Abstract
Primary cilia are unique sensory organelles that coordinate a wide variety of different signaling pathways to control cellular processes during development and in tissue homeostasis. Defects in function or assembly of these antenna-like structures are therefore associated with a broad range of developmental disorders and diseases called ciliopathies. Recent studies have indicated a major role of different populations of cilia, including nodal and cardiac primary cilia, in coordinating heart development, and defects in these cilia are associated with congenital heart disease. Here, we present an overview of the role of nodal and cardiac primary cilia in heart development.
Introduction
The heart is the first organ to form in the developing embryo to ensure distribution of nutrients and oxygen during fetal development.Citation1. Cardiogenesis is a complex process of highly coordinated events that include heart tube formation and looping, chamber septation, and maturation. The complexity of heart development is reflected by the high occurrence of congenital heart disease (CHD), which appear in almost 1% of all live births, thereby comprising the most common congenital disorder. Despite increasing knowledge and therapeutic advances, CHD causes 10% of all noninfectious infant deaths within the first year of life.Citation2,Citation3 The spectrum of CHD is broad and patients may have more than one heart abnormality. CHD can be characterized by multifaceted morphological and structural abnormalities including septal and valve defects, tetralogy of Fallot (TOF) and arterial transposition.Citation4,Citation5 CHD prognosis and mortality depend on the size, number, and type of defect(s) and the associated abnormalities.Citation2 An overview of common types of heart defects and their morphological characteristics is given in .
Table 1. Common types of congenital heart defects
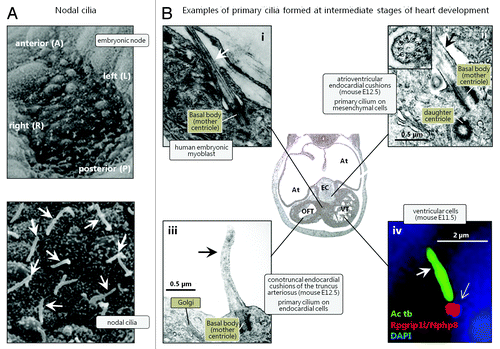
Throughout heart development, different types of cilia are expressed in a spatiotemporal manner to control various aspects of cardiogenesis. During gastrulation, motile and sensory cilia at the embryonic node () play a critical role in regulating signaling processes required for the establishment of left-right (L-R) organ asymmetry, a process which controls the initial stages of heart morphogenesis and connections to the vasculature.Citation6-Citation10 Consequently, defects in L-R signaling result in a variety of heart defects that arise from abnormal looping and remodeling of the primitive heart tube into a multi-chambered organ.Citation11-Citation19 In addition, primary cilia that are present in cardiomyocytes and within the developing heart, also known as cardiac primary cilia (), are compartmentalized with a series of receptor systems,Citation20-Citation22 which take part in regulating cellular signaling pathways important for the progressive differentiation, morphogenesis and maturation of the heart. This suggests that also cardiac primary cilia play a role in coordinating the signaling networks that are required for proper heart development. Here we present an overview of cilia, ciliopathies and heart development with focus on recent advances in understanding the role of different populations of cilia in coordinating signaling networks during heart development, and we discuss how defects in ciliary formation, motility, and sensory reception may lead to CHD.
Figure 1. Different populations of cilia in the developing heart. (A) Scanning electron microscopy images of nodal cilia (arrows) at the embryonic node. Reproduced from ref. Citation8 with permission. (B) Transmission electron microscopy (i, ii and iii) and immunofluorescence microscopy (IFM) (iv) images of cardiac primary cilia (arrows) emanating from the centrosomal mother centriole that functions as a basal body. In the IFM analysis, the primary cilium was marked with an antibody against acetylated α-tubulin (Ac tb; green), and the lower part of the cilium (open arrow) was marked with an antibody against Nephrocystin 8 (Rpgrip1l/Nphp8; red). Nuclei were marked with DAPI, which stains DNA (blue). Abbreviations: At: Atrium; EC: endocardial cushions; OFT: outflow tract; VT: ventricle. Reproduced from ref. Citation133 (i), Citation142(ii and iii), and Citation22 (iv) with permissions.
Cilia, Ciliopathies and CHD—An Overview
Cilia are membrane-bound, microtubule-based organelles that play important roles in motility and sensation. Cilia project from the surface of most animal cells, from protists to humans.Citation23 In vertebrates, cilia are generally divided into two types according to their axonemal arrangement and ability to engage active movement. Axonemes in multiciliated cells of mammalian epithelia (e.g., in brain ventricles, oviduct, and airways) usually have a 9+2 composition of microtubules, possess dynein arms, radial spokes, and are motile. Ciliary motility is regulated by outer (ODA) and inner (IDA) dynein arms that control ciliary beat frequency and form, respectively, and power the movement of fluid or substances over an epithelium. In contrast, non-motile primary cilia, which are found in a single copy on the surface of most quiescent cells in the body, usually have a 9+0 microtubule composition and lack dynein arms and radial spokes.Citation23 Both motile and primary cilia are subtended by a modified centriole called the basal body, which for primary cilia corresponds to the most mature (mother) centriole of the centrosome (), and rely on intraflagellar transport (IFT) for their assembly, length, maintenance, and signaling properties. IFT is a specialized bidirectional trafficking system with motor molecules, IFT complexes, and other adaptor proteins that move axonemal precursors (e.g., tubulin) and certain ciliary membrane proteins into and out of the cilium.Citation24 Canonical anterograde transport of proteins from the ciliary base to the tip is mediated by the heterotrimeric kinesin-2 motor protein, comprising Kif3a, Kif3b and Kap, whereas retrograde IFT is mediated by cytoplasmic dynein-2.Citation25,Citation26
Primary cilia play fundamental roles as chemo- and mechanosensory organelles and coordinate numerous signaling pathways, including the Hedgehog (Hh), Wingless-type Integration site (Wnt), Receptor Tyrosine Kinase (RTK), Notch, and Transforming Growth Factor β (TGFβ) signaling systems, as well as signaling through receptors for extracellular matrix proteins, ion channels, and a wide variety of different G-protein coupled receptors (GPCRs). By these means, primary cilia control cellular processes during development and in the adult organism.Citation20,Citation27-Citation36 In addition, specialized non-motile sensory cilia are present on neurons in the olfactory epithelium of the nasal cavity and on photoreceptor cells.Citation37,Citation38 Lastly, motile (nodal) cilia with ODA, also known as Left-Right dynein (LRD), are found during gastrulation at the embryonic node () and play a decisive role in laterality establishment.Citation6,Citation39 These cilia were originally reported to display 9+0 axonemes,Citation8 but nodal cilia with a 9+2 and even 9+4 axonemal structures have also been found in mouse, rabbit,Citation40,Citation41 and zebrafish.Citation42 Thus, motile as well as non-motile cilia with variable architecture of axonemal microtubules are present in multiple tissues and organs throughout the body where they regulate key events during development and in the adult. Therefore, defects in genes required for ciliary assembly, maintenance, motility, and/or sensory functions may lead to a series of syndromic diseases and developmental disorders referred to as ciliopathies. The clinical features of ciliopathies include laterality defects, airway dysfunction, sterility, cognitive disorders, skeletal bone, renal, hepatic, pancreatic, and brain defects, retinal degeneration, anosmia, obesity, and cancer.Citation43-Citation46
The multifaceted diseases and developmental defects associated with dysfunctional cilia reflect the complexity and importance of these organelles throughout life. Most importantly, defective cilia are also associated with CHD. Heart defects are observed in several ciliopathy syndromes, including Alström syndrome (ALMS), Bardet-Biedl syndrome (BBS), Meckel syndrome (MKS), Dandy-Walker syndrome (DWS), Joubert syndrome (JBTS), Ellis-van Creveld syndrome (EVC), McKusick-Kaufman syndrome (MKKS), Short-rib polydactyly syndromes (SRPS), Sensenbrenner syndrome (SS), and nephronophthisis (NPHP) (). In many cases, ciliopathy-associated CHDs display the typical heart defects observed in patients with laterality defects, yet several other types of CHD have been reported in ciliopathy patients, including septum defects, and aortic stenosis.Citation47-Citation51 As an example, NPHP proteins, the nephrocystins, critically regulate the recruitment and access of proteins to the cilium, such as through the formation of ciliary gating complexes.Citation52-Citation61 In this regard, NPHP2/inversin recruits and anchors NPHP3, NPHP9/Nek8, and the newly identified NPHP protein, Anks6, to a distinct region in the proximal cilium, the inversin compartment, and integrity of this module is essential for correct laterality establishment in organisms from zebrafish to humans.Citation15,Citation62-Citation68 Inversin was first discovered for its role in L-R establishmentCitation62 and, presumably, the inversin compartment impinges on the function of motile and/or sensory cilia at the embryonic node,Citation67,Citation69 which is critical in breaking the embryonic bilateral symmetry through the generation of a net leftward flow of the embryonic fluid during gastrulationCitation8 (see also below). Consequently, NPHP patients occasionally present with laterality defects and associated complex CHD.Citation13,Citation15,Citation47,Citation66,Citation68,Citation70-Citation72 At later stages of heart development, primary cilia may assist in coordinating signaling networks required for morphogenesis and maturation of the heart.
