Abstract
Cystic kidney diseases can cause end stage renal disease, affecting millions of individuals worldwide. They may arise early or later in life, are characterized by a spectrum of symptoms and can be caused by diverse genetic defects. The primary cilium, a microtubule-based organelle that can serve as a signaling antenna, has been demonstrated to have a significant role in ensuring correct kidney development and function. In the kidney, one of the signaling pathways that requires the cilium for normal development is Wnt signaling. In this review, the roles of primary cilia in relation to canonical and non-canonical Wnt/PCP signaling in cystic renal disease are described. The evidence of the associations between cilia, Wnt signaling and cystic renal disease is discussed and the significance of planar cell polarity-related mechanisms in cystic kidney disease is presented. Although defective Wnt signaling is not the only cause of renal disease, research is increasingly highlighting its importance, encouraging the development of Wnt-associated diagnostic and prognostic tools for cystic renal disease.
Kidney morphogenesis
The kidney, an organ of a characteristic shape composed of endothelial and epithelial cells that are contained within a stroma, is important for the maintenance of blood chemistry and fluid homeostasis. Kidney development is well conserved between mouse and human, with metanephric kidney development initiating at E10 in mouse and resulting in the distinctly branched kidney with the many nephron segments.
The process of metanephric kidney morphogenesis initiates with the branching of the ureteric bud within the metanephric mesenchyme (). While the ureteric bud is branching, nephron progenitors within the metanephric mesenchyme are triggered to proliferate, undergoing a mesenchymal to epithelial transition and forming renal vesicles.Citation1 Continuous mesenchyme signaling enables further branching of the ureteric bud, eventually giving rise to the collecting duct and ureter. Once renal vesicles have formed, they arrange into distinct shapes, first the comma- and later the S-shaped bodies (). Specific cell populations of these structures will give rise to the renal corpuscles, the proximal, distal and connecting tubules and the loops of Henle. At the same time, the ureteric bud develops into the collecting duct, renal pelvis and ureter.Citation1
Figure 1. Metanephric ureteric bud branching and nephron formation. Metanephric kidney development initiates upon invasion of the ureteric bud (black lines) in the metanephric mesenchyme (dark blue cells). Signals from the ureteric bud induce differentiation of the nephron progenitors to form the renal vesicles, while further ureteric bud branching is taking place. Renal vesicles will re-arrange into the distinct comma- and S-shaped bodies (dark blue cells). Specific cell populations of these structures will give rise to the glomeruli, the proximal and distal tubules and the loops of Henle, while the ureteric bud develops into the collecting duct. Cilia are present in the renal vesicles, comma-shaped and S-shaped bodies and nephrons (blue lines) and also form in the branching ureteric bud and colleting duct cells (black lines).
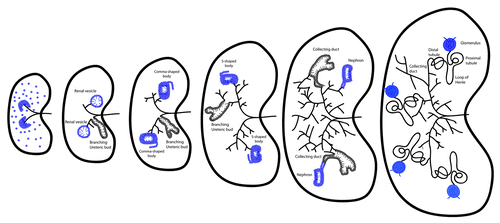
Cellular proliferation within the epithelial cell populations is critical for the lengthening of the tubule structures and an important aspect of kidney morphogenesis is that although tubules increase in length, their diameter remains constant. During kidney tubule elongation, cells divide in such a way that the mitotic spindle is within the plane or parallel to the proximal-distal axis of the epithelium. This process, known as oriented cell division (OCD), allows the elongation of kidney tubules without increasing tubule diameter. Kidney epithelial cells have also been shown to have planar polarity, such that when they elongate, their axis of elongation is vertical to the proximal-distal axis of the tubule.Citation2 These directed cell movements, cited as convergent extension, permit the kidney tubule to increase its length but maintain an appropriate diameter, crucial for kidney morphogenesis and function. The significance of OCD and convergent extension in the kidney is further highlighted by the fact that disruption of OCD resulted in dilated tubules and/or cystic kidneys in a number of animal models,Citation3-Citation5 while defects in convergent extension have been shown to give rise to abnormal kidneys.Citation6 Both processes are regulated by the non-canonical Wnt/Planar Cell Polarity (PCP) pathway, whose signaling has been associated with a cellular organelle called the cilium.
Ciliogenesis
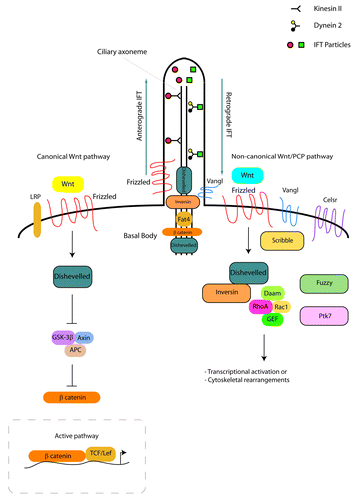
Cilia are evolutionarily-conserved, microtubule-based apical cellular protrusions that have been shown to form in almost all cell types. They are categorised as motile or immotile (primary) cilia, depending on whether their function is associated with movement or not. Cilia form in the G0 or G1 phases of the cell cycle, extending their axoneme from the centrosome at the cell surface into the ciliary lumen. The role of the mother centriole, a component of the centrosome and microtubule organizing center is crucial for ciliogenesis, as in order to nucleate a cilium, the mother centriole must differentiate into a basal body.Citation7 In the early stages of ciliogenesis, basal bodies associate with membrane compartments so as to permit the initiation of axonemal growth, emphasizing that basal bodies are the structures from which cilia arise. Ciliogenesis is an active process, with cilia being constantly formed and resorbed during the cell cycle. It depends on anterograde and retrograde motor proteins (kinesins and dyneins respectively) and a microtubule based transport system known as intraflagellar transport (IFT; ). Disturbance of IFT results in defective cilia formation and function.
Figure 2. Ciliogenesis and Wnt signaling in the kidney. Ciliogenesis depends on anterograde and retrograde transport involving kinesin II, dynein 2 and IFT proteins. The canonical Wnt signaling pathway (left side of figure) initiates when a Wnt ligand binds to a Frizzled receptor in the presence of LRP. This activates Dishevelled that inhibits the β-catenin destruction complex (GSK3β, APC, Axin). In the active pathway, β-catenin accumulates in the cytosol and causes the transcriptional activation of Wnt target genes. In the non-canonical Wnt/PCP signaling pathway in the kidney (right side of figure), the binding of a Wnt ligand will result in the activation of Frizzled and Dishevelled, leading to downstream cytoskeletal rearrangements or transcriptional activation through the activation of RhoA or Rac1. Core molecules (Vangl, Celsr, Scribble) are important for non-canonical Wnt/PCP signaling, while effector PCP molecules (Fuzzy, Fat4, Ptk7) are significant for kidney morphogenesis. A number of Wnt signaling proteins localize to the basal body (Dishevelled, β-catenin, Fat4) or base (Inversin, Dishevelled, Frizzled, Vangl) of kidney primary cilia.
Primary cilia bear a characteristic 9+0 microtubule arrangement, with 9 pairs of microtubules arranged in a circular pattern.Citation8 In the developing human kidney, they appear on the surfaces of non-proliferating cells.Citation9 Cilia have been found to gradually increase in mean length from 0.59 μm in renal vesicles to 0.81 μm in the S-shaped nephrons (), ultimately reaching 3.04 μm in length in mature fetal and post-natal nephrons. Some mechanistic insight on their role in kidney development and disease has been obtained by the discovery that many proteins important for the correct formation and function of kidney structures localize to the cilium or can be fully functional only if renal cilia are present and functional. Loss of cilia, malformed cilia or mutations in IFT genes can cause proliferative defects, affect fluid secretion and result in cystic renal disease.Citation10,Citation11 In the kidney in particular, the cilium is required for the timely regulation of Wnt signaling.Citation12 It has indeed been suggested that an active cilium acts as a repressor of the canonical Wnt pathway, permitting signaling from the non-canonical Wnt pathway and thus acting as a switch between the two.
