized by a predominance of the M1 phenotype at the interface with native tissue and M2 cells toward the endocardial surface of the remodeling samples. UBM implants were characterized by a mixed M1/M2 population which was located within the area closest to the native tissue. The superficial portion of the remodeling implant was populated with cells at the 4 week time point, however these cells did not stain positive for macrophage markers. This may indicate that bone marrow derived cells may influence remodeling through the differentiation of macrophages with respect to the ECM. It should be noted that macrophage phenotype occurs along a spectrum between M1 and M2 phenotypes and cells may express markers of both M1 and M2 phenotypes concurrently.Citation56,Citation57 Cells expressing both CD86 and CD206 concurrently were observed in both C-ECM and UBM implants. The exact implications of these cells in the process of cardiac tissue remodeling is largely unknown and a subject of significant interest.
An important question remains regarding the origin of the cells found in the remodeled UBM after 16 weeks. It has long been believed that resident cardiac cells are non-migratory and that there is little turnover within adult myocardium. However, there has been recent evidence that cardiac cells can migrate to areas of injury.Citation58,Citation59 In the present study, cardiomyocytes observed within remodeled UBM patches did not express GFP, indicating that the cells did not originate in the bone marrow. Preliminary hypotheses for the origin of these cells include cardiac progenitor cells or pre-existing cardiomyocytes within the surrounding tissue; however, future studies are required to determine the exact origin.
The primary limitations of this study stem from the use of a small animal model for cardiac tissue repair. The smaller size and lower pressures observed in the rat model do not provide an adequate prediction to the potential performance in a human setting. The current models for investigation of myocardial reconstruction and vascular treatments are juvenile sheep or piglets due to the similarities in the size, growth rates, and calcification issues as humans. However, the primary focus of this study was the differences in the cellular infiltration, distribution, and overall remodeling responses of the scaffolds as they are currently produced. The use of rat-derived ECM may have provided a more appropriate control for material performance, however, rat ECM scaffolds would not be feasible as a clinical material and the means of generating these scaffolds would not be applicable. Obvious differences exist between the mechanical structure and environment of the porcine organs of origin and the ultimate site of repair as well. However, the development of a chimera population allowed for more complete analysis of cell origin. Additionally, the use of a well-established RVOT surgical model for rats allowed for direct comparison to previous studies. Future experiments are necessary to investigate the performance of ECM patches in larger animals to more closely mimic the native environment of human hearts.
Conclusion
ECM scaffolds provide distinct advantages to currently used biomaterials for myocardial reconstruction. The ability to restore function in a cardiac location, however, is paramount to the long-term success of the scaffold. Previous studies have shown that artificial materials are completely encapsulated with fibrotic tissue and do not develop an endothelium. Wainwright et al. showed that C-ECM avoided fibrotic encapsulation, was integrated into the native tissue, and was able to support small areas of contractile cells with the development of an endothelial lining.Citation33 In contrast with previously held hypotheses, the results of the present study showed that UBM scaffolds may provide a more viable option for myocardial reconstruction than a scaffold derived from a cardiac location. The ability for the UBM patches to completely and rapidly degrade, while being replaced with newly formed site-appropriate tissue is a significant finding. These results are in direct contrast with results from the C-ECM patches, which showed integration into the cardiac tissue with little evidence of remodeling or presence of cardiomyocytes at the end of the study. Additional studies are needed to investigate both scaffolds on a more physiologically relevant scale, but this study provides evidence that the use of an organ-specific patch material in a cardiac location may not be the most appropriate approach in future studies.
Materials and Methods
Study design
Animals with confirmed chimerism (GFP+ bone marrow) were anesthetized and intubated for right ventricle outflow tract reconstruction surgery as previously described.Citation33,Citation42 A small (2–5 mm) defect was created in the RVOT of chimera rats and subsequently patched with one of the test materials (C-ECM, UBM, n = 5 for each material at each time point). The animals were monitored for 4, 8 and 16 weeks. MRI examination of the reconstructed area took place at 4, 8, and 16 weeks in all available animals for functional analysis. At the predetermined time points, animals were euthanized by injection of 1M KCl directly into the heart. The hearts were then removed and prepared for frozen histologic processing for staining with cardiac, endothelial, and macrophage specific cell markers.