Table 2. Human ciliopathies that include cardiac phenotypes
Major Steps in Heart Development
Two different cell populations of mesodermal origin, i.e., the first (FHF) and second (SHF) heart fields, participate in mammalian heart development in a spatiotemporal manner; cells from FHF contribute to the ventricles, the atria and the atrioventricular canal (AVC), whereas cells from SHF contribute to the outflow tract (OFT) and all other regions of the heart, except for the left ventricle ().Citation73-Citation75 At embryonic day (E) 6.5 in mice, myocardial progenitor cells undergo epithelial-to-mesenchymal transformation (EMT) and ingress through the primitive streak while giving rise to the mesodermal layer forming the FHF. On each side of the midline, the FHF resides as two patches of mesodermal cells that extend across the midline and at E7.5, they fuse to form a crescent-shaped epithelium called the cardiac crescent. Cells from SHF initially lie medially to FHF precursor cells at E7.0–7.5.Citation73 During embryonic folding at E8 in mice and day 21 in humans, the cardiac crescent fuses along the midline forming the early heart tube (reviewed in refs. Citation1 and Citation76).
Figure 2. Overview on developmental stages of the heart. The cardiac crescent is formed around day 15 in humans. Myocardial progenitors from the first heart field (FHF, red color) contribute to the ventricles, the atria and the atrioventricular canal (AVC). Progenitors from the second heart field (SHF, blue color) contribute to the outflow tract (OFT) and all other regions of the heart, except the left ventricle. A linear heart tube is formed around day 21 in humans. The heart tube loops and chambers are formed by ballooning of regions destined to become atrium and ventricles. Endocardial cushions form in the AVC and the OFT. These cushions will later transform into the semilunar and atrioventricular valves and will participate in the septation of the OFT. Abbreviations: Ao, Aorta; LA, left atrium; LV, left ventricle; OTC, outflow tract cushions; Pa, pulmonary artery; RA, right atrium; RV, right ventricle.
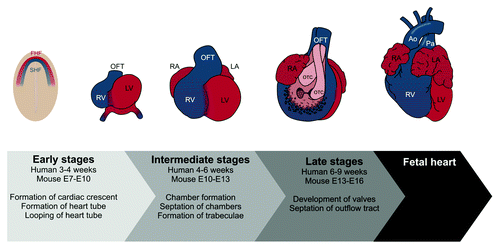
To form the cardiac chambers, the heart tube loops and expands regionally. Parts of the heart tube, which form the outer curvature of the looped heart tube, begin to gain a chamber myocardium gene expression profile, and the tissue expands in a balloon-like fashion to eventually become the atrial and ventricular chambers.Citation77 Specific parts of the heart tube retain a non-chamber myocardium gene expression profile, and function as “rings” of non-expanding myocardium, which participate in formation of the four-chambered heart.Citation78 This non-expanding myocardium consists of the inflow tract, the AVC and the OFT. Reciprocal signaling in the AVC and OFT between the endocardium and the myocardium induces endocardial cells to undergo endothelial to mesenchymal transformation (EndoMT), and invade the extracellular matrix, also known as the cardiac jelly, to form the endocardial cushions which will eventually be transformed into cardiac valves and participate in septation of the OFT.Citation79 Cells from the SHF and the neural crest also participate in septation of the OFT.Citation75,Citation80 The left and right atrium and ventricle become separated by muscular partitions, which grow from the atrial roof and the ventricular floor and meet endocardial cushions of the AVC and OFT.Citation81
Heart Development is Coordinated by Multiple Signaling Networks
Heart development is regulated by a complex network of inductive and inhibitory signals within the heart and from surrounding tissues that impact on cardiac specification, differentiation, and maturation. Initially, a series of craniolateral endoderm-derived inductive signals combined with inhibitory signals derived from midline structures regulate early cardiogenesis.Citation82 In all cases, the signals are controlled and interpreted by multiple signaling pathways, of which many are involved in coordination of several stages of heart development. As an example, the Hh signaling pathway plays an important role in establishing L-R asymmetry in vertebrates.Citation83-Citation85 In canonical Hh signaling, Hh ligands bind to and inhibit the activity of the transmembrane receptor Patched (Ptch), allowing another transmembrane receptor, Smoothened (Smo) to promote the activation of Gli transcription factors that control cellular processes during development and in tissue homeostasis.Citation86 The heart is the first organ to break the embryonic bilateral symmetry, and the asymmetric looping of the heart tube is important for correct chamber development and septation.Citation11 Targeted deletion of the Hh ligand gene, Sonic hedgehog (Shh), results in atrial and ventricular septum defects and abnormal development of the OFT in mice.Citation87 In agreement with this, atrial septum progenitor cells and cells of the pulmonary trunk show Hh responsiveness in mouse embryos.Citation88 Similarly, inhibition of Hh signaling in chicken embryos results in pulmonary atresia and stenosis as well as persistant truncus arteriosus (PTA).Citation89 In chicken embryos, SHH is expressed in the pharyngeal endoderm, adjacent to the SHF, where PTCH is expressed, suggesting a requirement for Hh signaling in SHF.Citation89 In line with this, tissue-specific deletion of Hh signaling components suggests that Hh signaling is required within the SHF and cardiac neural crest for normal development of the OFTCitation87,Citation90 and within the dorsal mesocardium for atrioventricular (AV) septation and AV valve development.Citation91
The TGFβ/Bone Morphogenic Protein (BMP) signaling network also plays a critical role during heart development.Citation92 This network comprises a multitude of different ligand types that act through the activation of a family of transmembrane receptor serine/threonine kinases, which in part activate Smad transcription factors to elicit different cellular responses during development and in tissue homeostasis.Citation92,Citation93 Nodal, Lefty1, and Lefty2 are all members of the TGFβ ligand superfamily. Like Shh, Nodal and Lefty1/2 are asymmetrically expressed in the node and involved in establishment of L-R asymmetry in the mouse embryo (see above, reviewed in ref. Citation10). In line with this, mutations in the NODAL and LEFTY2 genes cause heterotaxy and/or isolated CHD in humans.Citation94,Citation95 In vitro collagen gel experiments, using AV or OFT explants, suggest that TGFβ signaling is involved in EMT and EndoMT during the formation of endocardial cushions,Citation96-Citation98 and targeted mutation of genes encoding TGFβ ligands causes defects of the OFT (double outlet right ventricle (DORV)), abnormal semilunar valves, and AV cushions, supporting an involvement in development of the endocardial cushions.Citation99,Citation100 Null mutants for the TGFβ receptor genes, Tgfbr1 and Tgfbr2, are embryonic lethal in mice, but tissue specific deletion of TGFβ receptor genes support a role for TGFβ signaling in development of the OFT.Citation101,Citation102 Targeted deletion of Tgfbr1 or Tgfbr2 in neural crest cells leads to OFT defects (PTA, interrupted aortic arch and ventricular septal defect [VSD]), strongly suggesting that TGFβ signaling is necessary for cardiac neural crest cells to promote normal septation of the OFT.Citation101-Citation103 Other mouse models with targeted deletions in BMP/TGFβ signaling component genes support a role of this signaling network in heart development. Deletion of Smad6, which inhibits TGFβ/BMP signaling, causes valvular hyperplasia and OFT defects in mice.Citation104 Further, deletion of Ltbp1, encoding latent TGFβ-binding protein 1, causes OFT defects,Citation105 whereas cardiac specific deletion of Bmp2 causes AV cushion defects,Citation106 and cardiac specific deletion of Bmp4 results in OFT defects.Citation107 In humans, genomic deletion of TGFBR2Citation17 and point mutations in SMAD6Citation108 and SMAD2Citation109 have been associated with heart defects in the form of heterotaxy (TGFBR2, SMAD2) and valve defects (SMAD6).
