Abstract
Organ grafts developed in the xenogeneic pig scaffold are expected to resolve most issues of donor safety and ethical concerns about living-donor liver transplantation in Japan. We have been working on so-called “Yamaton” projects to develop transplantable organs using genetically engineered pigs. Our goal is to produce chimeric livers with human parenchyma in such pigs. The Yamaton-Liver project demonstrated the proof of concept by showing that rat–mouse chimeric livers could develop in mice and be successfully transplanted into syngeneic or allogeneic rats. Under conventional immunosuppression, the transplanted livers showed long-term function and protection against rejection. Because chimeric liver grafts have xenogeneic components, additional strategies, such as humanization of pig genes, induction of hematopoietic chimeras in donors, and replacement of pig endothelial cells with human ones, might be required in clinical use. Our projects still need to overcome various hurdles but can bring huge benefits to patients in the future.
The transplantation of livers harvested from living donors is a well established, widely used surgical technique in Japan. More than 6000 patients have already received this treatment with high graft and patient survival rates.Citation1 Living-donor liver transplantation was introduced in Japan as a last resort to rescue many patients who were waiting to die at a time when “brain-death” donors were unacceptable in our society. The program of living-donor liver transplantation started for pediatric patients, for whom parents volunteered as donors. The outcomes have been wonderful.Citation2,Citation3 However, as the application expanded to adult patients, the roles have been reversed. Adult children donate their livers to their parents who need larger grafts, thereby putting themselves at risk of developing liver insufficiency.Citation4
Alternative organ grafts are believed to resolve most concerns in organ transplantation.Citation5 Various approaches have already been tried to safely obtain sufficient numbers of functional organs, and organ engineering by using regenerative technology is regarded as a promising approach.Citation6 To create an organ, an appropriate scaffold for regeneration is vital to turning cell sources (stem cells, progenitor cells, and so on) into clinically available organs.Citation7 In addition to various biomaterials, the scaffold of xenogeneic animals has been attracting attention and widely studied today. Because of their size and anatomy,Citation8 pigs are regarded as the major candidate for clinical applications and have been investigated, following the studies with rodent models. The recent achievements in both rodent and pig models are summarized in .Citation9-Citation22
Table 1. Applications of pig as humanized-graft resource
One of the approaches is to utilize the organogenetic potential of scaffold animals. Although it is still hard to control complex of signals and interactions for organ development, this concept allows cells to make use of almost optimal environment. Scaffold animals have genetic impairment of specific organ developments and injected normal cells complement the niche of host animals. Kobayashi et al. injected rat wild-type pluripotent stem cells into the blastocyst of Pdx1(−/−) pancreatogenesis-disabled mouse, generating rat pancreas in the scaffold Pdx1(−/−) mouse.Citation9 Usui et al. also demonstrated the kidney generation by injecting wild-type mouse pluripotent stem cells into the blastocyst of Shall1(−/−) nephrogenesis-disabled mouse.Citation11 We transplanted rat primary hepatocytes into urokinase-type plasminogen activator/severe-combined immunodeficiency (uPA/SCID) mice and created chimeric livers with rat-origin parenchyma and mouse-origin non-parenchymal components.Citation10 Using a pig as a larger animal scaffold, Matsunari et al. injected blastomeres derived from allogeneic donor pig with normal pancreas development into the embryo from pancreatogenesis-disabled pig and produced normal pancreas in host pig.Citation12 Fisher et al. injected human hepatocytes directly into the fetal pig liver on gestational day 40 and again 1 week after birth, resulting in successful engraftment of human hepatocyte in postnatal pigs.Citation13 Although this concept enables effective development of organs, it is inevitable that some xenogeneic components remain in the engineered organs. Furthermore, the maintenance of transplanted human cells in a pig scaffold is also important. Suzuki et al. recently reported the generation of SCID pigCitation14 and this kind of transgenic advancement might resolve this issue.
Another approach is to utilize embryonic primordium derived from xenogeneic animals. Since such primordium has potential to develop a matured organ, it could continue the developmental process using recipient’s components after transplantation. In our collaborating kidney production group, Matsumoto et al. transplanted rat metanephroi into mouse omentum and demonstrated successful development of erythropoietin producing tissue from host mesenchymal stem cell population.Citation15 They also succeeded in eliminating the tissues of xenogeneic metanephroi using the suicide-inducible mouse as metanephron donor. Embryonic primordium also has lower transplant immunogenicity. Using pig pancreatic primordium, Hammerman transplanted it to diabetic rats and rhesus macaques and shown the improvement of glucose tolerance.Citation16 He also demonstrated xenogeneic islet cell transplantation without immunosuppression following primordium transplantation.