Bone marrow transplantation
Female Sprague Dawley rats were placed in a small box for whole body irradiation. An X-Ray Irradiator (XRad 320, Rangos Research Facility) was set to maximum amperage (12.5 A) and voltage (320 kV) and recipient animals were lethally irradiated (10 Gy at 1 Gy/min). Following irradiation, animals were transferred to an immune compromised animal room for the remainder of the study. A GFP + transgenic Sprague Dawley male donor rat was then euthanized, and the tibias and femurs from both legs were removed and placed in DMEM/F12 media containing 10% FBS, 1% PS, and 10,000 units of Heparin. Bone marrow was isolated by flushing the bones with media using a 23 gauge needle. Marrow and media isolate was centrifuged at 1,500 rpm for 7 min and resuspended in 10 mL of RBC lysis buffer for 10 min. The cell suspension was centrifuged again and resuspended in media, filtered through a 70 μm cell strainer, and a cell count was performed. A cell population of greater than 6 × 107 was confirmed and cells were then resuspended in 1 mL of media. The cell suspension was divided into two 1 mL syringes and placed on ice until ready for injection.
Irradiated rats were then rescued by injection of a minimum of 6 × 107 bone marrow cells after primary isolation. Animals were anesthetized and maintained at 2–3% Isoflurane at 1 L/min of O2 and a 25 gauge needle was inserted into the tail vein. Each rat received 0.5 mL of bone marrow cell suspension directly into the tail vein, and 0.2 g of Cefazolin was injected intramuscularly into the hind leg. Antibiotics were injected every other day for the ten days after bone marrow transfer, alternating injection sites between hind legs. At 30–60 d post-irradiation, a small sample of blood was taken from the tail vein and a blood smear was performed on a slide and less than 0.5 mL of blood was mixed with culture medium. The slide was observed under fluorescence for presence of GFP(+) cells, and the tube containing blood was centrifuged at 1500rpm for 7 min. The supernatant was aspirated and the cells were resuspended in RBC lysis buffer for ten minutes. The cells were centrifuged a second time and resuspended in a small volume of media for flow cytometry analysis. Cell suspensions were analyzed for both GFP presence and front and side scatter plots to identify GFP(+) WBC percentages. Chimera creation was verified by a GFP(+) WBC percentage above 93%.
Preparation of ECM patches
Whole pig hearts were obtained and subjected to a previously described method for decellularization.Citation39,Citation43 Following decellularization, the ventricular walls were separated and a small portion of the right ventricle near the apex was removed. This portion of the ventricle is much thinner than the surrounding myocardium and is most suitable for rat heart reconstruction. The ventricular wall was lyophilized overnight and 6mm diameter circular patches were cut from the tissue. The patches were packaged individually and sterilized using ethylene oxide gas prior to implantation. UBM sheets (Matristem™ Wound Sheets) were obtained from ACell, Inc. and were removed from sterile packaging, cut to 6mm diameter patches, and re-sterilized in a similar manner as the C-ECM patches.
Surgical repair of RVOT
After verification of chimerism, animals were prepared for RVOT reconstruction surgery as previously described.Citation33,Citation42 Anesthesia was induced by placing the rats in a small container with 3% Isoflurane in 2 L/minute of O2. A 16 G × 2″ angiocatheter sheath was inserted in the trachea. Proper insertion of the intubation catheter was ensured through inflation of lungs with a small ambubag. A rodent ventilation system (SAR-830/P) was set at approximately 75 breaths per minute and approximately 700 mL air/minute. Hair was removed from the chest of the rat and the site was sterilized with Iodine. An initial injection of 10 mg/kg lidocaine was delivered locally, and the Isoflurane was reduced to 1.5–2%. A 5cm incision was made in the chest with a #10 scalpel, and a thoracotomy was performed to expose the heart. The ribs were held open with an Alm retractor. A purse-string suture (with diameter of 5.0–6.0 mm) was placed in the free wall of the right ventricular outflow tract (RVOT) with 7–0 polypropylene sutures. Both ends of the stitch were passed through a 22-gauge plastic vascular cannula, which was used as a tourniquet. The tourniquet was tightened and the bulging part of the RVOT wall inside the purse-string stitch was resected. The tourniquet was then briefly released to verify a transmural defect was created in the RVOT as indicated by severe bleeding. One of the proposed patches was then sutured along the margin of the purse-string suture with over-and-over sutures with 7–0 polypropylene to cover the hole in the RVOT. After completion of suturing, the tourniquet was released and the purse-string stitch removed. The muscle layer was then closed with approximately 8 interrupted sutures (5–0 Surgipro). Prior to closure of the chest, the lungs were inflated to full capacity using a pediatric ambubag attached to the ventilator. Approximately 8 interrupted sutures were placed to close the skin and a local injection of 10 mg/kg lidocaine was delivered. Additionally, a dose of 200 mg/kg/d cefazolin was delivered to the thigh muscle, as well as 0.1 mg/kg buprenorphine (buprenex), subcutaneously. Doses of cefazolin were delivered once daily for 3 d post-operative and buprenorphine was delivered twice daily for the same time period.