Nodal Cilia and Heterotaxy
During gastrulation, both motile and immotile (primary) cilia are thought to play a critical role for the establishment of the L-R asymmetry of the body and proper placement and patterning of the internal organs and associated vasculature, including looping of the heart.Citation6,Citation39 Within the node, which is formed after definition of the dorsoventral and anteroposterior axes,Citation110,Citation111 motile nodal cilia () rotate synchronously to produce a leftward movement of embryonic fluid, i.e. the nodal flow. The correct positioning of nodal cilia is a prerequisite for the nodal flow, and a process that relies on planar cell polarity (PCP) signaling (reviewed inCitation39). The nodal flow is essential for defining lateral asymmetryCitation8,Citation67,Citation112,Citation113 in part through activation of the Nodal signaling cascade. Initially, the gene encoding actin binding lim protein 1 (Ablim1) is evenly expressed across the node but Ablim1 mRNA gradually disappears from the left side of the node in response to nodal flow, at least partially independent of the Nodal signaling cascade.Citation114 Simultaneously, Nodal accumulates at the left side of the embryo, conferring left-side specific Pitx2 expression and asymmetric morphogenesis.Citation115,Citation116
A total of 200–300 cilia generate the nodal flow in the mouse, but it has been suggested that as little as two motile cilia at the node can break the bilateral symmetry in the mouse embryo.Citation111 Several models have been introduced to explain how nodal flow regulates Nodal signaling and establishment of L-R asymmetry (reviewed in ref. Citation6). Two models suggest that nodal flow creates an asymmetric distribution of morphogens across the node resulting in accumulation of signaling molecules at the left side of the node — either as soluble molecules in the embryonic fluid or as encapsulated molecules that are delivered to the left side of the node in membrane-bound vesicles, also known as nodal vesicular parcels (NVPs) (reviewed in ref. Citation39). In the latter scenario, NVPs contain Shh and retinoic acid that induce an increase in the level of intracellular Ca2+ on the left side of the node and subsequent generation of L-R asymmetry.Citation117 A third model suggests that the nodal flow initiates a Ca2+ influx in crown cells on the left side of the node, via activation of the polycystins (PC2 and the PC1 homolog, PC1 like 1) in mechanosensory, immotile primary cilia.Citation7,Citation9,Citation118,Citation119 In support of the latter model, the asymmetric expression of Ablim1 at the node during the mid-headfold stage requires both a nodal flow and PC2, but the details of this relationship have not been resolved.Citation114 Further, it was recently shown that glycosylation of the Notch receptor in the crown cells by the N-acetylgalactosamine-type O-glycosylation enzyme GALNT11 is essential for the optimal ratio between motile and immotile cilia at the mouse node, in addition to correct Pitx2 expression in Xenopus embryos.Citation120 Importantly, however, the described models are not mutually exclusive, and since the breaking of bilateral symmetry is a crucial step in embryonic development, it is possible that several mechanisms exist to ascertain this process, up- and downstream of the nodal flow.Citation114,Citation121
The importance of nodal cilia in establishing L-R asymmetry was first revealed by the laboratories of Martina Brueckner and Nobutaka Hirokawa. Mouse embryos with deletion in either Kif3a or Kif3b displayed absence of nodal cilia with accompanied loss of nodal flow and L-R abnormalities, including defective cardiac looping,Citation8,Citation122 and mutation in Lrd, disrupting ciliary motility, produced a similar phenotype.Citation113,Citation123 Since these initial discoveries, several additional genes and gene products that regulate ciliary formation, maintenance, and function have been found to be critical for generating L-R asymmetry. These include genes encoding transcription factors such as Tbx6, Rfx3, Hfh-4, and ZIC3, which when defective lead to defects in formation of nodal cilia as well as situs abnormalities including situs inversus (SI) and heterotaxy.Citation16,Citation124-Citation126 Further, multiple genes important for ciliary positioning, motility and disassembly have been associated with situs abnormalities and heterotaxy, often associated with cardiac defects such as DORV, septal defects, transposition of the great arteries (TGA), TOF, single ventricle (SV), and coarctation of the aorta (CoA) in mice and/or zebrafish (reviewed in refs. Citation65,Citation127,Citation128). As an example, ciliary NPHP2/inversin is required for the generation of a proper nodal flow,Citation67 and potentially controls nodal cilia positioning.Citation39
In human studies, patients with mutations in genes required for ODA assembly and function often have motility-defective cilia and situs abnormalities.Citation128,Citation129 In a cohort of patients with ciliary motility defects, the incidence of heterotaxy was highly increased (6.3%) compared with the general population (0.004%). Further, the incidence of CHD is significantly increased in heterotaxy patients, when compared with the general population (57% vs. 1% respectively)Citation129,Citation130 with septal defects and TGA being the most prevalent CHDs.Citation129 In an ENU mutagenesis screen in mice,Citation131 a mutation in the gene encoding Dnah5 (a dynein heavy chain) was found to cause situs abnormalities, including heterotaxy (40%) and SI (35%), and many of the mice had associated CHD similar to those observed in the study by Kennedy and colleagues.Citation129 Together, these studies reflect the importance of nodal cilia and their motility in situs establishment.
In summary, mutations affecting the structure and function of nodal cilia lead to L-R asymmetry defects associated with CHD. However, it is important to realize that such mutations may also impact on the assembly and/or function of primary cilia formed within the developing heart, making it difficult to distinguish between the function of different populations of cilia in time and space during heart development; for example whether a given heart defect results from defective nodal and/or cardiac primary cilia. Nevertheless, as will be discussed below, multiple lines of evidence indicate that a series of cilia-based heart defects may be associated specifically with defects in cardiac primary cilia.
Cardiac Primary Cilia in Heart Development
Cardiac primary cilia were first discovered in 1969 when Rash and colleagues identified primary cilia throughout the embryonic and adult heart in chickens, mice, rabbits, and lizards.Citation132 Later, cardiac primary cilia were also observed in the embryonic and adult human heart,Citation133 as well as other parts of the cardiovascular system, including endothelial cells of the aortaCitation134 and cultured human umbilical vein endothelial cells.Citation135 The distribution of cardiac primary cilia during cardiogenesis is poorly resolved; however, a few papers have described a spatiotemporal distribution during heart development and morphogenesis. In E9.5 mouse embryos, where the early heart tube has looped and is contracting, primary cilia are lining the endothelium of both atria primordia, the forming ventricular trabeculations and the endothelium lining the developing endocardial cushions.Citation136 At E12.5, primary cilia are also found in the epicardium and in the mesenchymal cells of the endocardial cushions. The ventricular trabeculations remain ciliated, but the atria primordia are now less ciliated and the endothelium lining the endocardial cushion is de-ciliated.Citation136 For examples of cardiac primary cilia, please see . The re-absorption of cilia in the endothelium of the endocardial cushions is in agreement with other studies showing that primary cilia are resorbed in response to sheer stress.Citation135,Citation137-Citation139 Fluid shear stress is known to play important roles in trabeculation, cardiomyocyte proliferation, and establishment of the conduction system, and changes in the fluid flow leads to CHD.Citation140 Interestingly, mouse endothelial cells that form stunted primary cilia (e.g derived from the Tg737orpk mutant) or are mutated in Pkd1 show impaired response to shear stress,Citation141 and mice with mutations in Kif3a, Lrd, Pkd2Citation136 and Ift88/Tg737Citation21,Citation142 have defects in chamber maturation.Citation21,Citation136,Citation142
A variety of different vesicle trafficking pathways regulate the assembly, maintenance, and sensory function of cilia.Citation143,Citation144 Often, the proximal part of the cilium is placed within an invagination of the plasma membrane known as the ciliary pocket, which functions as the ciliary platform for exocytosis of Golgi-derived vesicles and for clathrin-dependent endocytosis (CDE).Citation145 Available evidence suggests that exocytosis is required for the targeting of transmembrane receptors and ion channels to the ciliary membrane, and CDE may regulate ciliary signaling through the internalization and/or recycling of receptors at the ciliary base.Citation20,Citation146 In support of a critical role of cilia in heart development, mutations in genes involved in trafficking of vesicles to and from the cilium are associated with CHD. Mutant mouse embryos lacking the Golgi-associated protein, Gmap210, which is required for Ift20-mediated trafficking and localization of PC2 to the cilium,Citation147 die around the time of birth and show heart defects including VSD and TOF.Citation147 In agreement with this, Pkd2−/− mouse embryos display heart defects reminiscent of those observed in Gmap210−/− mouse embryos.Citation148 Further, Pifo−/− E7.5 mouse embryos, which lack Pitchfork that interacts with components of the endocytic machinery, including the small GTPases Rab8, Rab11 and Arl13b, and induces the resorption of the primary cilium before entry into mitosis,Citation19 display double-ciliated cells in the nodal pit as well as L-R defects with associated cardiac defects, including DORV and right ventricle hypoplasia.Citation19 Other proteins that are important for intracellular trafficking pathways in relation to cilia and have been associated with CHD include the Alström syndrome protein 1, ALSM1, and the sorting Nexin, SNX10.Citation149,Citation150 ALMS1 localizes to the pericentriolar region together with Rab11 and early endosomes, and in isolated fibroblasts from ALMS patients the kinetics of transferrin uptake by CDE is increased compared with that in wild type (wt) fibroblasts.Citation149 SNX10 affects the ciliary localization of Rab8a, and in snx10 mutant zebrafish, cardiac looping is affected.Citation150 In support of a role for nexins in cardiogenesis, a patient with TOF was recently found to have a rare genomic deletion which include the SNX8 gene.Citation130 In relation to the different functions of nodal and cardiac primary cilia at different time points during development, it is likely that some of the heart defects found in ciliary mutants may be caused by events later in heart development, and could thus be triggered by defects in cardiac primary cilia.Citation21,Citation136
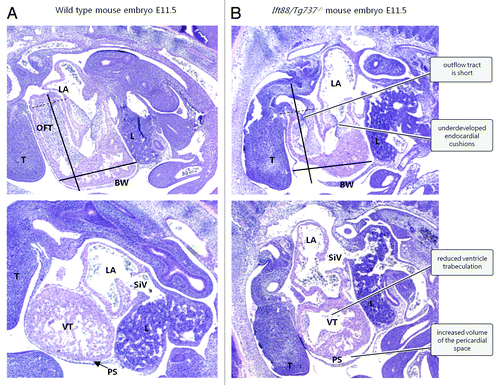
Mutations in other genes involved in ciliogenesis also causes CHD. Ift88/Tg737−/− mouse embryos with stunted cilia develop severe CHD including atrial septal defects (ASD), VSD, atrioventricular septal defect (AVSD), and OFT septal defectsCitation21,Citation136,Citation142; defects that are all associated with malformed endocardial cushions (). Development of the endocardial cushions depends on EndoMT, a cellular process whereby endothelial cells lose the epithelial phenotype and differentiate into mesenchymal cells that ingress into the underlying cardiac jelly.Citation151 The endocardial cushions in Kif3a−/− and Pkd2−/− mouse embryos are acellular and embryos with mutation in the Ift88/Tg737 gene have a decreased amount of mesenchymal cells in the endocardial cushions. Furthermore, endocardial cells derived from Ift88/Tg737−/− mice show disturbances in EndoMT.Citation136,Citation142,Citation151 Taken together, this suggests that cardiac primary cilia are involved in formation and development of the endocardial cushions. Indeed, the regulation of signaling networks during heart development has in some cases been linked to the function of cardiac primary cilia,Citation20-Citation22 indicating the complexity by which different populations of cilia may contribute to the formation the heart in a spatiotemporal manner. However, we still know little as to how cardiac primary cilia contribute to heart development independently of nodal cilia and downstream of situs establishment, and whether CHD may arise exclusively by defects in the sensory function of primary cilia in the tissues of the developing heart.