The other approach to use animal scaffolds is to utilize their organ for decellularization. Organ framework after decellularization provides appropriate architecture of organ and potential interactions as a scaffold for cell regeneration. Ott et al. decellularized rat heartCitation17 and lung,Citation20 and recellularized them with rat cardiocytes and lung cells, respectively, demonstrating their functions. Ross et al. transplanted murine embryonic stem cells into rat decellularized kidney.Citation18 Uygun et al. also demonstrated engineering liver using rat decellularized liver matrix and rat primary hepatocytes.Citation19 In porcine models, Orlando et al. decellularized porcine kidney and transplanted the acellular scaffold to pig recipients with vessel reconstructions.Citation21 Yagi et al. demonstrated the decellularization of porcine liver and successful engraftment of primary hepatocytes in vitro.Citation22 However, this strategy still requires further advancement in control of cell engraftment, proliferation and differentiation in scaffolds in vitro.
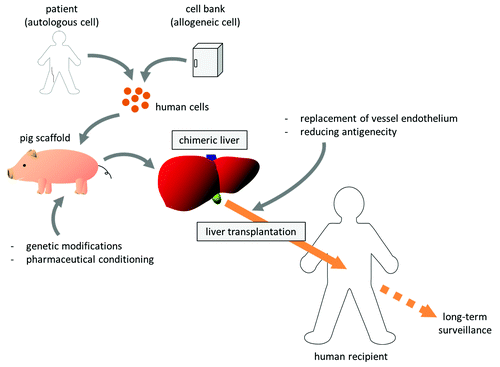
Our projects to develop new therapeutic methods reliant on pig organ donors have been dubbed “Yamaton” by Eiji Kobayashi, from the Japanese words “yamato” (an old word for Japan) and “ton” (pig). Our final goal is, using such pigs as scaffolds, to develop transplantable humanized organs (). We have been focusing on the chimeric organs produced by repopulation of transplanted cells within the xenogeneic organ which has impaired parenchyma, and working with proof-of-concept studies using rodent models as well as development of transgenic pigs.
To verify our concept to create and transplant the chimeric organ of liver, we developed a xenochimeric liver (XCL) in a mouse by repopulation with rat hepatocytes and successfully transplanted these rat–mouse XCLs into rats, with vessel reconstruction.Citation10 Hepatocytes from luciferase transgenic or luciferase/LacZ double-transgenic rats were transplanted into 20- to 30-d-old uPA/SCID mice, whose native hepatocytes were damaged by accumulation of uPA, to create XCLs with rat hepatocytes. The XCLs were then transplanted into wild-type Lewis (syngeneic recipient) and Nagase analbuminemia (allogeneic recipient) rats. Conventional immunosuppression with tacrolimus and cyclophosphamide significantly improved graft survival and reduced histological rejection of XCLs relative to transplantation without immunosuppression. Rat albumin production was maintained in the recipients for 4 mo after transplantation, and ultrasonography revealed patent circulation in the transplanted grafts for 6 mo. Ki67 staining revealed the regenerative potential of transplanted XCLs after hepatectomy of the host native liver; however, immune reactions still remained in the mouse-origin structures. These results indicated that tissue specific impairment of liver facilitated development of chimeric organs and such organ grafts were transplantable. It is also suggested that rat-origin parenchyma could maintain its functions and regenerative capacity after transplantation. Although the immunosuppression was effective, our results showed the importance of additional strategies to prevent severe rejection from the recipient toward xenogeneic components.