Cardiac MRI
Cardiac MRI (Horizontal bore 7-T MRI system, Bruker Biospin 70/30) was performed for detailed assessment of cardiac function of all hearts treated with ECM. Animals were anesthetized with 1.5 to 2% Isoflurane in oxygen gas via nose cone during MRI imaging. Animal body temperature, heart rate, respiratory rate, and arterial oxygen saturation were continuously monitored using a vital monitoring system. The total scanning time for each animal was approximately 45 to 60 min. Under electrocardiogram and respiratory gating, right (left lateral image plane) and left ventricular (long and short axis image planes) wall motions were recorded by a FLASH cine image sequence. Cardiac MRI was performed at 4, 8 and 16 weeks after ECM patch implantation and images were compared with those taken from a native heart. All videos detailed a minimum of one full cardiac cycle so that distinct measurements could be taken from the left and right ventricles throughout systole and diastole. Cardiac function was assessed by calculation of ejection fraction and end diastolic volume from the LV, as well as shortening fraction from RV outflow tract where graft was implanted using OsiriX software. A repeated measures, one way analysis of variance (ANOVA) was performed on all samples to determine significant differences (p < 0.05) from native values for ejection fraction, RV shortening fraction, and LV end diastolic volume.
Specimen processing
At time points of 4, 8 and 16 weeks, animals were euthanized by injection of 5mL of 1M KCl directly into the heart. The hearts were then removed and fixed in 4% paraformaldehyde for 24 h. Hearts were then moved to 30% sucrose for another 24 h. The hearts were then cut in half through the patched area, frozen in OCT solution at -80°C, and sections were cut at 8 µm thickness and placed onto slides for future staining.
Immunofluorescent staining
All specimens were permeabilized with 0.1 M glycine, 0.5% Triton X-100 in PBS for 15 min. The specimens were then washed five times in 1× PBS and then incubated with 1% goat serum for 1 h. After the hour, the specimens were again washed three times in 1% BSA. The primary antibodies [α-actinin (Sigma aldrich, A7811), connexin 43 (Abcam, ab11370), von Willebrand factor (Abcam, ab6994), α-smooth muscle actin (Abcam, ab7817), and GFP (Invitrogen, G10362)] were then added and incubated for 2 h at room temperature and then washed five times in 1% BSA. After these washes, the secondary antibodies (AlexaFluor A21125-594 and AlexaFluor A11008-488, respectively) and Draq5 for nuclear staining were added and incubated for another two hours. Hoechst (1 mg/100 mL) solution was then added for 30 sec and then washed five times in 1% BSA. Slides were covered in mounting medium, coverslipped, and sealed until imaging.
Macrophage phenotype analysis
Macrophage staining was performed on samples in order to describe the immune response of the ECM patches as recently described.Citation48,Citation60 Antibodies for CD68 (pan-macrophage), CD86 (M1), and CD206 (M2) were used for an investigation of the M1 and M2 macrophage phenotypes. Prior to staining, samples were submerged in a solution of methanol to quench the GFP signal. After the elimination of GFP signal was verified, slides were washed in PBS and then incubated in a blocking serum consisting of horse serum, BSA, Triton X-100, and Tween 20. Blocking solution was removed and a 1:150 dilution of mouse anti-rat CD68, rabbit anti-CD86, and goat anti-CD206 antibodies in blocking solution was added to the slides at 4°C overnight. The following day, the slides were washed in PBS three times to remove primary antibodies. Secondary antibodies were added to blocking solution at the following concentrations; donkey anti-goat AlexaFluor 488 and donkey anti-mouse AlexaFluor 594 (1:200), donkey anti-rabbit Perp Cy5.5 (1:300). Secondary antibodies were added to the slides and allowed to incubate at room temperature for 1 h. Slides were washed three times in PBS to remove the secondary antibody. Mounting media with DAPI and coverslips were then added to each slide prior to imaging.