Figure 3. Defects in ciliogenesis lead to CHD in Ift88/Tg737−/− mouse embryos (E11.5). Upper panels: longitudinal mid-sagittal sections. Lower panels: comparable longitudinal para-sagittal sections. Abbreviations: BW, body wall; L, liver; LA, left atrium; OFT, outflow tract; PS, pericardial space (arrow); SiV, sinus venosus; T, tongue; VT, ventricle. The black bars have identical dimensions in wt (A) and mutant (B) embryos. The distal end of the OFT is marked with a dotted line. The Figure is modified from ref. Citation21 with permission.
Cardiac Primary Cilia and Coordination of Hedgehog Signaling
Hh signaling is coordinated by primary cilia in a variety of different cell types to regulate tissue patterning and homeostasis in vertebrates (reviewed in refs. Citation29,Citation86,Citation152,Citation153). The cilium functions as a unique compartment for the continuous turnover of Hh signaling components, such as Ptch-1, Smo, and Gli transcription factors and other regulatory proteins to control cellular processes. Smo becomes localized to the primary cilium through the binding of Hh ligands to Ptch-1, which then induces Smo-dependent activation of Gli transcription factors in the cilium. Consequently, defects in ciliary assembly or turnover of Hh components in the cilium lead to a plethora of developmental disorders and diseases, including CHD.Citation21,Citation22,Citation136,Citation142
A number of observations have specifically linked cardiac primary cilia to the regulation of Hh signaling. Components in this pathway localize to primary cilia in embryonic hearts as well as in cardiomyocytes differentiating from stem cells in vitro (),Citation21,Citation22,Citation142 and defective ciliary assembly is associated with defective cardiomyogenesisCitation21 as well as Hh-related heart defects that might be independent of nodal cilia, including ventricular and endocardial cushion-derived defects.Citation122,Citation142,Citation154 Further, 60% of patients with mutations in genes encoding the EVC proteins, EVC1/EVC2, which interact with Smo at the primary cilium to transduce Hh signalingCitation155-Citation159, display CHD, including AVSD and ASD.Citation47,Citation51 In line with these findings, EVC proteins are co-expressed in the OFT and the mesenchymal structures of the atrial septa and AV cushions during heart development in miceCitation51; areas that are known to be ciliated during cardiogenesis.Citation136,Citation142
Figure 4. Primary cilia and signaling pathways in cardiomyogenesis and heart development. (A) Schematic drawing of signaling pathways in the cilium. (B) Immunofluorescence microscopy (IFM) analysis of Hedgehog (Hh) and Platelet-Derived Growth Factor Receptor α (PDGFRα) signaling components in ventricular primary cilia in transverse embryonic mouse heart sections at E11.5. Primary cilia (arrows) were marked with an antibody against acetylated α-tubulin (Ac tb; green). Signaling components (red) were marked with antibodies against transcription factors in Hh signaling (Gli2 and Gli3; upper and middle row images) and PDGFRα (lower row images). Nuclei were marked with DAPI, which stains DNA (blue). Reproduced from ref. Citation21 (i) and ref. Citation22 (ii and iii) with permissions. (C) Left image: Light microscopy analysis of a beating cluster of cardiomyocytes differentiated from mouse embryonic stem cells and IFM image (inset) of a primary cilium (arrow) marked with glutamylated α-tubulin (Glu tb; green) in cardiomyocytes expressing α-actinin (red). Nuclei were marked with DAPI (blue). Right images: IFM analysis on the localization of Hedgehog and Transforming Growth Factor β (TGFβ) signaling components to primary cilia (Ac tb, open arrows) in stem cells undergoing cardiomyogenesis. The ciliary pocket area is indicated with arrow heads. Hh signaling components (Smoothened [Smo], Patched-1 [Ptch-1] and Gli2, all green) localizes along the entire length of the cilium (red) - nuclei were marked with DAPI (blue) (upper row images). TGFβ signaling components (TGFβ Receptor I [TGFβ-RI], phospho-Smad2/3 [pSmad2/3] and Smad4, all green) localize around the ciliary pocket area (lower row images). For Smad4 localization, the cilium is indicated with blue fluorescence. Reproduced from refs. Citation20,Citation21 with permissions.
![Figure 4. Primary cilia and signaling pathways in cardiomyogenesis and heart development. (A) Schematic drawing of signaling pathways in the cilium. (B) Immunofluorescence microscopy (IFM) analysis of Hedgehog (Hh) and Platelet-Derived Growth Factor Receptor α (PDGFRα) signaling components in ventricular primary cilia in transverse embryonic mouse heart sections at E11.5. Primary cilia (arrows) were marked with an antibody against acetylated α-tubulin (Ac tb; green). Signaling components (red) were marked with antibodies against transcription factors in Hh signaling (Gli2 and Gli3; upper and middle row images) and PDGFRα (lower row images). Nuclei were marked with DAPI, which stains DNA (blue). Reproduced from ref. Citation21 (i) and ref. Citation22 (ii and iii) with permissions. (C) Left image: Light microscopy analysis of a beating cluster of cardiomyocytes differentiated from mouse embryonic stem cells and IFM image (inset) of a primary cilium (arrow) marked with glutamylated α-tubulin (Glu tb; green) in cardiomyocytes expressing α-actinin (red). Nuclei were marked with DAPI (blue). Right images: IFM analysis on the localization of Hedgehog and Transforming Growth Factor β (TGFβ) signaling components to primary cilia (Ac tb, open arrows) in stem cells undergoing cardiomyogenesis. The ciliary pocket area is indicated with arrow heads. Hh signaling components (Smoothened [Smo], Patched-1 [Ptch-1] and Gli2, all green) localizes along the entire length of the cilium (red) - nuclei were marked with DAPI (blue) (upper row images). TGFβ signaling components (TGFβ Receptor I [TGFβ-RI], phospho-Smad2/3 [pSmad2/3] and Smad4, all green) localize around the ciliary pocket area (lower row images). For Smad4 localization, the cilium is indicated with blue fluorescence. Reproduced from refs. Citation20,Citation21 with permissions.](/cms/asset/f36e2ddf-40f1-453b-927d-cef8295862fe/kogg_a_10927483_f0004.gif)
Septation of the ventricles is also dependent on the gene Fantom (Ftm)Citation22 that encodes the ciliary base protein Rpgrip1l/Nphp8, which is required for proper ciliogenesis, PCP, and Shh signaling, and when mutated leads to a series of ciliopathies.Citation22,Citation52-Citation54,Citation160-Citation165 In Ftm null mouse embryos, 33% of all analyzed embryos had VSD in the membranous part of the septum, 81.5% had defects in the muscular part, and all analyzed embryos had reduced atrial and ventricular wall thickness.Citation22 In the ciliated areas of Ftm−/− embryonic hearts at E11.5, the rate of proliferation and Shh signaling was markedly reduced. In contrast, no change was observed in non-ciliated areas,Citation22 suggesting that Ftm relies on cardiac primary cilia for signaling, and that the heart phenotypes in the Ftm−/− mouse are primary cilia-dependent. Ftm seems to be involved in the processing of Gli3,Citation161 and in agreement with this, the amount of unprocessed Gli3 is 10-fold higher in Ftm−/− hearts compared with heart of control littermates.Citation22 The cardiac phenotypes described above suggest that Hh signaling is important for proliferation and differentiation of cardiomyocytes and are thus in agreement with the previous findings that primary cilia in part coordinate Hh signaling during the differentiation of stem cells into cardiomyocytes.Citation21,Citation166 Finally, defects in proper trafficking of Smo and Ptch1 in and out of the cilium due to mutations in Ift25 lead to cardiac phenotypes in mice reminiscent of those found in Hh signaling mutants, including defects involving the ventricle, OFT and AVC.Citation154
Cardiac Primary Cilia and Coordination of TGFβ Signaling
The superfamily of TGFβ/BMP signaling pathways is involved in a vast majority of cellular processes and is therefore fundamentally important during development and in tissue homeostasis.Citation92 The functional output of these pathways highly relies on their extensive cross-talking with other receptor-mediated signaling systems, leading to synergistic or antagonistic effects on tissue patterning and organ function.Citation92 Consequently, deregulation of TGFβ/BMP activity almost invariably leads to pathologies in the adult as well as severe developmental defects, including malformation of the OFT, AVC and septa.Citation19,Citation167 Interestingly, defects in these areas are also found in cilia-related mutants. Further, genomic deletion and duplication of the human gene encoding TGFβ Receptor II, TGFBR2, causes heterotaxy,Citation17 whereas endothelial cells derived from Ift88/Tg737−/− mouse embryos show defective TGFβ signaling upon sheer induced EndoMT.Citation151 These results indicate that TGFβ signaling is important at multiple stages during heart development, i.e., both up- and downstream of L-R determination.