Although technological advances have encouraged us to create human chimeric livers in pigs, we still need to overcome the important issue of rejection as shown in our rodent work. At the present state of the technology, it would be necessary to combine supportive therapies to prevent rejection at the time of transplant into human recipients since the blood vessels come from the pig. Modifications of major pig genes, such as knockout of α-1,3-galactosyltransferase and insertion of human decay accelerating factor, have already been achieved, but other genes still cause rejection.Citation23 Unlike harvesting organs from human donors, engineering chimeric organs gives us opportunities for preconditioning or for additional modifications in the scaffold pig. Therefore, we might replace the pig vascular endothelial cells with human cells in vivo before harvest by ablation of the host endothelium and transplantation of recipient-derived endothelial progenitor cells.Citation24 Beschorner et al. demonstrated in sheep and pig xenocombination that heart grafts, harvested from pigs receiving sheep marrow cells during their fetal stage, showed prevention of acute vascular rejection after transplantation into sheep recipients.Citation25 It might be possible to reduce the immunogenicity of XCLs by induction of hematopoietic chimerism in donor pigs with bone marrow cells derived from human recipients. In addition, further improvement of immunosuppression would be beneficial.Citation26
Recent dramatic advances in regenerative technology now provide us a variety of new techniques and insights, and give us hope and motivation to open new frontiers of therapy. Chimeric organ engineering has the large advantages in effective development of target organ with appropriate three-dimensional structure, transplantable vessel system and physiological human parenchyma. Although it is still required to overcome the issues of xeno-antigenicity and xenozoonosis, once they are resolved, human-pig chimeric organs can replace the current human grafts.
| Abbreviations: | ||
| XCL | = | xenochimeric liver |
| uPA/SCID | = | urokinase-type plasminogen activator / severe-combined immunodeficiency |
Disclosure of Conflicts of Interest
EK is a chief scientific adviser to Otsuka Pharmaceutical Factory Inc. (Naruto, Japan).
References
- Japanese Liver Transplantation Society Home Page. . http://jlts.umin.ac.jp/
- Mizuta K, Urahashi T, Ihara Y, Sanada Y, Wakiya T, Yamada N, et al. Living donor liver transplantation in children with cholestatic liver disease: a single-center experience. Transplant Proc 2012; 44:469 - 72; http://dx.doi.org/10.1016/j.transproceed.2011.11.014; PMID: 22410047
- Egawa H, Tanabe K, Fukushima N, Date H, Sugitani A, Haga H. Current status of organ transplantation in Japan. Am J Transplant 2012; 12:523 - 30; http://dx.doi.org/10.1111/j.1600-6143.2011.03822.x; PMID: 22054061
- Umeshita K, Fujiwara K, Kiyosawa K, Makuuchi M, Satomi S, Sugimachi K, et al, Japanese Liver Transplantation Society. Operative morbidity of living liver donors in Japan. Lancet 2003; 362:687 - 90; http://dx.doi.org/10.1016/S0140-6736(03)14230-4; PMID: 12957090
- Soto-Gutierrez A, Wertheim JA, Ott HC, Gilbert TW. Perspectives on whole-organ assembly: moving toward transplantation on demand. J Clin Invest 2012; 122:3817 - 23; http://dx.doi.org/10.1172/JCI61974; PMID: 23114604
- Orlando G, Wood KJ, Stratta RJ, Yoo JJ, Atala A, Soker S. Regenerative medicine and organ transplantation: past, present, and future. Transplantation 2011; 91:1310 - 7; http://dx.doi.org/10.1097/TP.0b013e318219ebb5; PMID: 21505379
- Lee SJ, Atala A. Scaffold technologies for controlling cell behavior in tissue engineering. Biomed Mater 2013; 8:010201; http://dx.doi.org/10.1088/1748-6041/8/1/010201; PMID: 23355718
- Kobayashi E, Hishikawa S, Teratani T, Lefor AT. The pig as a model for translational research: overview of porcine animal models at Jichi Medical University. Transplant Res 2012; 1:8; http://dx.doi.org/10.1186/2047-1440-1-8; PMID: 23369409