Acknowledgments
Funding for this study was provided by NIH Grant R03EB009237 (TWG), the support of the William G. McGowan Charitable Fund's support to the McGowan Institute for Regenerative Medicine, and Children’s Hospital of Pittsburgh of UPMC, as well as NIH Training Grants T32EB001026-06 from the National Institute of Biomedical Imaging And Bioengineering (NTR) and T32HL076124-05 entitled “Cardiovascular Bioengineering Training Program” (NTR). The authors would like to recognize contributions from Dr Lei Yang at Children’s Hospital of Pittsburgh as well as Dr John Wainwright.
Disclosure of Potential Conflicts of Interest
TWG served on the Scientific Advisory Board for ACell, Inc. during the completion of this work, and is now the Vice President of Research and Development.
References
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al, American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008; 117:e25 - 146; http://dx.doi.org/10.1161/CIRCULATIONAHA.107.187998; PMID: 18086926
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al, American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation 2013; 127:e6 - 245; http://dx.doi.org/10.1161/CIR.0b013e31828124ad; PMID: 23239837
- Report of the New England Regional Infant Cardiac Program. Report of the New England Regional Infant Cardiac Program. Pediatrics 1980; 65:375 - 461; PMID: 7355042
- Ozawa T, Mickle DA, Weisel RD, Koyama N, Ozawa S, Li RK. Optimal biomaterial for creation of autologous cardiac grafts. Circulation 2002; 106:Suppl 1 I176 - 82; PMID: 12354729
- Ozawa T, Mickle DA, Weisel RD, Koyama N, Wong H, Ozawa S, et al. Histologic changes of nonbiodegradable and biodegradable biomaterials used to repair right ventricular heart defects in rats. J Thorac Cardiovasc Surg 2002; 124:1157 - 64; http://dx.doi.org/10.1067/mtc.2002.127449; PMID: 12447182
- Breymann T, Blanz U, Wojtalik MA, Daenen W, Hetzer R, Sarris G, et al. European Contegra multicentre study: 7-year results after 165 valved bovine jugular vein graft implantations. Thorac Cardiovasc Surg 2009; 57:257 - 69; http://dx.doi.org/10.1055/s-0029-1185513; PMID: 19629887
- Konuma T, Devaney EJ, Bove EL, Gelehrter S, Hirsch JC, Tavakkol Z, et al. Performance of CryoValve SG decellularized pulmonary allografts compared with standard cryopreserved allografts. Ann Thorac Surg 2009; 88:849 - 54, discussion 554-5; http://dx.doi.org/10.1016/j.athoracsur.2009.06.003; PMID: 19699910
- Hickey EJ, Veldtman G, Bradley TJ, Gengsakul A, Manlhiot C, Williams WG, et al. Late risk of outcomes for adults with repaired tetralogy of Fallot from an inception cohort spanning four decades. Eur J Cardiothorac Surg 2009; 35:156 - 64, discussion 164; http://dx.doi.org/10.1016/j.ejcts.2008.06.050; PMID: 18848456
- Rajani B, Mee RB, Ratliff NB. Evidence for rejection of homograft cardiac valves in infants. J Thorac Cardiovasc Surg 1998; 115:111 - 7; http://dx.doi.org/10.1016/S0022-5223(98)70449-0; PMID: 9451053
- Dora CD, Dimarco DS, Zobitz ME, Elliott DS. Time dependent variations in biomechanical properties of cadaveric fascia, porcine dermis, porcine small intestine submucosa, polypropylene mesh and autologous fascia in the rabbit model: implications for sling surgery. J Urol 2004; 171:1970 - 3; http://dx.doi.org/10.1097/01.ju.0000121377.61788.ad; PMID: 15076323
- Butler CE, Langstein HN, Kronowitz SJ. Pelvic, abdominal, and chest wall reconstruction with AlloDerm in patients at increased risk for mesh-related complications. Plast Reconstr Surg 2005; 116:1263 - 75, discussion 1276-7; http://dx.doi.org/10.1097/01.prs.0000181692.71901.bd; PMID: 16217466