We recently showed a function of the primary cilium in regulating canonical TGFβ signaling through the activation of Smad2/3 transcription factors at the ciliary pocket.Citation20 This signaling pathway is regulated by CDE where internalization of ligand-bound TGFβ receptors in clathrin coated vesicles (CCVs) and early endosomes (EEs) allows Smad2/3 to become activated by receptor-mediated phosphorylation.Citation168,Citation169 In several cases, cardiac primary cilia emerge from a ciliary pocketCitation133,Citation142 and during the differentiation of murine carcinoma stem cells as well as human embryonic stem cells (hESC) into cardiomyocytes, TGFβ receptors and activated Smad2/3 accumulate at the ciliary base ().Citation20 Further, the TGFβ ligand, TGF-β1, stimulates the differentiation of stem cells into cardiomyocytes, and mouse embryonic fibroblasts derived from mice with a hypomorphic mutation in Ift88/Tg737 (Tg737orpk) show decreased TGFβ signaling associated with reduced CDE activity at the ciliary base.Citation20 These findings support the conclusion that cardiac primary cilia play a direct role in coordinating TGFβ signaling during cardiomyogenesis. It remains to be investigated whether BMP signaling also is associated with the primary cilium during heart development.
Cardiac Primary Cilia and Coordination of PDGF Signaling
PDGF signaling is mediated by a series of ligands (PDGF-AA, -AB, -BB, -CC, and -DD), which bind to and activate either homo- or hetero-dimers of the RTKs, Platelet Derived Growth Factor Receptor α (PDGFRα) and Platelet Derived Growth Factor Receptor β (PDGFRβ).Citation170 Previous studies showed that PDGFRα specifically localizes to primary cilia in a number of cell types and tissues, including the heart,Citation22,Citation171-Citation176 and that activation of the receptor and its downstream effectors, Mek1/2-Erk1/2-Rsk and Pi3K-Akt, is initiated in the cilium to regulate cell cycle control and directional cell migration in fibroblasts.Citation171,Citation177,Citation178
The recent finding that PDGFRα localizes to primary cilia in E11.5 mouse heart ventriclesCitation22 (), indicates that part of the PDGF signaling network is associated with cardiac primary cilia during heart development. Ciliary localization of PDGFRα is downregulated in both Ftm- and Shh-negative ventricles, suggesting that PDGFRα signaling acts downstream of Hh signaling in cardiac primary cilia and that defects in these cilia are associated with reduced ventricular cell proliferation leading to diminished ventricular wall thickness and VSD.Citation22 Interestingly, PDGFRα and its specific ligands, PDGF-A/C,Citation179 localize to the ventricles and the OFT and AV cushions, and the myocardium of the OFT, VS, and AS during heart development.Citation180,Citation181 These regions correspond to ciliated areas of the heart, further emphasizing a link between PDGFRα signaling and the primary cilium. In particular, the spatiotemporal expression of PDGFRα and PDGF-A/C ligands suggests a role of this signaling pathway during remodeling of the myocardium and in septation of atria, ventricles, and OFT,Citation180,Citation182,Citation183 and in agreement with this, alteration in PDGF signaling affects myofibril differentiation and migration.Citation184,Citation185 Furthermore, studies from mice show that mutated or absent PDGFRα, results in prenatal death and heart defects including thinned myocardium, and septa, valve, OFT, and aortic arch malformations.Citation186-Citation188 In conclusion, part of the PDGF signaling system might be specifically coordinated by cardiac primary cilia, potentially in a network with Hh and other signaling pathways to coordinate cardiogenesis.
Cardiac Primary Cilia and Other Signaling Pathways
In addition to Hh, TGFβ, and PDGFRα signaling, multiple other signaling networks critically regulate heart development, including Wnt and Notch signaling.Citation82,Citation189-Citation194 The Wnt signaling network consists of a highly complex signaling arrangement that traditionally is divided into canonical and non-canonical Wnt signaling.Citation195 In canonical signaling, the binding of Wnt-ligands to Frizzled (Fz) receptors facilitates stabilization of β-catenin, which mediates transcription of canonical Wnt target genes in cell proliferation and differentiation. Non-canonical Wnt signaling operates independently of β-catenin via Dishevelled (Dvl) and its various interaction partners, such as Vangl2 and Celsr, resulting in polarization of cells and tissues, e.g. PCP, to promote cell migration and convergent extension movements.Citation2,Citation189,Citation192,Citation193,Citation196 The link between Wnt signaling and the primary cilium is highly controversial.Citation32,Citation35,Citation197 However, many Wnt signaling components, such as Fz3, Dvl, and β-catenin, localize to the ciliary/centrosomal axis, and dysregulated Wnt signaling has been reported in cells and tissues with disrupted ciliogenesis or basal body integrity.Citation198-Citation200 Moreover, in the mouse inner ear, establishment of PCP was shown to depend on the formation of functional primary cilia.Citation201 However, other studies in mice and zebrafish reveal no obvious canonical or non-canonical Wnt phenotypes in cilia mutants,Citation202,Citation203 emphasizing the importance of further studies in this area.
Multiple PCP components are strongly expressed in the OFT during early heart development, and when defective, result in OFT and ventricular defects.Citation2,Citation189 Mutations in Vangl2 disrupt migration of cells into the OFT and this is associated with impaired OFT myocardialization as well as ventricular and OFT septal defects.Citation189 The localization of Vangl2 during heart development is dependent on Scribble, another PCP component,Citation204 and in agreement with this, Scribble phenotypes are reminiscent of Vangl2 mutants.Citation204,Citation205 In support of the idea that non-canonical Wnt signaling is associated with cardiac primary cilia, Gmap210−/− mouse embryos with impaired ciliogenesis due to impaired ciliary targeting have cardiac phenotypes resembling those observed in multiple PCP mutants, including Vangl2, Dvl2, and Scribble mutant mice.Citation147 Although kidney-specific depletion of Ift20 in mice resulted in defective Wnt signaling in the affected tissues,Citation206 it remains to be determined whether the cardiac phenotypes observed in the Gmap210-/- mouse are directly associated with defective non-canonical Wnt signaling. Interestingly, NPHP2–4 and Ftm/Rpgrip1l/NPHP8 all seem to impair canonical Wnt-signaling while promoting non-canonical Wnt responses.Citation14,Citation165,Citation200,Citation207-Citation209 As an example, NPHP2/inversin and the structurally related diversin in zebrafish are required for convergence extension movement in vertebrates, and depletion of either result in cardiac defects.Citation190,Citation208 Together, these aspects suggest that inversin and other NPHPs are implicated in heart development by controlling Wnt signaling, but whether this is coordinated by cardiac primary cilia remains speculative at this point.
During cardiogenesis, Notch signaling is important for development of the OFT and ventricular trabeculation.Citation194 Cell-cell contact is required for Notch signaling as the ligands for the Notch receptors (1–4) are transmembrane proteins and include proteins from the Delta and Jagged family. Ligand binding to the Notch receptors induces cleavage and release of NICD, the intracellular part of the receptor that translocates to the nucleus to regulate gene transcription.Citation194,Citation210 Recently, Notch signaling was associated with the primary cilium during skin development, as Notch3 and presenilin-2 localize to the primary cilium in suprabasal epidermal cells, and defective IFT results in altered Notch signaling.Citation31 The expression of Notch components correlates with ciliated areas of the heart during development, and defective Notch signaling leads to cardiac phenotypes reminiscent of cilia-related phenotypes.Citation194,Citation210,Citation211 It was recently shown that glycosylation of the Notch receptor is important for specifying non-motile primary cilia in the frog gastrocoel roof plate (frog node),Citation120 although a direct link between Notch signaling and primary cilia in cardiomyogenesis and heart development remains to be proven.
Concluding Remarks and Perspectives
Primary and motile cilia are required throughout heart development. Initially, at E8 in the mouse, non-motile sensory cilia on crown cells at the periphery of the node and motile cilia at the center of the node are required for generating the L-R asymmetry of the embryo and correct looping of the heart. At later stages, primary cilia in the developing heart may coordinate signaling pathways important for organizing morphogenesis and maturation of the heart. Consequently, defects in assembly or function of nodal and cardiac primary cilia may lead to CHD.
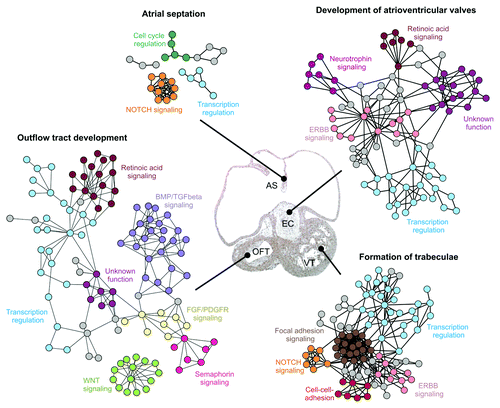
Solexa-based transcriptomics of murine stem cells differentiating into beating clusters of cardiomyocytes showed that this process involves time-dependent differential expression of genes within the Mitogen Activated Protein Kinase (MAPK), Wnt, TGFβ/BMP, Hh, Epidermal Growth Factor Receptor (EGFR) and Notch signaling pathways,Citation20 and both Hh and TGFβ signaling are associated with primary cilia forming within these clusters of cardiomyocytes.Citation20,Citation21 Lage et al. (2010) performed a genome-wide systematic mapping of protein-protein interaction networks involved in different stages of heart development based on mouse models.Citation212 In this study, high-confidence experimental interactome data suggested that heart development is controlled by communication within and between a defined set of signaling pathways (). As such, these signaling pathways seem to function as recycled signaling modules, which integrate into higher-order networks to control the different stages of heart development. Keeping in mind that such an analysis only covers part of the signaling networks involved in heart development, we note that many of these cross-talking functional modules include signaling pathways known to be coordinated by the primary cilium. These signaling modules include TGFβ/BMP signaling, Fibroblast Growth Factor Receptor (FGFR) signaling, PDGFR signaling, Notch signaling, EGFR signaling and Wnt signaling among others.