Dissecting the Wnt signaling pathways
One of the signaling pathways that has been shown to require the primary cilium for correct development of the kidney is the Wnt signaling pathway, a highly conserved pathway involved in a variety of biological processes in various species. Wnts are small, secreted glycoproteins whose binding to a receptor can activate a signaling cascade. There are three signaling cascades, one β-catenin-dependent (the canonical Wnt pathway) and two β-catenin-independent (the non-canonical Wnt/planar cell polarity [PCP] pathway and the calcium signaling pathway). The two Wnt signaling cascades that have been most thoroughly studied in kidney development and disease are the canonical and non-canonical/PCP Wnt signaling pathways.
The canonical Wnt signaling pathway initiates when a canonical Wnt ligand binds to a Frizzled (Fz) receptor in the presence of lipoprotein receptor-related protein 5 or 6 (LRP5 or 6). This results in the activation of Dishevelled (Dvl) that inhibits the destruction complex, consisting of the glycogen synthase kinase 3β (GSK3β), adenomatous polyposis coli (APC), Axin and casein kinase 1 (CK1) proteins. All these proteins are inhibitors of β-catenin. When this pathway is inactive, β-catenin is targeted through phosphorylation for proteosomal degradation. When the pathway is active, such destruction is inhibited, resulting in the accumulation of β-catenin in the cytosol and the subsequent transcriptional activation of Wnt target genes ().
Besides the canonical Wnt signaling pathway, the other widely studied downstream Wnt signaling pathway in the kidney is the PCP pathway, also referred to as the non-canonical Wnt/PCP pathway. In the kidney, the interaction of inversin (Inv/NPHP2) with Dvl is key for the activation of the non-canonical Wnt/PCP pathway. The binding of a non-canonical Wnt ligand will result in the activation of Fz and Dvl. This will lead to the activation of a signaling cascade, resulting in downstream cytoskeletal rearrangements or transcriptional activation through the activation of RhoA or Rac1 ().
It has become apparent that disruption of the non-canonical Wnt/PCP signaling pathway is detrimental for correct orientation of cells along the epithelium plane of a tissue. PCP is the uniform organization of cells within the epithelial plane across a tissue, parallel to the basement membrane and perpendicular to apical-basal polarity. It is most easily observed in the arrangement of the hair follicles, the position of the stereocilia within the cochlea, the patterning of wing hairs in Drosophila and it has also been increasingly associated with normal morphogenesis in the kidney. Defects in non-canonical Wnt/PCP signaling cause abnormal planar cell polarization. Since the balance between canonical Wnt and non-canonical Wnt/PCP signaling is thought to be important for cyst formation and renal disease in mouse models, by its association with Wnt signaling pathways, the primary cilium is implicated as a key player in regulating this balance.
In an attempt to understand the mechanisms behind the activation of canonical Wnt vs. non-canonical Wnt/PCP signaling in kidney development and disease, the role of a number of Wnt ligands and their interacting molecules has been carefully investigated. Although it was initially thought that a single Wnt ligand could activate only the canonical or the non-canonical Wnt/PCP signaling pathway, it is now becoming obvious that most Wnt ligands are able to activate both pathways. The choice of downstream pathway activation is dependent on as yet unknown factors. Wnt4, a ligand important for canonical Wnt signaling, is a key regulator of the mesenchymal to epithelial transition as it controls the transition of pre-tubular aggregates into renal vesicles.Citation13 Treatment of mouse and human podocytes with Wnt5a, a non-canonical Wnt ligand, resulted in the recruitment of Dvl2 to the plasma membrane and the movement of the Dishevelled-associated activator of morphogenesis 1 (Daam1) to actin-based stress fibers, showing that PCP signaling affects podocyte shape and motility.Citation14 Wnt5a was also required so as to enable Dvl2 to recruit β−arr2, resulting in the internalisation of Fz4 in a human embryonic kidney cell line.Citation15 Nevertheless, in other systems Wnt5a has been shown to be required for the canonical Wnt signaling pathway, affecting the activation of β-catenin.Citation16-Citation18
Wnt9b, a very important Wnt ligand in kidney morphogenesis, acts upstream of Wnt4 and is required for the development of mesonephric and metanephric tubules and the caudal extension of the Mullerian duct.Citation19 Depletion of Wnt9b resulted in abnormal PCP of the kidney epithelium and an increased diameter of tubules.Citation2 Cell divisions were also randomly oriented, implicating Wnt9b in the convergent extension and OCD processes. It was subsequently shown that Wnt9b signaling can cause the mesenchymal-to-epithelial progenitors to differentiate or proliferate, depending on the activity of the transcription factor Six2.Citation20 Although Wnt9b appears to be an important ligand for non-canonical Wnt/PCP signaling, it has also been associated with canonical Wnt signaling, as mis-expression of Wnt9b disrupted kidney function by activating canonical Wnt signaling in mouse embryos.Citation21
Experiments in zebrafish and Xenopus suggested that Wnt11 signals through the non-canonical Wnt/PCP pathway to regulate convergent extension movements during gastrulation.Citation22,Citation23 During metanephric mouse kidney development, Wnt11 regulates ureteric branching morphogenesis in a signaling pathway that is reciprocally dependent upon Ret/Gdnf signaling.Citation24 Wnt11 was recently shown to be regulated by TGF-β through Smad3 in primary and immortalised renal epithelial cells.Citation25 This resulted in the activation of mesenchymal gene expression through the c-Jun N-terminal kinase (JNK) pathway. Wnt signaling is thus emerging as a major player for kidney development and function and the study of Wnt ligands and their receptors has enabled a better understanding of their exact role in kidney morphogenesis. So far, Wnt11 is the only ligand that has been demonstrated to exclusively activate non-canonical Wnt/PCP signaling in the kidney.
The Overlap Between Canonical Wnt and Non-Canonical Wnt/PCP Signaling Pathways
Although the canonical Wnt and the non-canonical Wnt/PCP pathways trigger different downstream cascades and have diverse transcriptional targets, many of their upstream components (Fz, Dvl) are identical. Fzs are the principal receptors for the Wnt ligands;Citation26 a number of Fz receptors exist and it appears that their function might be tissue-dependent. In kidney development, Fz4 functions redundantly with Fz8 with double homozygote mouse embryos exhibiting renal hypoplasia.Citation27 When studied in a cell culture system, Fz4 was able to activate both the canonical and non-canonical Wnt/PCP pathway, although another study demonstrated that while Fz4 and Fz5 may be capable of activating the PCP signaling pathway, Fz3 and Fz6 were important for PCP signaling.Citation28 Fz3 was subsequently shown to localize to primary cilia in mouse embryonic fibroblasts,Citation29 indicating that the activation of Fz receptors in cilia might be important for the regulation of Wnt signaling.
The effect of Dvl proteins in both Wnt signaling pathways is also complicated. Three highly conserved mammalian Dvl genes exist that demonstrate wide spatial and temporal expression during development and in adult tissues.Citation30 In mouse kidneys, Dvl2 was recently shown to interact with Dapper3 and cause Dvl2 downregulation,Citation31 while mutations in the PDZ domains of Dvl2 downregulated non-canonical Wnt/PCP signaling in Xenopus.Citation32 At the same time, the interaction of Dvl with NPHP4 and Inv regulated subcellular amounts of Dvl; in the absence of NPHP4, an increase in Dvl amounts in the cytoplasm of renal epithelial cells was detected, suggesting the favoring of the canonical Wnt signaling pathway.Citation33 In multi-ciliated, non-renal mucosal epithelial cells, Dvl regulates apical docking and planar polarization of basal bodies and controls polarized ciliary beating.Citation34 This demonstrates a requirement for components of the Wnt signaling cascade for correct cilium function and provides a link with planar polarization. It should be noted however, that the cilia that are present on mucosal surfaces are motile and hence their function and potentially regulatory mechanisms can be different to the immotile, sensory cilia that are present in the kidney. Nevertheless, Dvl1 localizes to the bottom part of primary cilia and Dvl3 is present at the basal body of cilia in mouse embryonic fibroblasts.Citation29 This localization is lost in inv−/− cells and it correlates with the disappearance of phosphorylated β-catenin from the basal body of the cilium. So although the Dvl proteins have been shown to localize to cilia, it is not yet fully understood what their roles in ciliogenesis and kidney function are, nor is it clear how the interaction of Dvl with components of the cascade can trigger either the canonical or the non-canonical Wnt/PCP pathway.