- Kobayashi T, Yamaguchi T, Hamanaka S, Kato-Itoh M, Yamazaki Y, Ibata M, et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell 2010; 142:787 - 99; http://dx.doi.org/10.1016/j.cell.2010.07.039; PMID: 20813264
- Hata T, Uemoto S, Fujimoto Y, Murakami T, Tateno C, Yoshizato K, et al. Transplantation of engineered chimeric liver with autologous hepatocytes and xenobiotic scaffold. Ann Surg 2013; 257:542 - 7; http://dx.doi.org/10.1097/SLA.0b013e31825c5349; PMID: 22691372
- Usui J, Kobayashi T, Yamaguchi T, Knisely AS, Nishinakamura R, Nakauchi H. Generation of kidney from pluripotent stem cells via blastocyst complementation. Am J Pathol 2012; 180:2417 - 26; http://dx.doi.org/10.1016/j.ajpath.2012.03.007; PMID: 22507837
- Matsunari H, Nagashima H, Watanabe M, Umeyama K, Nakano K, Nagaya M, et al. Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. Proc Natl Acad Sci U S A 2013; 110:4557 - 62; http://dx.doi.org/10.1073/pnas.1222902110; PMID: 23431169
- Fisher JE, Lillegard JB, McKenzie TJ, Rodysill BR, Wettstein PJ, Nyberg SL. In utero transplanted human hepatocytes allow postnatal engraftment of human hepatocytes in pigs. Liver Transpl 2013; 19:328 - 35; http://dx.doi.org/10.1002/lt.23598; PMID: 23280879
- Suzuki S, Iwamoto M, Saito Y, Fuchimoto D, Sembon S, Suzuki M, et al. Il2rg gene-targeted severe combined immunodeficiency pigs. Cell Stem Cell 2012; 10:753 - 8; http://dx.doi.org/10.1016/j.stem.2012.04.021; PMID: 22704516
- Matsumoto K, Yokoo T, Matsunari H, Iwai S, Yokote S, Teratani T, et al. Xenotransplanted embryonic kidney provides a niche for endogenous mesenchymal stem cell differentiation into erythropoietin-producing tissue. Stem Cells 2012; 30:1228 - 35; http://dx.doi.org/10.1002/stem.1101; PMID: 22488594
- Hammerman MR. Development of a novel xenotransplantation strategy for treatment of diabetes mellitus in rat hosts and translation to non-human primates. Organogenesis 2012; 8:41 - 8; http://dx.doi.org/10.4161/org.20930; PMID: 22699748
- Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med 2008; 14:213 - 21; http://dx.doi.org/10.1038/nm1684; PMID: 18193059
- Ross EA, Williams MJ, Hamazaki T, Terada N, Clapp WL, Adin C, et al. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol 2009; 20:2338 - 47; http://dx.doi.org/10.1681/ASN.2008111196; PMID: 19729441
- Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med 2010; 16:814 - 20; http://dx.doi.org/10.1038/nm.2170; PMID: 20543851
- Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med 2010; 16:927 - 33; http://dx.doi.org/10.1038/nm.2193; PMID: 20628374
- Orlando G, Farney AC, Iskandar SS, Mirmalek-Sani SH, Sullivan DC, Moran E, et al. Production and implantation of renal extracellular matrix scaffolds from porcine kidneys as a platform for renal bioengineering investigations. Ann Surg 2012; 256:363 - 70; http://dx.doi.org/10.1097/SLA.0b013e31825a02ab; PMID: 22691371
- Yagi H, Fukumitsu K, Fukuda K, Kitago M, Shinoda M, Obara H, et al. Human-scale whole-organ bioengineering for liver transplantation: a regenerative medicine approach. Cell Transplant 2013; 22:231 - 42; http://dx.doi.org/10.3727/096368912X654939; PMID: 22943797
- Breimer ME. Gal/non-Gal antigens in pig tissues and human non-Gal antibodies in the GalT-KO era. Xenotransplantation 2011; 18:215 - 28; http://dx.doi.org/10.1111/j.1399-3089.2011.00644.x; PMID: 21848538
- Follenzi A, Benten D, Novikoff P, Faulkner L, Raut S, Gupta S. Transplanted endothelial cells repopulate the liver endothelium and correct the phenotype of hemophilia A mice. J Clin Invest 2008; 118:935 - 45; PMID: 18274668
- Beschorner WE, Sudan DL, Radio SJ, Yang T, Franco KL, Hill AC, et al. Heart xenograft survival with chimeric pig donors and modest immune suppression. Ann Surg 2003; 237:265 - 72; http://dx.doi.org/10.1097/01.SLA.0000048456.81319.DA; PMID: 12560785
- Ekser B, Klein E, He J, Stolz DB, Echeverri GJ, Long C, et al. Genetically-engineered pig-to-baboon liver xenotransplantation: histopathology of xenografts and native organs. PLoS One 2012; 7:e29720; http://dx.doi.org/10.1371/journal.pone.0029720; PMID: 22247784