Figure 5. Examples of signaling networks involved in development of specific anatomical structures of the heart. Protein-protein interaction networks involved in atrial septation, outflow tract development, atrioventricular valve development and formation of trabeculae are shown. Functional clusters within the networks are color coded. Tissues affected by the networks are marked in a hematoxylin-eosin stained frontal section of the heart from a 37 d human embryo. Abbreviations: AS, atrial septum; EC, endocardial cushions; OFT, outflow tract; VT, ventricle. The figure is modified from ref. Citation212
Because so many different signaling systems are associated with primary cilia,Citation20,Citation21,Citation31,Citation171,Citation172,Citation175,Citation200,Citation213-Citation216 we suggest that primary cilia may function as signaling hubs in the spatiotemporal cross-talking between diverse signaling networks during heart development. Future work should therefore focus on how primary cilia are involved in the integration and cross-talking between different signaling networks and how this may impact on different stages of heart development. Here, it will be important to differentiate between signaling involving cilia in the node and signaling involving primary cilia in cardiac tissues at later developmental stages. Thus, it will be important to include analysis with conditional knockout of primary cilia from various heart tissues at different time points and investigate how cardiac primary cilia function in the progressive differentiation, morphogenesis and maturation of the heart independent of nodal cilia and early L-R specification.
| Abbreviations: | ||
| Ablim1 | = | actin binding lim protein 1 |
| AoS | = | aortic stenosis |
| ALMS | = | Alström syndrome |
| ASD | = | atrial septal defect |
| AV | = | atrioventricular |
| AVC | = | atrioventricular canal |
| AVCD | = | atrioventricular canal defect |
| AVS | = | aortic valve stenosis |
| AVSD | = | atrioventricular septal defect |
| BBS | = | Bardet-Biedl syndrome |
| BMP | = | Bone Morphogenic Protein |
| CA | = | common atrium |
| CCV | = | clathrin coated vesicle |
| CDE | = | clathrin dependent endocytosis |
| CHD | = | congenital heart disease |
| DWS | = | Dandy-Walker syndrome |
| CoA | = | coarctation of the aorta |
| DORV | = | double outlet right ventricle |
| Dvl | = | Dishevelled |
| E | = | embryonic day |
| EE | = | early endosome |
| EGFR | = | Epidermal Growth Factor Receptor |
| EMT | = | epithelial-to-mesenchymal transformation |
| EndoMT | = | endothelial-to-mesenchymal transformation |
| EVC | = | Ellis-van Creveld syndrome |
| FGFR | = | Fibroblast Growth Factor Receptor |
| FHF | = | first heart field |
| Ftm | = | Fantom |
| Fz | = | Frizzled |
| GPCRs | = | G-protein coupled receptors |
| hESC | = | human embryonic stem cells |
| Hh | = | Hedgehog |
| HLHS | = | hypoplastic left heart syndrome |
| IDA | = | inner dynein arm |
| IFT | = | intraflagellar transport |
| JBTS | = | Joubert syndrome |
| L-R | = | left-right |
| LRD | = | left-right dynein |
| MAPK | = | Mitogen Activated Protein Kinase |
| MKKS | = | McKusick-Kaufman syndrome |
| MKS | = | Meckel syndrome |
| NPHP | = | nephronophthisis |
| NVP | = | nodal vesicular parcels |
| ODA | = | outer dynein arm |
| OFD | = | Orofaciodigital syndrome |
| OFT | = | outflow tract |
| PC | = | polycystin |
| PCP | = | planar cell polarity |
| PDA | = | patent ductus arteriosus |
| PDGFR | = | Platelet Derived Growth Factor Receptor |
| PDGFRα | = | Platelet Derived Growth Factor Receptor alpha |
| PDGFRβ | = | Platelet Derived Growth Factor Receptor beta |
| Pifo | = | Pitchfork |
| PLSVC | = | persistent left superior vena cava |
| PS | = | pulmonary stenosis |
| PTA | = | persistent truncus arteriosus |
| Ptch | = | Patched |
| RTK | = | Receptor Tyrosine Kinase |
| SHF | = | second heart field |
| Shh | = | Sonic hedgehog |
| SI | = | Situs Inversus |
| Smo | = | Smoothened |
| SRPS | = | Short-rib polydactyly syndrome |
| SS | = | Sensenbrenner syndrome |
| SV | = | single ventricle |
| TGA | = | transposition of the great arteries |
| TGFβ | = | Transforming Growth Factor beta |
| TOF | = | tetralogy of Fallot |
| VSD | = | ventricular septal defect |
| Wnt | = | Wingless-type Integration site |
| wt | = | wildtype |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank all authors who have given permission for their micrographs to be used in the article. We apologize to those investigators whose work has not been cited due to space limitations. This work was supported by grants from the Lundbeck Foundation, The Novo Nordisk Foundation, The Danish Council for Independent Research, Nordforsk, The Danish Cancer Society, The Danish Heart Association and the UCPH Excellence Programme for Interdisciplinary Research (2016 Funds), University of Copenhagen, Denmark.
References
- Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet 2005; 6:826 - 35; http://dx.doi.org/10.1038/nrg1710; PMID: 16304598
- Wu G, Ge J, Huang X, Hua Y, Mu D.. Planar cell polarity signaling pathway in congenital heart diseases. J Biomed Biotechnol. 2011; 2011:589414
- Erdogan F, Larsen LA, Zhang L, Tümer Z, Tommerup N, Chen W, Jacobsen JR, Schubert M, Jurkatis J, Tzschach A, et al. High frequency of submicroscopic genomic aberrations detected by tiling path array comparative genome hybridisation in patients with isolated congenital heart disease. J Med Genet 2008; 45:704 - 9; http://dx.doi.org/10.1136/jmg.2008.058776; PMID: 18713793
- Kaltenbrun E, Tandon P, Amin NM, Waldron L, Showell C, Conlon FL. Xenopus: An emerging model for studying congenital heart disease. Birth Defects Res A Clin Mol Teratol 2011; 91:495 - 510; http://dx.doi.org/10.1002/bdra.20793; PMID: 21538812
- Fraser CD Jr.. A clinical commentary on the article “biomaterials advances in patches for congenital heart defect repair” : cardiac bioengineering for congenital heart disease: time for progress. J Cardiovasc Transl Res 2011; 4:655 - 7; http://dx.doi.org/10.1007/s12265-011-9294-y; PMID: 21845479
- Norris DP. Cilia, calcium and the basis of left-right asymmetry. BMC Biol 2012; 10:102; http://dx.doi.org/10.1186/1741-7007-10-102; PMID: 23256866
- McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 2003; 114:61 - 73; http://dx.doi.org/10.1016/S0092-8674(03)00511-7; PMID: 12859898
- Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 1998; 95:829 - 37; http://dx.doi.org/10.1016/S0092-8674(00)81705-5; PMID: 9865700
- McGrath J, Brueckner M. Cilia are at the heart of vertebrate left-right asymmetry. Curr Opin Genet Dev 2003; 13:385 - 92; http://dx.doi.org/10.1016/S0959-437X(03)00091-1; PMID: 12888012
- Komatsu Y, Mishina Y. Establishment of left-right asymmetry in vertebrate development: the node in mouse embryos. Cell Mol Life Sci 2013; 70:4659 - 66; http://dx.doi.org/10.1007/s00018-013-1399-9; PMID: 23771646
- Ramsdell AF. Left-right asymmetry and congenital cardiac defects: getting to the heart of the matter in vertebrate left-right axis determination. Dev Biol 2005; 288:1 - 20; http://dx.doi.org/10.1016/j.ydbio.2005.07.038; PMID: 16289136
- Onoufriadis A, Paff T, Antony D, Shoemark A, Micha D, Kuyt B, Schmidts M, Petridi S, Dankert-Roelse JE, Haarman EG, et al, UK10K. Splice-site mutations in the axonemal outer dynein arm docking complex gene CCDC114 cause primary ciliary dyskinesia. Am J Hum Genet 2013; 92:88 - 98; http://dx.doi.org/10.1016/j.ajhg.2012.11.002; PMID: 23261303
- French VM, van de Laar IM, Wessels MW, Rohe C, Roos-Hesselink JW, Wang G, Frohn-Mulder IM, Severijnen LA, de Graaf BM, Schot R, et al. NPHP4 variants are associated with pleiotropic heart malformations. Circ Res 2012; 110:1564 - 74; http://dx.doi.org/10.1161/CIRCRESAHA.112.269795; PMID: 22550138
- Bergmann C, Fliegauf M, Brüchle NO, Frank V, Olbrich H, Kirschner J, Schermer B, Schmedding I, Kispert A, Kränzlin B, et al. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet 2008; 82:959 - 70; http://dx.doi.org/10.1016/j.ajhg.2008.02.017; PMID: 18371931