The Significance of Core and Effector PCP Proteins for Kidney Development and Function
Besides the Wnt ligands that are required to activate the non-canonical Wnt/PCP signaling pathway and its upstream components such as Fz and Dvl, a number of core and effector PCP proteins play significant roles in the kidney. Core PCP proteins such as Vangl and Celsr have been shown to be required for kidney morphogenesis and function. Although the exact mechanisms by which altered expression or absence of the core PCP proteins could lead to cyst formation have not yet been fully explained, it is becoming clear that their expression is important for kidney development.
Vangl2 has been shown to be important for normal morphogenesis of the ureteric bud and metanephric mesenchyme-derived structuresCitation35 and it localizes to the base of the cilium in kidney cells.Citation36 Furthermore, in zebrafish, the WT-1 interacting protein (Wtip) localized to the basal body of cilia and its knockdown resulted in pronephric cysts and mis-oriented cell division in anterior and middle pronephros. This phenotype was suggestive of a potential interaction between Wtip and Vangl2 proteins.Citation37
Celsr1 belongs to the family of atypical cadherins with important roles in PCP during brain and epithelia development and it was found to be expressed in the distal tubule and pronephric duct in Xenopus embryos.Citation38 Another PCP associated protein, Scribble, on the other hand, is a cytoplasmic scaffold protein that is expressed in the adherens junctions and the lateral membrane of polarized renal epithelial cells in a E-cadherin-dependent manner.Citation39 The vertebrate Scribble protein was shown to control spatial cellular orientation through PCP signaling in zebrafish.Citation40 Nevertheless, although important for pronephros development, Scribble seems to be dispensable for podocyte function and development, since podocyte-specific Scribble knockout mice do not manifest any podocyte-related abnormalities.Citation41
The inversion of embryonic turning (inv) mutation producing mice with situs inversus and cystic kidneysCitation42 has provided a further link between cysts, cilia and Wnt signaling. The Inv protein (also known as NPHP2) displayed high expression in the liver and kidneys; its human homolog was later found to cause nephronophthisis type 2.Citation43 Inv localizes to the base of primary cilia and is required for the localization of NPHP3 and Nek8/NPHP9 at the base of the cilium.Citation44 Knockdown of Inv in Xenopus affected pronephros extension and distal tubule differentiation and Inv was shown to be required for the recruitment of Dvl in response to Fz8 and to be important in pronephros morphogenesis.Citation45 It was thus assumed that Inv inhibited canonical Wnt signaling. However, no change in canonical Wnt signaling was recorded in mouse inv/inv kidneys, raising the possibility that Inv functions in non-canonical Wnt/PCP signaling.Citation46
Furthermore, the non-canonical Wnt/PCP signaling pathway contains many other proteins that are equally important for the correct establishment of PCP and are known as effector proteins. Dishevelled-associated activators of morphogenesis (DAAM) belong to the family of formin proteins, the actin assembly factors with roles in cell motility, division and adhesion.Citation47 Two eukaryotic Daam proteins are known, Daam1 and Daam2. Both are considered important for PCP. Daam1 depletion in zebrafish resulted in the reduction of kidney tubulogenesis and altered kidney morphogenesis.Citation48 Daam1 was shown to be expressed in human podocytes,Citation14 but the function and expression pattern of Daam2 in the kidney remains largely unknown.
A second group of effector PCP molecules that mostly function downstream of Dvl and could play potentially important roles in correct kidney formation have been characterized in some detail. The exact mode of operation of these components is not clear; they are believed to either function upstream of the core PCP proteins and confer global polarity or they could be regulating PCP by operating on a separate, parallel pathway. Fuzzy, an effector molecule of the PCP pathway was recently shown to be required for the recruitment of Dvl2 to the cilium in Rab8-associated vesicles.Citation49 Loss of Fuzzy resulted in loss of Dvl1 from the basal body of the cilium in kidney cell lines and hyperactivation of the canonical Wnt pathway, suggesting that besides its role in PCP, Fuzzy is required for Dvl recruitment to the cilium and canonical Wnt signaling activity.
Another PCP-associated gene, fat4, is required for OCD and tubule elongation during kidney development; its loss results in cystic kidneys in mice.Citation3 Fat4 localizes to primary cilia and it is hypothesized to act in a partially redundant fashion with Vangl2 during cyst formation. It is proposed that the role of Fat4 in murine kidney development is enabled via its receptor-ligand interaction with the myosin-related protein, Dchs1.Citation50 Similarly, protein tyrosine kinase-7 (PTK-7) is a conserved transmembrane protein with a characteristic role in convergent extension and cell motility.Citation51 Both canonical and non-canonical Wnt signaling defects were observed in Xenopus, zebrafish and Drosophila when Ptk7 expression was perturbed.Citation52,Citation53 Although PTK7 demonstrated high mRNA expression levels in the human kidney,Citation54 its exact role in kidney development and function remains unknown.
The studies of the core and effector PCP molecules have thus demonstrated their significance in both embryonic and adult kidneys. Core PCP proteins are also expressed in podocytes, the highly polarized glomerular epithelial cells.Citation35 PCP signaling was shown to be important for nephrin endocytosis and glomerular maturationCitation55 and Wnt signaling overactivation in adult kidneys contributed to podocyte dysfunction. Dysfunctional podocytes have been associated with proteinuria.Citation56,Citation57 The relationship between Wnt signaling and podocyte dysfunction was validated by the recent discovery that inappropriate stimulation of Wnt5a might lead to proteinuriaCitation55 and it highlights the significance of balanced, regulated Wnt signaling for correct tissue formation and function.