- Chen SG, Tzeng YS, Wang CH. Treatment of severe burn with DermACELL(®), an acellular dermal matrix. Int J Burns Trauma 2012; 2:105 - 9; PMID: 23071908
- Freedman BE. Full incorporation of Strattice™ Reconstructive Tissue Matrix in a reinforced hiatal hernia repair: a case report. J Med Case Rep 2012; 6:234; http://dx.doi.org/10.1186/1752-1947-6-234; PMID: 22876792
- Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, et al. Tissue-engineered lungs for in vivo implantation. Science 2010; 329:538 - 41; http://dx.doi.org/10.1126/science.1189345; PMID: 20576850
- Quarti A, Nardone S, Colaneri M, Santoro G, Pozzi M. Preliminary experience in the use of an extracellular matrix to repair congenital heart diseases. Interact Cardiovasc Thorac Surg 2011; 13:569 - 72; http://dx.doi.org/10.1510/icvts.2011.280016; PMID: 21979987
- Kelly DJ, Rosen AB, Schuldt AJ, Kochupura PV, Doronin SV, Potapova IA, et al. Increased myocyte content and mechanical function within a tissue-engineered myocardial patch following implantation. Tissue Eng Part A 2009; 15:2189 - 201; http://dx.doi.org/10.1089/ten.tea.2008.0430; PMID: 19231971
- Kochupura PV, Azeloglu EU, Kelly DJ, Doronin SV, Badylak SF, Krukenkamp IB, et al. Tissue-engineered myocardial patch derived from extracellular matrix provides regional mechanical function. Circulation 2005; 112:Suppl I144 - 9; PMID: 16159807
- Badylak SF, Kochupura PV, Cohen IS, Doronin SV, Saltman AE, Gilbert TW, et al. The use of extracellular matrix as an inductive scaffold for the partial replacement of functional myocardium. Cell Transplant 2006; 15:Suppl 1 S29 - 40; http://dx.doi.org/10.3727/000000006783982368; PMID: 16826793
- Robinson KA, Li J, Mathison M, Redkar A, Cui J, Chronos NA, et al. Extracellular matrix scaffold for cardiac repair. Circulation 2005; 112:Suppl I135 - 43; PMID: 16159805
- Ota T, Gilbert TW, Badylak SF, Schwartzman D, Zenati MA. Electromechanical characterization of a tissue-engineered myocardial patch derived from extracellular matrix. J Thorac Cardiovasc Surg 2007; 133:979 - 85; http://dx.doi.org/10.1016/j.jtcvs.2006.11.035; PMID: 17382638
- Soto-Gutierrez A, Zhang L, Medberry C, Fukumitsu K, Faulk D, Jiang H, et al. A whole-organ regenerative medicine approach for liver replacement. Tissue Eng Part C Methods 2011; 17:677 - 86; http://dx.doi.org/10.1089/ten.tec.2010.0698; PMID: 21375407
- Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med 2010; 16:814 - 20; http://dx.doi.org/10.1038/nm.2170; PMID: 20543851
- Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med 2010; 16:927 - 33; http://dx.doi.org/10.1038/nm.2193; PMID: 20628374
- Elliott MJ, Haw MP, Jacobs JP, Bailey CM, Evans JN, Herberhold C. Tracheal reconstruction in children using cadaveric homograft trachea. Eur J Cardiothorac Surg 1996; 10:707 - 12; http://dx.doi.org/10.1016/S1010-7940(96)80328-9; PMID: 8905270
- Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, et al. Clinical transplantation of a tissue-engineered airway. Lancet 2008; 372:2023 - 30; http://dx.doi.org/10.1016/S0140-6736(08)61598-6; PMID: 19022496
- Remlinger NT, Czajka CA, Juhas ME, Vorp DA, Stolz DB, Badylak SF, et al. Hydrated xenogeneic decellularized tracheal matrix as a scaffold for tracheal reconstruction. Biomaterials 2010; 31:3520 - 6; http://dx.doi.org/10.1016/j.biomaterials.2010.01.067; PMID: 20144481