- Zhou W, Dai J, Attanasio M, Hildebrandt F. Nephrocystin-3 is required for ciliary function in zebrafish embryos. Am J Physiol Renal Physiol 2010; 299:F55 - 62; http://dx.doi.org/10.1152/ajprenal.00043.2010; PMID: 20462968
- Hadjantonakis AK, Pisano E, Papaioannou VE. Tbx6 regulates left/right patterning in mouse embryos through effects on nodal cilia and perinodal signaling. PLoS One 2008; 3:e2511; http://dx.doi.org/10.1371/journal.pone.0002511; PMID: 18575602
- Fakhro KA, Choi M, Ware SM, Belmont JW, Towbin JA, Lifton RP, Khokha MK, Brueckner M. Rare copy number variations in congenital heart disease patients identify unique genes in left-right patterning. Proc Natl Acad Sci U S A 2011; 108:2915 - 20; http://dx.doi.org/10.1073/pnas.1019645108; PMID: 21282601
- Pennekamp P, Karcher C, Fischer A, Schweickert A, Skryabin B, Horst J, Blum M, Dworniczak B. The ion channel polycystin-2 is required for left-right axis determination in mice. Curr Biol 2002; 12:938 - 43; http://dx.doi.org/10.1016/S0960-9822(02)00869-2; PMID: 12062060
- Kinzel D, Boldt K, Davis EE, Burtscher I, Trümbach D, Diplas B, Attié-Bitach T, Wurst W, Katsanis N, Ueffing M, et al. Pitchfork regulates primary cilia disassembly and left-right asymmetry. Dev Cell 2010; 19:66 - 77; http://dx.doi.org/10.1016/j.devcel.2010.06.005; PMID: 20643351
- Clement CA, Ajbro KD, Koefoed K, Vestergaard ML, Veland IR, Henriques de Jesus MP, Pedersen LB, Benmerah A, Andersen CY, Larsen LA, et al. TGF-β signaling is associated with endocytosis at the pocket region of the primary cilium. Cell Rep 2013; 3:1806 - 14; http://dx.doi.org/10.1016/j.celrep.2013.05.020; PMID: 23746451
- Clement CA, Kristensen SG, Møllgård K, Pazour GJ, Yoder BK, Larsen LA, Christensen ST. The primary cilium coordinates early cardiogenesis and hedgehog signaling in cardiomyocyte differentiation. J Cell Sci 2009; 122:3070 - 82; http://dx.doi.org/10.1242/jcs.049676; PMID: 19654211
- Gerhardt C, Lier JM, Kuschel S, Rüther U. The ciliary protein Ftm is required for ventricular wall and septal development. PLoS One 2013; 8:e57545; http://dx.doi.org/10.1371/journal.pone.0057545; PMID: 23469020
- Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol 2007; 69:377 - 400; http://dx.doi.org/10.1146/annurev.physiol.69.040705.141236; PMID: 17009929
- Pedersen LB, Schrøder JM, Satir P, Christensen ST. The ciliary cytoskeleton. Compr Physiol 2012; 2:779 - 803; PMID: 23728985
- Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol 2008; 85:23 - 61; http://dx.doi.org/10.1016/S0070-2153(08)00802-8; PMID: 19147001
- Ishikawa H, Marshall WF. Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol 2011; 12:222 - 34; http://dx.doi.org/10.1038/nrm3085; PMID: 21427764
- Soetedjo L, Glover DA, Jin H. Targeting of vasoactive intestinal peptide receptor 2, VPAC2, a secretin family G-protein coupled receptor, to primary cilia. Biol Open 2013; 2:686 - 94; http://dx.doi.org/10.1242/bio.20134747; PMID: 23862016
- Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci U S A 2008; 105:4242 - 6; http://dx.doi.org/10.1073/pnas.0711027105; PMID: 18334641
- Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 2010; 11:331 - 44; http://dx.doi.org/10.1038/nrg2774; PMID: 20395968
- Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci 2010; 123:499 - 503; http://dx.doi.org/10.1242/jcs.050377; PMID: 20144997
- Ezratty EJ, Stokes N, Chai S, Shah AS, Williams SE, Fuchs E. A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell 2011; 145:1129 - 41; http://dx.doi.org/10.1016/j.cell.2011.05.030; PMID: 21703454
- Lienkamp S, Ganner A, Walz G. Inversin, Wnt signaling and primary cilia. Differentiation 2012; 83:S49 - 55; http://dx.doi.org/10.1016/j.diff.2011.11.012; PMID: 22206729
- Seeger-Nukpezah T, Liebau MC, Höpker K, Lamkemeyer T, Benzing T, Golemis EA, Schermer B. The centrosomal kinase Plk1 localizes to the transition zone of primary cilia and induces phosphorylation of nephrocystin-1. PLoS One 2012; 7:e38838; http://dx.doi.org/10.1371/journal.pone.0038838; PMID: 22701722
- Christensen ST, Clement CA, Satir P, Pedersen LB. Primary cilia and coordination of receptor tyrosine kinase (RTK) signalling. J Pathol 2012; 226:172 - 84; http://dx.doi.org/10.1002/path.3004; PMID: 21956154
- Oh EC, Katsanis N. Context-dependent regulation of Wnt signaling through the primary cilium. J Am Soc Nephrol 2013; 24:10 - 8; http://dx.doi.org/10.1681/ASN.2012050526; PMID: 23123400
- Praetorius HA, Leipziger J. Primary cilium-dependent sensing of urinary flow and paracrine purinergic signaling. Semin Cell Dev Biol 2013; 24:3 - 10; http://dx.doi.org/10.1016/j.semcdb.2012.10.003; PMID: 23085624
- Jenkins PM, McEwen DP, Martens JR. Olfactory cilia: linking sensory cilia function and human disease. Chem Senses 2009; 34:451 - 64; http://dx.doi.org/10.1093/chemse/bjp020; PMID: 19406873
- Roepman R, Wolfrum U. Protein networks and complexes in photoreceptor cilia. Subcell Biochem 2007; 43:209 - 35; http://dx.doi.org/10.1007/978-1-4020-5943-8_10; PMID: 17953396
- Hirokawa N, Tanaka Y, Okada Y. Cilia, KIF3 molecular motor and nodal flow. Curr Opin Cell Biol 2012; 24:31 - 9; http://dx.doi.org/10.1016/j.ceb.2012.01.002; PMID: 22285930
- Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell 2007; 12:767 - 78; http://dx.doi.org/10.1016/j.devcel.2007.03.004; PMID: 17488627
- Feistel K, Blum M. Three types of cilia including a novel 9+4 axoneme on the notochordal plate of the rabbit embryo. Dev Dyn 2006; 235:3348 - 58; http://dx.doi.org/10.1002/dvdy.20986; PMID: 17061268
- Gao C, Wang G, Amack JD, Mitchell DR. Oda16/Wdr69 is essential for axonemal dynein assembly and ciliary motility during zebrafish embryogenesis. Dev Dyn 2010; 239:2190 - 7; http://dx.doi.org/10.1002/dvdy.22355; PMID: 20568242
- Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol 2007; 23:345 - 73; http://dx.doi.org/10.1146/annurev.cellbio.23.090506.123249; PMID: 17506691
- Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med 2011; 364:1533 - 43; http://dx.doi.org/10.1056/NEJMra1010172; PMID: 21506742
- Waters AM, Beales PL. Ciliopathies: an expanding disease spectrum. Pediatr Nephrol 2011; 26:1039 - 56; http://dx.doi.org/10.1007/s00467-010-1731-7; PMID: 21210154
- Ibañez-Tallon I, Heintz N, Omran H. To beat or not to beat: roles of cilia in development and disease. Hum Mol Genet 2003; 12:R27 - 35; http://dx.doi.org/10.1093/hmg/ddg061; PMID: 12668594
- Digilio MC, Marino B, Ammirati A, Borzaga U, Giannotti A, Dallapiccola B. Cardiac malformations in patients with oral-facial-skeletal syndromes: clinical similarities with heterotaxia. Am J Med Genet 1999; 84:350 - 6; http://dx.doi.org/10.1002/(SICI)1096-8628(19990604)84:4<350::AID-AJMG8>3.0.CO;2-E; PMID: 10340650
- Elmali M, Ozmen Z, Ceyhun M, Tokatlioğlu O, Incesu L, Diren B. Joubert syndrome with atrial septal defect and persistent left superior vena cava. Diagn Interv Radiol 2007; 13:94 - 6; PMID: 17562515
- Hills CB, Kochilas L, Schimmenti LA, Moller JH. Ellis-van Creveld syndrome and congenital heart defects: presentation of an additional 32 cases. Pediatr Cardiol 2011; 32:977 - 82; http://dx.doi.org/10.1007/s00246-011-0006-9; PMID: 21533779
- Slavotinek AM, Biesecker LG. Phenotypic overlap of McKusick-Kaufman syndrome with bardet-biedl syndrome: a literature review. Am J Med Genet 2000; 95:208 - 15; http://dx.doi.org/10.1002/1096-8628(20001127)95:3<208::AID-AJMG5>3.0.CO;2-J; PMID: 11102925
- Sund KL, Roelker S, Ramachandran V, Durbin L, Benson DW. Analysis of Ellis van Creveld syndrome gene products: implications for cardiovascular development and disease. Hum Mol Genet 2009; 18:1813 - 24; http://dx.doi.org/10.1093/hmg/ddp098; PMID: 19251731
- Williams CL, Li C, Kida K, Inglis PN, Mohan S, Semenec L, Bialas NJ, Stupay RM, Chen N, Blacque OE, et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol 2011; 192:1023 - 41; http://dx.doi.org/10.1083/jcb.201012116; PMID: 21422230