The Links between Wnt Signaling, Cilia and Cystic Renal Disease
Cystic renal diseases are characterized by the formation of cysts in the nephron and collecting duct with most patients requiring dialysis and potentially a kidney transplant.Citation58 A number of diverse mechanisms have been associated with cystic renal diseases and it has recently emerged that defects in primary cilia and inappropriate Wnt signaling may contribute to the formation of cysts. Autosomal dominant polycystic kidney disease (ADPKD) is the most common form of polycystic kidney disease with an incidence of 1 in 800 live births. There are two types of ADPKD: type I accounts for more than 80% of ADPKD cases and is caused by mutations in the Pkd1 gene and type II accounts for the remaining cases and results from mutations in the Pkd2 gene.Citation59 The Pkd1 gene encodes the protein polycystin-1 (PC-1). PC-1 is a membrane receptor that localizes to the primary cilium in renal epithelial cellsCitation60 and it interacts with many signaling proteins, such as β-catenin.Citation61,Citation62 The relationship between defective Wnt signaling and ADPKD is emphasized by the fact that frizzled-related protein 4 was upregulated in human ADPKD and mouse models,Citation63 while DKK3, a β-catenin antagonist, was subsequently identified as a potential modifier of disease severity in ADPKD.Citation64 Polycystin-2 (PC-2), the product of the Pkd2 gene is on the other hand a calcium-permeable channel proteinCitation65 that also localizes to the primary cilium and interacts with PC-1.Citation60 The localization of the PC-1/PC-2 complex in the primary cilium is proposed to be crucial for its function as a mechanosensory antenna of fluid flow in the kidney. It has indeed been shown that PC-2 expression in nodal cilia is important for sensing nodal flow and thus establishing left-right asymmetry.Citation66
Autosomal recessive polycystic kidney disease (ARPKD) is the recessive form of PKD that has an incidence of 1 in 20 000 and is associated with perinatal and early infantile death. It manifests as extreme bilateral enlargement of cystic kidneys in utero associated with hepatic ductal plate abnormalities and pulmonary hypoplasia.Citation67 ARPKD patients display a great range of disease severity, with the most severe cases resulting in death in utero or after birth and the milder cases surviving into their 30s. The disease is caused by mutation in the Pkhd1 gene, which encodes a large, modular membrane protein, known as fibrocystin (polyductin). Fibrocystin is required for normal ureteric bud and liver ductal plate branching morphogenesis and its expression leads to appropriate organ differentiation and development.Citation68 Although the exact function of fibrocystin remains unknown, it interacts with other cystic proteins (PC-1 and PC-2) at the cell and ciliary membranes, to form a multiprotein complex that is thought to act as a mechanosensory receptor.Citation69 The oak ridge polycystic kidney (orpk) mouse that resembles human ARPKD is caused by a mutation in the intraflagellar transport 88 (Ift88) gene, which is known to be crucial for ciliogenesis, as it forms part of the IFT complex. These mice have a complex phenotype that includes polycystic kidneys with shortened cilia on renal epithelial cells and represent a clear association between polycystic kidney disease and cilia malformation and malfunction.Citation70,Citation71 Furthermore, IFT88 is required for correct convergent extension of the cochlear duct and the establishment of epithelial PCP; lack of IFT88 expression results in incorrect basal body positioning in mice.Citation72 Another ARPKD mouse model, the congenital polycystic kidney (cpk) mouse, has a mutation in Cystin, a gene whose protein product localizes to the primary cilium. Cpk mice manifest defective ciliogenesis and develop renal cysts,Citation73,Citation74 providing further links between this cellular organelle and cystic renal disease.
Another disease that is classified under the umbrella of cystic renal diseases and has drawn the interest of many researchers is familial nephronophthisis (NPHP). NPHP is a recessive disorder that can be distinguished into juvenile, infantile and adolescent and it is mainly caused by mutations in the Nphp1, Nphp2, and Nphp3 genes, respectively. Nevertheless, at least 13 genes have been associated with the NPHP phenotype.Citation75 Most NPHP proteins localize to the cilium or the basal body and mutations in these genes affect ciliary function in both kidney and other cell types.Citation76 The Nphp2 (Inv) gene in particular, has provided evidence for the association between cilia, Wnt signaling and kidney disease, as it localizes to the basal body of the cilium and, as described above, its lack of expression results in the disruption of apical-basal polarity in renal epithelia and the prevalence of the canonical Wnt signaling pathway.Citation77
Medullary cystic kidney disease on the other hand is less extensively studied. It is caused by mutations in the medullary cystic kidney disease 1 (Mckd1) or medullary cystic kidney disease 2 (Mckd2) genes and is characterized by bilaterally smaller kidneys. It is a rare autosomal dominant condition that eventually leads to end stage renal disease.Citation78 The uromodulin protein that when mutated gives rise to MCKD2, is expressed in the primary cilia of renal tubules, where it co-localizes with Kif3a and NPHP1.Citation79 Furthermore, the hepatocyte nuclear factor 1-β (HNF1β) mutations are accountable for various renal abnormalities including cystic renal disease. HNF1β is a transcription factor that acts as the master regulator of many cystic genes, such as Pkhd1, Pkd2, Mckd2, and Ift88.Citation80 Its expression in multiple organs could provide an explanation for the pleiotropic phenotypes of HNF1β mutations. In the kidney, HNF1β acts upstream of Wnt9b and is involved in the mesenchymal to epithelial transition in nephrogenesis.Citation81 Increased β-catenin expression was observed in fetuses with HNF1β mutations,Citation82 showing that HNF1β can regulate Wnt signaling, though it is not understood precisely how.
It is clear that there are a number of other genes and factors that contribute to cystic renal diseases and affect their manifestation and severity. Studies on a number of genes important for IFT trafficking and correct cilium signaling have emphasized the links between cilia, Wnt signaling and renal cysts. Inactivation of an IFT component, IFT20, resulted in postnatal cystic kidneys lacking cilia; mis-oriented cell division and increased canonical Wnt signaling were also observed.Citation83 Deletion of another IFT gene, Ift140, also led to renal cyst formation and disruption of several genes important for the canonical Wnt and Hedgehog (Hh) signaling pathways.Citation11
Besides IFT genes, the kidney-specific inactivation of kif3a, an anterograde motor protein critical for ciliogenesis, resulted in viable offspring that developed cystic kidneys and displayed increased β catenin expression.Citation84 The Bardet-Biedl syndrome (BBS) proteins that together form the BBSome complex at the basal body of the cilium also have roles in kidney disease. BBS2 was shown to be important for both ciliogenesis and normal kidney formation.Citation85,Citation86 BBS3 localizes to the basal body of cilia and modulates Wnt signaling in cell culture,Citation87 while BBS1, BBS4, and BBS6 genetically interact with Vangl2, providing an association between BBS proteins and PCP.Citation36 Intriguingly, BBS1, BBS4, and BBS6 also affect β-catenin expression in zebrafish.Citation88 Further associations between cilia and PCP have been provided by the discovery that mutation of the Ofd1 gene gives rise to polycystic kidneys and results in convergent extension defects and shorter cilia.Citation89 These defects were worsened by the loss of Wnt11 and Vangl2. Finally, the juvenile cystic kidney (jck) mouse carrying a missense mutation in Nek8/NPHP9 manifested cysts in multiple nephron segments, with the Nek8 protein localizing along the entire length of renal cilia.Citation90,Citation91 In these mice, overall repression of canonical Wnt signaling was observed in osteocytes;Citation92 whether this holds true for kidney cells remains to be seen.
It is thus becoming apparent that disturbance of normal ciliary architecture and function is associated with the formation of renal cysts. This means that correct cilia formation is imperative for normal kidney development and function. At the same time, the signaling pathways that operate through the cilia should also be working so as to enable complete cascade activation and avoid defects in proliferation and fluid flow. Many different genes have been shown to play a role in the formation, maintenance and correct signaling of cilia and have been shown to affect canonical Wnt and non-canonical Wnt/PCP signaling. Indeed, ciliary genes could act in a parallel pathway or downstream of the classical PCP proteins, in a manner that allows them to be categorised as kidney-specific effectors of PCP. Since many of the mutated proteins that give rise to the disease are expressed in the cilium or the basal body, some of the cystic renal diseases such as PKD and NPHP are now being classified as Ciliopathies.
The Story So Far and the Future
The primary cilium and, in particular, its basal body are essential organelles governing the activity of various signaling pathways, one of which is Wnt signaling. The interconnections between Wnt signaling, vesicle trafficking and ciliogenesis have been described for a number of proteins in variable experimental settings. Based on the fact that proteins crucial for renal cystic disease (PC-1, PC-2, fibrocystin) have been shown to localize to the basal body or within the cilium, cilia are considered to be important for kidney morphogenesis and function. The primary cilium is proposed to act as a mechanosensor in kidney tubules, regulating the homeostasis of renal epithelial cells. At the same time, many signaling components crucial for the canonical Wnt and the non-canonical Wnt/PCP signaling pathways (Vangl, Fz, Dvl) have been found to also localize to cilia, besides their primary localization to cell membranes. It should be noted however that although malformed or malfunctioning cilia have been associated with renal cystic formation, vertebrates lacking functional polycystins do not have abnormal cilia.Citation93 It was recently demonstrated that loss of cilia following the inactivation of polycystins suppresses cyst growth, with the severity of cystic renal disease related to the length of time between loss of polycystin proteins and loss of cilia.Citation94 It thus remains possible that polycystins might play a role in the signaling or sensory role of renal cilia but the series of events and the associations with signaling pathways remain unclear. At the same time, polycystins are also expressed in the basolateral membrane and other cystogenic proteins display non-ciliary expression, hence there could be ciliary and non-ciliary pathways involved in the pathogenesis of renal cysts.