- Sellaro TL, Ravindra AK, Stolz DB, Badylak SF. Maintenance of hepatic sinusoidal endothelial cell phenotype in vitro using organ-specific extracellular matrix scaffolds. Tissue Eng 2007; 13:2301 - 10; http://dx.doi.org/10.1089/ten.2006.0437; PMID: 17561801
- Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G, et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A 2010; 16:2565 - 80; http://dx.doi.org/10.1089/ten.tea.2009.0730; PMID: 20408765
- Soto-Gutierrez A, Wertheim JA, Ott HC, Gilbert TW. Perspectives on whole-organ assembly: moving toward transplantation on demand. J Clin Invest 2012; 122:3817 - 23; http://dx.doi.org/10.1172/JCI61974; PMID: 23114604
- Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials 2011; 32:3233 - 43; http://dx.doi.org/10.1016/j.biomaterials.2011.01.057; PMID: 21296410
- Gilbert TW. Strategies for tissue and organ decellularization. J Cell Biochem 2012; 113:2217 - 22; http://dx.doi.org/10.1002/jcb.24130; PMID: 22415903
- Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials 2009; 30:5409 - 16; http://dx.doi.org/10.1016/j.biomaterials.2009.06.045; PMID: 19608268
- Wainwright JM, Hashizume R, Fujimoto KL, Remlinger NT, Pesyna C, Wagner WR, et al. Right ventricular outflow tract repair with a cardiac biologic scaffold. Cells Tissues Organs 2012; 195:159 - 70; http://dx.doi.org/10.1159/000331400; PMID: 22025093
- Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med 2008; 14:213 - 21; http://dx.doi.org/10.1038/nm1684; PMID: 18193059
- Lehr EJ, Rayat GR, Chiu B, Churchill T, McGann LE, Coe JY, et al. Decellularization reduces immunogenicity of sheep pulmonary artery vascular patches. J Thorac Cardiovasc Surg 2011; 141:1056 - 62; PMID: 20637475
- Xiong Y, Chan WY, Chua AW, Feng J, Gopal P, Ong YS, et al. Decellularized Porcine Saphenous Artery for Small-Diameter Tissue-Engineered Conduit Graft. Artif Organs 2013; 9; PMID: 23566255
- Lofland GK, O’Brien JE Jr., Gandy KL, Dennis PA, Marshall JA, Mastbergen RK, et al. Initial pediatric cardiac experience with decellularized allograft patches. Ann Thorac Surg 2012; 93:968 - 71; http://dx.doi.org/10.1016/j.athoracsur.2011.09.039; PMID: 22364987
- Singelyn JM, DeQuach JA, Christman KL. Injectable myocardial matrix as a scaffold for myocardial tissue engineering. Conf Proc IEEE Eng Med Biol Soc 2009; 2009:2406 - 8; PMID: 19964956
- Wainwright JM, Czajka CA, Patel UB, Freytes DO, Tobita K, Gilbert TW, et al. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng Part C Methods 2010; 16:525 - 32; http://dx.doi.org/10.1089/ten.tec.2009.0392; PMID: 19702513
- Remlinger NT, Wearden PD, Gilbert TW. Procedure for decellularization of porcine heart by retrograde coronary perfusion. J Vis Exp 2012; e50059; PMID: 23242494
- Ota T, Gilbert TW, Schwartzman D, McTiernan CF, Kitajima T, Ito Y, et al. A fusion protein of hepatocyte growth factor enhances reconstruction of myocardium in a cardiac patch derived from porcine urinary bladder matrix. J Thorac Cardiovasc Surg 2008; 136:1309 - 17; http://dx.doi.org/10.1016/j.jtcvs.2008.07.008; PMID: 19026821
- Fujimoto KL, Guan J, Oshima H, Sakai T, Wagner WR. In vivo evaluation of a porous, elastic, biodegradable patch for reconstructive cardiac procedures. Ann Thorac Surg 2007; 83:648 - 54; http://dx.doi.org/10.1016/j.athoracsur.2006.06.085; PMID: 17258002
- Remlinger NT, Wearden PD, Gilbert TW. Procedure for decellularization of porcine heart by retrograde coronary perfusion. J Vis Exp 2012; 70:e50059; PMID: 23242494