It has been hypothesized that cilia regulate the balance between canonical Wnt and non-canonical Wnt/PCP signaling, possibly through the regulation of Dvl. The exact mechanisms which lead to Dvl and Fz activating either the canonical or non-canonical Wnt signaling haven’t yet been clarified but they are thought to be tissue and time dependent. Although core PCP molecules have been shown to be important for kidney development, the precise role of effector PCP proteins remains elusive. It is clear though that many of the proteins that can give rise to cystic renal disease have a role in Wnt signaling. PC-1 has been shown to modulate Wnt signaling,Citation62,Citation95 while the NPHP2 and NPHP3 proteins negatively regulate canonical Wnt signaling.Citation96,Citation97 The BBS proteins have also been demonstrated to regulate the canonical Wnt pathway.Citation87,Citation88 Furthermore, over-activation of canonical Wnt signaling results in a cystic kidney phenotypeCitation98,Citation99 but loss of Wnt signaling can also give rise to renal cysts.Citation100,Citation101 In addition, Wnt4 was upregulated in mouse models of Adriamycin nephropathyCitation56 and mutations in Wnt4 were detected in renal hypodysplasia patients.Citation102 It is thus obvious that the canonical Wnt pathway must be finely balanced in the kidney, as unbalanced Wnt signaling will result in cystogenesis.
A number of genes that are known to give rise to cystic kidneys have also been shown to cause defects in cilia and PCP signaling; such genes include Pkd1, Bbs4, Bbs6 and Ofd1. PCP signaling was also found to be required for nephrin endocytosis and glomerular maturation,Citation55 further highlighting the significance of correct PCP for normal kidney function. It should however be noted that although mouse models of cystic renal disease have provided an association between cilia, cysts and Wnt signaling, not all of the ciliary genes demonstrated to cause kidney defects in animal models, have been shown to be causative of cystic renal disease in humans. This could be because of species differences between human and mouse or perhaps more detailed screening of these genes in the cystic renal disease patient population is required. It is obvious though that the mechanisms of renal cyst formation and kidney morphogenesis entail a number of potentially interlinked biological processes, with ciliogenesis playing a significant role in correct kidney function. As cilia are found in many cell types, mutations in ciliary or cilia-related genes could affect a number of tissues, resulting in a wide variety of phenotypes and diseases extending beyond the kidney.
Extensive research over the past decade has demonstrated that the signaling pathways that are transmitted through cilia are crucial for kidney morphogenesis and renal disease. Strides in live imaging and gene knockout techniques have very convincingly demonstrated that disturbance of either the canonical Wnt or non-canonical Wnt/PCP signaling pathways will have detrimental effects in both pediatric and adult renal disease. Nevertheless, most of the work has been performed on animal models and the demonstration of the importance of Wnt signaling for human renal function is only now emerging. Future work will hopefully dissect the mechanisms of activation of canonical Wnt vs. non-canonical Wnt/PCP signaling and determine the significance of primary cilia. Although cilia and defective Wnt signaling are not the only mechanism associated with renal cyst formation, they clearly play an important role. The development of better animal models and more detailed human studies will allow both a greater understanding of the mechanisms of cystic renal disease and will hopefully lead to the establishment of accurate diagnostic and prognostic tools, which can relieve the suffering of cystic renal disease patients and their families.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
I would like to thank Charlotte Dean and Dominic Norris for critical reading of the manuscript.
References
- Carroll TJ, Das A. Planar cell polarity in kidney development and disease. Organogenesis 2011; 7:180 - 90; http://dx.doi.org/10.4161/org.7.3.18320; PMID: 22027435
- Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet 2009; 41:793 - 9; http://dx.doi.org/10.1038/ng.400; PMID: 19543268
- Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet 2008; 40:1010 - 5; http://dx.doi.org/10.1038/ng.179; PMID: 18604206
- Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M. Defective planar cell polarity in polycystic kidney disease. Nat Genet 2006; 38:21 - 3; http://dx.doi.org/10.1038/ng1701; PMID: 16341222
- Luyten A, Su X, Gondela S, Chen Y, Rompani S, Takakura A, Zhou J. Aberrant regulation of planar cell polarity in polycystic kidney disease. J Am Soc Nephrol 2010; 21:1521 - 32; http://dx.doi.org/10.1681/ASN.2010010127; PMID: 20705705
- Lienkamp SS, Liu K, Karner CM, Carroll TJ, Ronneberger O, Wallingford JB, Walz G. Vertebrate kidney tubules elongate using a planar cell polarity-dependent, rosette-based mechanism of convergent extension. Nat Genet 2012; 44:1382 - 7; http://dx.doi.org/10.1038/ng.2452; PMID: 23143599
- Kim S, Dynlacht BD. Assembling a primary cilium. Curr Opin Cell Biol 2013; 25:506 - 11; http://dx.doi.org/10.1016/j.ceb.2013.04.011; PMID: 23747070
- Rosenbaum J. Intraflagellar transport. Curr Biol 2002; 12:R125; http://dx.doi.org/10.1016/S0960-9822(02)00703-0; PMID: 11864582
- Saraga-Babić M, Vukojević K, Bočina I, Drnašin K, Saraga M. Ciliogenesis in normal human kidney development and post-natal life. Pediatr Nephrol 2012; 27:55 - 63; http://dx.doi.org/10.1007/s00467-011-1941-7; PMID: 21688189
- Harris PC, Torres VE. Polycystic kidney disease. Annu Rev Med 2009; 60:321 - 37; http://dx.doi.org/10.1146/annurev.med.60.101707.125712; PMID: 18947299
- Jonassen JA, SanAgustin J, Baker SP, Pazour GJ. Disruption of IFT complex A causes cystic kidneys without mitotic spindle misorientation. J Am Soc Nephrol 2012; 23:641 - 51; http://dx.doi.org/10.1681/ASN.2011080829; PMID: 22282595
- Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev 2011; 25:201 - 13; http://dx.doi.org/10.1101/gad.2008011; PMID: 21289065
- Kispert A, Vainio S, McMahon AP. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development 1998; 125:4225 - 34; PMID: 9753677
- Babayeva S, Zilber Y, Torban E. Planar cell polarity pathway regulates actin rearrangement, cell shape, motility, and nephrin distribution in podocytes. Am J Physiol Renal Physiol 2011; 300:F549 - 60; http://dx.doi.org/10.1152/ajprenal.00566.2009; PMID: 20534871
- Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, Caron MG, Barak LS, Nusse R, Lefkowitz RJ. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science 2003; 301:1391 - 4; http://dx.doi.org/10.1126/science.1082808; PMID: 12958364
- Niu CC, Zhao C, Zhang XL, Pan J, Zhao C, Wu WR, Li ZQ, Liu T, Yang Z, Si WK. Wnt5a enhances the response of CML cells to Imatinib Mesylate through JNK activation and γ-catenin inhibition. Leuk Res 2013; S0145:00240 - 3; PMID: 23972517
- van Amerongen R, Fuerer C, Mizutani M, Nusse R. Wnt5a can both activate and repress Wnt/β-catenin signaling during mouse embryonic development. Dev Biol 2012; 369:101 - 14; http://dx.doi.org/10.1016/j.ydbio.2012.06.020; PMID: 22771246
- Kwack MH, Kim MK, Kim JC, Sung YK. Wnt5a attenuates Wnt/β-catenin signalling in human dermal papilla cells. Exp Dermatol 2013; 22:229 - 31; http://dx.doi.org/10.1111/exd.12101; PMID: 23489428
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 2005; 9:283 - 92; http://dx.doi.org/10.1016/j.devcel.2005.05.016; PMID: 16054034
- Karner CM, Das A, Ma Z, Self M, Chen C, Lum L, Oliver G, Carroll TJ. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development 2011; 138:1247 - 57; http://dx.doi.org/10.1242/dev.057646; PMID: 21350016
- Kiefer SM, Robbins L, Rauchman M. Conditional expression of Wnt9b in Six2-positive cells disrupts stomach and kidney function. PLoS One 2012; 7:e43098; http://dx.doi.org/10.1371/journal.pone.0043098; PMID: 22912798
- Heisenberg CP, Tada M, Rauch GJ, Saúde L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 2000; 405:76 - 81; http://dx.doi.org/10.1038/35011068; PMID: 10811221
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development 2000; 127:2227 - 38; PMID: 10769246
- Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development 2003; 130:3175 - 85; http://dx.doi.org/10.1242/dev.00520; PMID: 12783789
- Zhang P, Cai Y, Soofi A, Dressler GR. Activation of Wnt11 by transforming growth factor-β drives mesenchymal gene expression through non-canonical Wnt protein signaling in renal epithelial cells. J Biol Chem 2012; 287:21290 - 302; http://dx.doi.org/10.1074/jbc.M112.357202; PMID: 22556418
- van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development 2009; 136:3205 - 14; http://dx.doi.org/10.1242/dev.033910; PMID: 19736321
- Ye X, Wang Y, Rattner A, Nathans J. Genetic mosaic analysis reveals a major role for frizzled 4 and frizzled 8 in controlling ureteric growth in the developing kidney. Development 2011; 138:1161 - 72; http://dx.doi.org/10.1242/dev.057620; PMID: 21343368
- Yu H, Ye X, Guo N, Nathans J. Frizzled 2 and frizzled 7 function redundantly in convergent extension and closure of the ventricular septum and palate: evidence for a network of interacting genes. Development 2012; 139:4383 - 94; http://dx.doi.org/10.1242/dev.083352; PMID: 23095888
- Veland IR, Montjean R, Eley L, Pedersen LB, Schwab A, Goodship J, Kristiansen K, Pedersen SF, Saunier S, Christensen ST. Inversin/Nephrocystin-2 is required for fibroblast polarity and directional cell migration. PLoS One 2013; 8:e60193; http://dx.doi.org/10.1371/journal.pone.0060193; PMID: 23593172
- Wynshaw-Boris A. Dishevelled: in vivo roles of a multifunctional gene family during development. Curr Top Dev Biol 2012; 101:213 - 35; http://dx.doi.org/10.1016/B978-0-12-394592-1.00007-7; PMID: 23140631
- Xue H, Xiao Z, Zhang J, Wen J, Wang Y, Chang Z, Zhao J, Gao X, Du J, Chen YG. Disruption of the Dapper3 gene aggravates ureteral obstruction-mediated renal fibrosis by amplifying Wnt/β-catenin signaling. J Biol Chem 2013; 288:15006 - 14; http://dx.doi.org/10.1074/jbc.M113.458448; PMID: 23580654
- McCoy KE, Zhou X, Vize PD. Non-canonical wnt signals antagonize and canonical wnt signals promote cell proliferation in early kidney development. Dev Dyn 2011; 240:1558 - 66; http://dx.doi.org/10.1002/dvdy.22626; PMID: 21465621
- Burcklé C, Gaudé HM, Vesque C, Silbermann F, Salomon R, Jeanpierre C, Antignac C, Saunier S, Schneider-Maunoury S. Control of the Wnt pathways by nephrocystin-4 is required for morphogenesis of the zebrafish pronephros. Hum Mol Genet 2011; 20:2611 - 27; http://dx.doi.org/10.1093/hmg/ddr164; PMID: 21498478
- Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet 2008; 40:871 - 9; http://dx.doi.org/10.1038/ng.104; PMID: 18552847
- Yates LL, Papakrivopoulou J, Long DA, Goggolidou P, Connolly JO, Woolf AS, Dean CH. The planar cell polarity gene Vangl2 is required for mammalian kidney-branching morphogenesis and glomerular maturation. Hum Mol Genet 2010; 19:4663 - 76; http://dx.doi.org/10.1093/hmg/ddq397; PMID: 20843830
- Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet 2005; 37:1135 - 40; http://dx.doi.org/10.1038/ng1644; PMID: 16170314
- Bubenshchikova E, Ichimura K, Fukuyo Y, Powell R, Hsu C, Morrical SO, Sedor JR, Sakai T, Obara T. Wtip and Vangl2 are required for mitotic spindle orientation and cloaca morphogenesis. Biol Open 2012; 1:588 - 96; http://dx.doi.org/10.1242/bio.20121016; PMID: 23213452
- Zhang B, Tran U, Wessely O. Expression of Wnt signaling components during Xenopus pronephros development. PLoS One 2011; 6:e26533; http://dx.doi.org/10.1371/journal.pone.0026533; PMID: 22028899
- Navarro C, Nola S, Audebert S, Santoni MJ, Arsanto JP, Ginestier C, Marchetto S, Jacquemier J, Isnardon D, Le Bivic A, et al. Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene 2005; 24:4330 - 9; http://dx.doi.org/10.1038/sj.onc.1208632; PMID: 15806148
- Skouloudaki K, Puetz M, Simons M, Courbard JR, Boehlke C, Hartleben B, Engel C, Moeller MJ, Englert C, Bollig F, et al. Scribble participates in Hippo signaling and is required for normal zebrafish pronephros development. Proc Natl Acad Sci U S A 2009; 106:8579 - 84; http://dx.doi.org/10.1073/pnas.0811691106; PMID: 19439659
- Hartleben B, Widmeier E, Wanner N, Schmidts M, Kim ST, Schneider L, Mayer B, Kerjaschki D, Miner JH, Walz G, et al. Role of the polarity protein Scribble for podocyte differentiation and maintenance. PLoS One 2012; 7:e36705; http://dx.doi.org/10.1371/journal.pone.0036705; PMID: 22586490
- Mochizuki T, Saijoh Y, Tsuchiya K, Shirayoshi Y, Takai S, Taya C, Yonekawa H, Yamada K, Nihei H, Nakatsuji N, et al. Cloning of inv, a gene that controls left/right asymmetry and kidney development. Nature 1998; 395:177 - 81; http://dx.doi.org/10.1038/26006; PMID: 9744276
- Otto EA, Schermer B, Obara T, O’Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet 2003; 34:413 - 20; http://dx.doi.org/10.1038/ng1217; PMID: 12872123
- Shiba D, Manning DK, Koga H, Beier DR, Yokoyama T. Inv acts as a molecular anchor for Nphp3 and Nek8 in the proximal segment of primary cilia. Cytoskeleton (Hoboken) 2010; 67:112 - 9; PMID: 20169535
- Lienkamp S, Ganner A, Boehlke C, Schmidt T, Arnold SJ, Schäfer T, Romaker D, Schuler J, Hoff S, Powelske C, et al. Inversin relays Frizzled-8 signals to promote proximal pronephros development. Proc Natl Acad Sci U S A 2010; 107:20388 - 93; http://dx.doi.org/10.1073/pnas.1013070107; PMID: 21059920