- Sellaro TL, Ranade A, Faulk DM, McCabe GP, Dorko K, Badylak SF, et al. Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels. Tissue Eng Part A 2010; 16:1075 - 82; http://dx.doi.org/10.1089/ten.tea.2008.0587; PMID: 19845461
- Gilbert TW, Gilbert S, Madden M, Reynolds SD, Badylak SF. Morphologic assessment of extracellular matrix scaffolds for patch tracheoplasty in a canine model. Ann Thorac Surg 2008; 86:967 - 74, discussion 967-74; http://dx.doi.org/10.1016/j.athoracsur.2008.04.071; PMID: 18721593
- Nieponice A, McGrath K, Qureshi I, Beckman EJ, Luketich JD, Gilbert TW, et al. An extracellular matrix scaffold for esophageal stricture prevention after circumferential EMR. Gastrointest Endosc 2009; 69:289 - 96; http://dx.doi.org/10.1016/j.gie.2008.04.022; PMID: 18657808
- Gilbert TW, Nieponice A, Spievack AR, Holcomb J, Gilbert S, Badylak SF. Repair of the thoracic wall with an extracellular matrix scaffold in a canine model. J Surg Res 2008; 147:61 - 7; http://dx.doi.org/10.1016/j.jss.2007.04.035; PMID: 17950323
- Brown BN, Londono R, Tottey S, Zhang L, Kukla KA, Wolf MT, et al. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater 2012; 8:978 - 87; http://dx.doi.org/10.1016/j.actbio.2011.11.031; PMID: 22166681
- Keane TJ, Londono R, Turner NJ, Badylak SF. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 2012; 33:1771 - 81; http://dx.doi.org/10.1016/j.biomaterials.2011.10.054; PMID: 22137126
- Badylak SF, Park K, Peppas N, McCabe G, Yoder M. Marrow-derived cells populate scaffolds composed of xenogeneic extracellular matrix. Exp Hematol 2001; 29:1310 - 8; http://dx.doi.org/10.1016/S0301-472X(01)00729-9; PMID: 11698127
- Zantop T, Gilbert TW, Yoder MC, Badylak SF. Extracellular matrix scaffolds are repopulated by bone marrow-derived cells in a mouse model of achilles tendon reconstruction. J Orthop Res 2006; 24:1299 - 309; http://dx.doi.org/10.1002/jor.20071; PMID: 16649228
- Beattie AJ, Gilbert TW, Guyot JP, Yates AJ, Badylak SF. Chemoattraction of progenitor cells by remodeling extracellular matrix scaffolds. Tissue Eng Part A 2009; 15:1119 - 25; http://dx.doi.org/10.1089/ten.tea.2008.0162; PMID: 18837648
- Silva KD, Gamelli RL, Shankar R. Bone marrow stem cell and progenitor response to injury. Wound Repair Regen 2001; 9:495 - 500; http://dx.doi.org/10.1046/j.1524-475x.2001.00495.x; PMID: 11896991
- Valentin JE, Stewart-Akers AM, Gilbert TW, Badylak SF. Macrophage participation in the degradation and remodeling of extracellular matrix scaffolds. Tissue Eng Part A 2009; 15:1687 - 94; http://dx.doi.org/10.1089/ten.tea.2008.0419; PMID: 19125644
- Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A 2008; 14:1835 - 42; http://dx.doi.org/10.1089/ten.tea.2007.0264; PMID: 18950271
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004; 25:677 - 86; http://dx.doi.org/10.1016/j.it.2004.09.015; PMID: 15530839
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8:958 - 69; http://dx.doi.org/10.1038/nri2448; PMID: 19029990
- Malliaras K, Zhang Y, Seinfeld J, Galang G, Tseliou E, Cheng K, et al. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med 2013; 5:191 - 209; http://dx.doi.org/10.1002/emmm.201201737; PMID: 23255322
- Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, et al. Transient regenerative potential of the neonatal mouse heart. Science 2011; 331:1078 - 80; http://dx.doi.org/10.1126/science.1200708; PMID: 21350179
- Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 2009; 30:1482 - 91; http://dx.doi.org/10.1016/j.biomaterials.2008.11.040; PMID: 19121538