- Sugiyama N, Tsukiyama T, Yamaguchi TP, Yokoyama T. The canonical Wnt signaling pathway is not involved in renal cyst development in the kidneys of inv mutant mice. Kidney Int 2011; 79:957 - 65; http://dx.doi.org/10.1038/ki.2010.534; PMID: 21248711
- Prokop A, Sánchez-Soriano N, Gonçalves-Pimentel C, Molnár I, Kalmár T, Mihály J. DAAM family members leading a novel path into formin research. Commun Integr Biol 2011; 4:538 - 42; PMID: 22046456
- Miller RK, Canny SG, Jang CW, Cho K, Ji H, Wagner DS, Jones EA, Habas R, McCrea PD. Pronephric tubulogenesis requires Daam1-mediated planar cell polarity signaling. J Am Soc Nephrol 2011; 22:1654 - 64; http://dx.doi.org/10.1681/ASN.2010101086; PMID: 21804089
- Zilber Y, Babayeva S, Seo JH, Liu JJ, Mootin S, Torban E. The PCP effector Fuzzy controls cilial assembly and signaling by recruiting Rab8 and Dishevelled to the primary cilium. Mol Biol Cell 2013; 24:555 - 65; http://dx.doi.org/10.1091/mbc.E12-06-0437; PMID: 23303251
- Mao Y, Mulvaney J, Zakaria S, Yu T, Morgan KM, Allen S, Basson MA, Francis-West P, Irvine KD. Characterization of a Dchs1 mutant mouse reveals requirements for Dchs1-Fat4 signaling during mammalian development. Development 2011; 138:947 - 57; http://dx.doi.org/10.1242/dev.057166; PMID: 21303848
- Yen WW, Williams M, Periasamy A, Conaway M, Burdsal C, Keller R, Lu X, Sutherland A. PTK7 is essential for polarized cell motility and convergent extension during mouse gastrulation. Development 2009; 136:2039 - 48; http://dx.doi.org/10.1242/dev.030601; PMID: 19439496
- Peradziryi H, Kaplan NA, Podleschny M, Liu X, Wehner P, Borchers A, Tolwinski NS. PTK7/Otk interacts with Wnts and inhibits canonical Wnt signalling. EMBO J 2011; 30:3729 - 40; http://dx.doi.org/10.1038/emboj.2011.236; PMID: 21772251
- Hayes M, Naito M, Daulat A, Angers S, Ciruna B. Ptk7 promotes non-canonical Wnt/PCP-mediated morphogenesis and inhibits Wnt/β-catenin-dependent cell fate decisions during vertebrate development. Development 2013; 140:1807 - 18; http://dx.doi.org/10.1242/dev.090183; PMID: 23533179
- Park SK, Lee HS, Lee ST. Characterization of the human full-length PTK7 cDNA encoding a receptor protein tyrosine kinase-like molecule closely related to chick KLG. J Biochem 1996; 119:235 - 9; http://dx.doi.org/10.1093/oxfordjournals.jbchem.a021228; PMID: 8882711
- Babayeva S, Rocque B, Aoudjit L, Zilber Y, Li J, Baldwin C, Kawachi H, Takano T, Torban E. Planar cell polarity pathway regulates nephrin endocytosis in developing podocytes. J Biol Chem 2013; 288:24035 - 48; http://dx.doi.org/10.1074/jbc.M113.452904; PMID: 23824190
- Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y. Wnt/β-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol 2009; 20:1997 - 2008; http://dx.doi.org/10.1681/ASN.2009010019; PMID: 19628668
- Kato H, Gruenwald A, Suh JH, Miner JH, Barisoni-Thomas L, Taketo MM, Faul C, Millar SE, Holzman LB, Susztak K. Wnt/β-catenin pathway in podocytes integrates cell adhesion, differentiation, and survival. J Biol Chem 2011; 286:26003 - 15; http://dx.doi.org/10.1074/jbc.M111.223164; PMID: 21613219
- Bacallao RL, McNeill H. Cystic kidney diseases and planar cell polarity signaling. Clin Genet 2009; 75:107 - 17; http://dx.doi.org/10.1111/j.1399-0004.2008.01148.x; PMID: 19215242
- Wilson PD. Mouse models of polycystic kidney disease. Curr Top Dev Biol 2008; 84:311 - 50; http://dx.doi.org/10.1016/S0070-2153(08)00606-6; PMID: 19186247
- Patel A, Honoré E. Polycystins and renovascular mechanosensory transduction. Nat Rev Nephrol 2010; 6:530 - 8; http://dx.doi.org/10.1038/nrneph.2010.97; PMID: 20625375
- Qian F, Boletta A, Bhunia AK, Xu H, Liu L, Ahrabi AK, Watnick TJ, Zhou F, Germino GG. Cleavage of polycystin-1 requires the receptor for egg jelly domain and is disrupted by human autosomal-dominant polycystic kidney disease 1-associated mutations. Proc Natl Acad Sci U S A 2002; 99:16981 - 6; http://dx.doi.org/10.1073/pnas.252484899; PMID: 12482949
- Lal M, Song X, Pluznick JL, Di Giovanni V, Merrick DM, Rosenblum ND, Chauvet V, Gottardi CJ, Pei Y, Caplan MJ. Polycystin-1 C-terminal tail associates with beta-catenin and inhibits canonical Wnt signaling. Hum Mol Genet 2008; 17:3105 - 17; http://dx.doi.org/10.1093/hmg/ddn208; PMID: 18632682
- Romaker D, Puetz M, Teschner S, Donauer J, Geyer M, Gerke P, Rumberger B, Dworniczak B, Pennekamp P, Buchholz B, et al. Increased expression of secreted frizzled-related protein 4 in polycystic kidneys. J Am Soc Nephrol 2009; 20:48 - 56; http://dx.doi.org/10.1681/ASN.2008040345; PMID: 18945944
- Liu M, Shi S, Senthilnathan S, Yu J, Wu E, Bergmann C, Zerres K, Bogdanova N, Coto E, Deltas C, et al. Genetic variation of DKK3 may modify renal disease severity in ADPKD. J Am Soc Nephrol 2010; 21:1510 - 20; http://dx.doi.org/10.1681/ASN.2010030237; PMID: 20616171
- González-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci U S A 2001; 98:1182 - 7; http://dx.doi.org/10.1073/pnas.98.3.1182; PMID: 11252306
- Yoshiba S, Shiratori H, Kuo IY, Kawasumi A, Shinohara K, Nonaka S, Asai Y, Sasaki G, Belo JA, Sasaki H, et al. Cilia at the node of mouse embryos sense fluid flow for left-right determination via Pkd2. Science 2012; 338:226 - 31; http://dx.doi.org/10.1126/science.1222538; PMID: 22983710
- Wilson PD, Goilav B. Cystic disease of the kidney. Annu Rev Pathol 2007; 2:341 - 68; http://dx.doi.org/10.1146/annurev.pathol.2.010506.091850; PMID: 18039103
- Onuchic LF, Furu L, Nagasawa Y, Hou X, Eggermann T, Ren Z, Bergmann C, Senderek J, Esquivel E, Zeltner R, et al. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am J Hum Genet 2002; 70:1305 - 17; http://dx.doi.org/10.1086/340448; PMID: 11898128
- Kim I, Fu Y, Hui K, Moeckel G, Mai W, Li C, Liang D, Zhao P, Ma J, Chen XZ, et al. Fibrocystin/polyductin modulates renal tubular formation by regulating polycystin-2 expression and function. J Am Soc Nephrol 2008; 19:455 - 68; http://dx.doi.org/10.1681/ASN.2007070770; PMID: 18235088
- Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol 2002; 12:R378 - 80; http://dx.doi.org/10.1016/S0960-9822(02)00877-1; PMID: 12062067
- Lehman JM, Michaud EJ, Schoeb TR, Aydin-Son Y, Miller M, Yoder BK. The Oak Ridge Polycystic Kidney mouse: modeling ciliopathies of mice and men. Dev Dyn 2008; 237:1960 - 71; http://dx.doi.org/10.1002/dvdy.21515; PMID: 18366137
- Jones C, Roper VC, Foucher I, Qian D, Banizs B, Petit C, Yoder BK, Chen P. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet 2008; 40:69 - 77; http://dx.doi.org/10.1038/ng.2007.54; PMID: 18066062
- Tao B, Bu S, Yang Z, Siroky B, Kappes JC, Kispert A, Guay-Woodford LM. Cystin localizes to primary cilia via membrane microdomains and a targeting motif. J Am Soc Nephrol 2009; 20:2570 - 80; http://dx.doi.org/10.1681/ASN.2009020188; PMID: 19850956
- Hou X, Mrug M, Yoder BK, Lefkowitz EJ, Kremmidiotis G, D’Eustachio P, Beier DR, Guay-Woodford LM. Cystin, a novel cilia-associated protein, is disrupted in the cpk mouse model of polycystic kidney disease. J Clin Invest 2002; 109:533 - 40; PMID: 11854326
- Halbritter J, Porath J, Diaz K, Braun D, Kohl S, Chaki M, Allen S, Soliman N, Hildebrandt F, Otto E. Identification of 99 novel mutations in a worldwide cohort of 1,056 patients with a nephronophthisis-related ciliopathy. Hum Genet 2013; 132:1 - 4; PMID: 23001594
- Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med 2011; 364:1533 - 43; http://dx.doi.org/10.1056/NEJMra1010172; PMID: 21506742
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Krönig C, Schermer B, Benzing T, Cabello OA, Jenny A, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet 2005; 37:537 - 43; http://dx.doi.org/10.1038/ng1552; PMID: 15852005
- Loftus H, Ong AC. Cystic kidney diseases: many ways to form a cyst. Pediatr Nephrol 2013; 28:33 - 49; http://dx.doi.org/10.1007/s00467-012-2221-x; PMID: 22736301
- Zaucke F, Boehnlein JM, Steffens S, Polishchuk RS, Rampoldi L, Fischer A, Pasch A, Boehm CWA, Baasner A, Attanasio M, et al. Uromodulin is expressed in renal primary cilia and UMOD mutations result in decreased ciliary uromodulin expression. Hum Mol Genet 2010; 19:1985 - 97; http://dx.doi.org/10.1093/hmg/ddq077; PMID: 20172860
- Gresh L, Fischer E, Reimann A, Tanguy M, Garbay S, Shao X, Hiesberger T, Fiette L, Igarashi P, Yaniv M, et al. A transcriptional network in polycystic kidney disease. EMBO J 2004; 23:1657 - 68; http://dx.doi.org/10.1038/sj.emboj.7600160; PMID: 15029248
- Lokmane L, Heliot C, Garcia-Villalba P, Fabre M, Cereghini S. vHNF1 functions in distinct regulatory circuits to control ureteric bud branching and early nephrogenesis. Development 2010; 137:347 - 57; http://dx.doi.org/10.1242/dev.042226; PMID: 20040500
- Haumaitre C, Fabre M, Cormier S, Baumann C, Delezoide AL, Cereghini S. Severe pancreas hypoplasia and multicystic renal dysplasia in two human fetuses carrying novel HNF1β/MODY5 mutations. Hum Mol Genet 2006; 15:2363 - 75; http://dx.doi.org/10.1093/hmg/ddl161; PMID: 16801329
- Jonassen JA, San Agustin J, Follit JA, Pazour GJ. Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J Cell Biol 2008; 183:377 - 84; http://dx.doi.org/10.1083/jcb.200808137; PMID: 18981227
- Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proceedings of the National Academy of Sciences of the United States of America 2003; 2003/04/04:5286-5291.
- Nishimura DY, Fath M, Mullins RF, Searby C, Andrews M, Davis R, Andorf JL, Mykytyn K, Swiderski RE, Yang B, et al. Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc Natl Acad Sci U S A 2004; 101:16588 - 93; http://dx.doi.org/10.1073/pnas.0405496101; PMID: 15539463
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peränen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 2007; 129:1201 - 13; http://dx.doi.org/10.1016/j.cell.2007.03.053; PMID: 17574030
- Wiens CJ, Tong Y, Esmail MA, Oh E, Gerdes JM, Wang J, Tempel W, Rattner JB, Katsanis N, Park HW, et al. Bardet-Biedl syndrome-associated small GTPase ARL6 (BBS3) functions at or near the ciliary gate and modulates Wnt signaling. J Biol Chem 2010; 285:16218 - 30; http://dx.doi.org/10.1074/jbc.M109.070953; PMID: 20207729
- Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson SS, Kato M, Beachy PA, Beales PL, DeMartino GN, Fisher S, et al. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet 2007; 39:1350 - 60; http://dx.doi.org/10.1038/ng.2007.12; PMID: 17906624
- Ferrante MI, Romio L, Castro S, Collins JE, Goulding DA, Stemple DL, Woolf AS, Wilson SW. Convergent extension movements and ciliary function are mediated by ofd1, a zebrafish orthologue of the human oral-facial-digital type 1 syndrome gene. Hum Mol Genet 2009; 18:289 - 303; http://dx.doi.org/10.1093/hmg/ddn356; PMID: 18971206
- Sohara E, Luo Y, Zhang J, Manning DK, Beier DR, Zhou J. Nek8 regulates the expression and localization of polycystin-1 and polycystin-2. 2008; 19:469 - 476
- Trapp ML, Galtseva A, Manning DK, Beier DR, Rosenblum ND, Quarmby LM. Defects in ciliary localization of Nek8 is associated with cystogenesis. Pediatr Nephrol 2008; 23:377 - 87; http://dx.doi.org/10.1007/s00467-007-0692-y; PMID: 18189147
- Sabbagh Y, Graciolli FG, O’Brien S, Tang W, dos Reis LM, Ryan S, Phillips L, Boulanger J, Song W, Bracken C, et al. Repression of osteocyte Wnt/β-catenin signaling is an early event in the progression of renal osteodystrophy. J Bone Miner Res 2012; 27:1757 - 72; http://dx.doi.org/10.1002/jbmr.1630; PMID: 22492547
- Winyard P, Jenkins D. Putative roles of cilia in polycystic kidney disease. Biochim Biophys Acta 2011; 1812:1256 - 62; http://dx.doi.org/10.1016/j.bbadis.2011.04.012; PMID: 21586324
- Ma M, Tian X, Igarashi P, Pazour GJ, Somlo S. Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat Genet 2013; 45:1004 - 12; http://dx.doi.org/10.1038/ng.2715; PMID: 23892607
- Kim E, Arnould T, Sellin LK, Benzing T, Fan MJ, Grüning W, Sokol SY, Drummond I, Walz G. The polycystic kidney disease 1 gene product modulates Wnt signaling. J Biol Chem 1999; 274:4947 - 53; http://dx.doi.org/10.1074/jbc.274.8.4947; PMID: 9988738
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Krönig C, Schermer B, Benzing T, Cabello OA, Jenny A, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet 2005; 37:537 - 43; http://dx.doi.org/10.1038/ng1552; PMID: 15852005
- Bergmann C, Fliegauf M, Brüchle NO, Frank V, Olbrich H, Kirschner J, Schermer B, Schmedding I, Kispert A, Kränzlin B, et al. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet 2008; 82:959 - 70; http://dx.doi.org/10.1016/j.ajhg.2008.02.017; PMID: 18371931
- Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, Kahn A, Vandewalle A, Perret C. Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene 2001; 20:5972 - 81; http://dx.doi.org/10.1038/sj.onc.1204825; PMID: 11593404
- Qian CN, Knol J, Igarashi P, Lin F, Zylstra U, Teh BT, Williams BO. Cystic renal neoplasia following conditional inactivation of apc in mouse renal tubular epithelium. J Biol Chem 2005; 280:3938 - 45; http://dx.doi.org/10.1074/jbc.M410697200; PMID: 15550389
- Marose TD, Merkel CE, McMahon AP, Carroll TJ. β-catenin is necessary to keep cells of ureteric bud/Wolffian duct epithelium in a precursor state. Dev Biol 2008; 314:112 - 26; http://dx.doi.org/10.1016/j.ydbio.2007.11.016; PMID: 18177851
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 2000; 407:535 - 8; http://dx.doi.org/10.1038/35035124; PMID: 11029008
- Vivante A, Mark-Danieli M, Davidovits M, Harari-Steinberg O, Omer D, Gnatek Y, Cleper R, Landau D, Kovalski Y, Weissman I, et al. Renal hypodysplasia associates with a WNT4 variant that causes aberrant canonical WNT signaling. J Am Soc Nephrol 2013; 24:550 - 8; http://dx.doi.org/10.1681/ASN.2012010097; PMID: 23520208