- Huang L, Szymanska K, Jensen VL, Janecke AR, Innes AM, Davis EE, Frosk P, Li C, Willer JR, Chodirker BN, et al. TMEM237 is mutated in individuals with a Joubert syndrome related disorder and expands the role of the TMEM family at the ciliary transition zone. Am J Hum Genet 2011; 89:713 - 30; http://dx.doi.org/10.1016/j.ajhg.2011.11.005; PMID: 22152675
- Patzke S, Redick S, Warsame A, Murga-Zamalloa CA, Khanna H, Doxsey S, Stokke T. CSPP is a ciliary protein interacting with Nephrocystin 8 and required for cilia formation. Mol Biol Cell 2010; 21:2555 - 67; http://dx.doi.org/10.1091/mbc.E09-06-0503; PMID: 20519441
- Garcia-Gonzalo FR, Corbit KC, Sirerol-Piquer MS, Ramaswami G, Otto EA, Noriega TR, Seol AD, Robinson JF, Bennett CL, Josifova DJ, et al. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet 2011; 43:776 - 84; http://dx.doi.org/10.1038/ng.891; PMID: 21725307
- Sang L, Miller JJ, Corbit KC, Giles RH, Brauer MJ, Otto EA, Baye LM, Wen X, Scales SJ, Kwong M, et al. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell 2011; 145:513 - 28; http://dx.doi.org/10.1016/j.cell.2011.04.019; PMID: 21565611
- Warburton-Pitt SR, Jauregui AR, Li C, Wang J, Leroux MR, Barr MM. Ciliogenesis in Caenorhabditis elegans requires genetic interactions between ciliary middle segment localized NPHP-2 (inversin) and transition zone-associated proteins. J Cell Sci 2012; 125:2592 - 603; http://dx.doi.org/10.1242/jcs.095539; PMID: 22393243
- Roepman R, Letteboer SJ, Arts HH, van Beersum SE, Lu X, Krieger E, Ferreira PA, Cremers FP. Interaction of nephrocystin-4 and RPGRIP1 is disrupted by nephronophthisis or Leber congenital amaurosis-associated mutations. Proc Natl Acad Sci U S A 2005; 102:18520 - 5; http://dx.doi.org/10.1073/pnas.0505774102; PMID: 16339905
- Szymanska K, Johnson CA. The transition zone: an essential functional compartment of cilia. Cilia 2012; 1:10; http://dx.doi.org/10.1186/2046-2530-1-10; PMID: 23352055
- Delous M, Baala L, Salomon R, Laclef C, Vierkotten J, Tory K, Golzio C, Lacoste T, Besse L, Ozilou C, et al. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet 2007; 39:875 - 81; http://dx.doi.org/10.1038/ng2039; PMID: 17558409
- Craige B, Tsao CC, Diener DR, Hou Y, Lechtreck KF, Rosenbaum JL, Witman GB. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J Cell Biol 2010; 190:927 - 40; http://dx.doi.org/10.1083/jcb.201006105; PMID: 20819941
- Mochizuki T, Saijoh Y, Tsuchiya K, Shirayoshi Y, Takai S, Taya C, Yonekawa H, Yamada K, Nihei H, Nakatsuji N, et al. Cloning of inv, a gene that controls left/right asymmetry and kidney development. Nature 1998; 395:177 - 81; http://dx.doi.org/10.1038/26006; PMID: 9744276
- Shiba D, Manning DK, Koga H, Beier DR, Yokoyama T. Inv acts as a molecular anchor for Nphp3 and Nek8 in the proximal segment of primary cilia. Cytoskeleton (Hoboken) 2010; 67:112 - 9; PMID: 20169535
- Shiba D, Yamaoka Y, Hagiwara H, Takamatsu T, Hamada H, Yokoyama T. Localization of Inv in a distinctive intraciliary compartment requires the C-terminal ninein-homolog-containing region. J Cell Sci 2009; 122:44 - 54; http://dx.doi.org/10.1242/jcs.037408; PMID: 19050042
- Fukui H, Shiba D, Asakawa K, Kawakami K, Yokoyama T. The ciliary protein Nek8/Nphp9 acts downstream of Inv/Nphp2 during pronephros morphogenesis and left-right establishment in zebrafish. FEBS Lett 2012; 586:2273 - 9; http://dx.doi.org/10.1016/j.febslet.2012.05.064; PMID: 22687244
- Hoff S, Halbritter J, Epting D, Frank V, Nguyen TM, van Reeuwijk J, Boehlke C, Schell C, Yasunaga T, Helmstädter M, et al. ANKS6 is a central component of a nephronophthisis module linking NEK8 to INVS and NPHP3. Nat Genet 2013; 45:951 - 6; http://dx.doi.org/10.1038/ng.2681; PMID: 23793029
- Okada Y, Nonaka S, Tanaka Y, Saijoh Y, Hamada H, Hirokawa N. Abnormal nodal flow precedes situs inversus in iv and inv mice. Mol Cell 1999; 4:459 - 68; http://dx.doi.org/10.1016/S1097-2765(00)80197-5; PMID: 10549278
- Otto EA, Schermer B, Obara T, O’Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet 2003; 34:413 - 20; http://dx.doi.org/10.1038/ng1217; PMID: 12872123
- Watanabe D, Saijoh Y, Nonaka S, Sasaki G, Ikawa Y, Yokoyama T, Hamada H. The left-right determinant Inversin is a component of node monocilia and other 9+0 cilia. Development 2003; 130:1725 - 34; http://dx.doi.org/10.1242/dev.00407; PMID: 12642479
- Tory K, Rousset-Rouvière C, Gubler MC, Morinière V, Pawtowski A, Becker C, Guyot C, Gié S, Frishberg Y, Nivet H, et al. Mutations of NPHP2 and NPHP3 in infantile nephronophthisis. Kidney Int 2009; 75:839 - 47; http://dx.doi.org/10.1038/ki.2008.662; PMID: 19177160
- Donaldson MD, Warner AA, Trompeter RS, Haycock GB, Chantler C. Familial juvenile nephronophthisis, Jeune’s syndrome, and associated disorders. Arch Dis Child 1985; 60:426 - 34; http://dx.doi.org/10.1136/adc.60.5.426; PMID: 4015147
- Okada M, Sugimoto K, Shimada Y, Fujita S, Yanagida H, Yagi K, Takemura T. Association of INVS (NPHP2) mutation in an adolescent exhibiting nephronophthisis (NPH) and complete situs inversus. Clin Nephrol 2008; 69:135 - 41; http://dx.doi.org/10.5414/CNP69135; PMID: 18218308
- Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell 2001; 1:435 - 40; http://dx.doi.org/10.1016/S1534-5807(01)00040-5; PMID: 11702954
- Meilhac SM, Esner M, Kelly RG, Nicolas JF, Buckingham ME. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev Cell 2004; 6:685 - 98; http://dx.doi.org/10.1016/S1534-5807(04)00133-9; PMID: 15130493
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell 2003; 5:877 - 89; http://dx.doi.org/10.1016/S1534-5807(03)00363-0; PMID: 14667410
- Srivastava D. Making or breaking the heart: from lineage determination to morphogenesis. Cell 2006; 126:1037 - 48; http://dx.doi.org/10.1016/j.cell.2006.09.003; PMID: 16990131
- Christoffels VM, Habets PE, Franco D, Campione M, de Jong F, Lamers WH, Bao ZZ, Palmer S, Biben C, Harvey RP, et al. Chamber formation and morphogenesis in the developing mammalian heart. Dev Biol 2000; 223:266 - 78; http://dx.doi.org/10.1006/dbio.2000.9753; PMID: 10882515
- Moorman AF, Christoffels VM. Cardiac chamber formation: development, genes, and evolution. Physiol Rev 2003; 83:1223 - 67; PMID: 14506305
- Lin CJ, Lin CY, Chen CH, Zhou B, Chang CP. Partitioning the heart: mechanisms of cardiac septation and valve development. Development 2012; 139:3277 - 99; http://dx.doi.org/10.1242/dev.063495; PMID: 22912411
- Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to normal aorticopulmonary septation. Science 1983; 220:1059 - 61; http://dx.doi.org/10.1126/science.6844926; PMID: 6844926
- Anderson RH, Webb S, Brown NA, Lamers W, Moorman A. Development of the heart: (2) Septation of the atriums and ventricles. Heart 2003; 89:949 - 58; http://dx.doi.org/10.1136/heart.89.8.949; PMID: 12860885
- Abu-Issa R, Kirby ML. Heart field: from mesoderm to heart tube. Annu Rev Cell Dev Biol 2007; 23:45 - 68; http://dx.doi.org/10.1146/annurev.cellbio.23.090506.123331; PMID: 17456019
- Schilling TF, Concordet JP, Ingham PW. Regulation of left-right asymmetries in the zebrafish by Shh and BMP4. Dev Biol 1999; 210:277 - 87; http://dx.doi.org/10.1006/dbio.1999.9214; PMID: 10357891
- Meyers EN, Martin GR. Differences in left-right axis pathways in mouse and chick: functions of FGF8 and SHH. Science 1999; 285:403 - 6; http://dx.doi.org/10.1126/science.285.5426.403; PMID: 10411502
- Levin M, Johnson RL, Stern CD, Kuehn M, Tabin C. A molecular pathway determining left-right asymmetry in chick embryogenesis. Cell 1995; 82:803 - 14; http://dx.doi.org/10.1016/0092-8674(95)90477-8; PMID: 7671308
- Robbins DJ, Fei DL, Riobo NA. The Hedgehog signal transduction network. Sci Signal 2012; 5:re6; http://dx.doi.org/10.1126/scisignal.2002906; PMID: 23074268
- Washington Smoak I, Byrd NA, Abu-Issa R, Goddeeris MM, Anderson R, Morris J, Yamamura K, Klingensmith J, Meyers EN. Sonic hedgehog is required for cardiac outflow tract and neural crest cell development. Dev Biol 2005; 283:357 - 72; http://dx.doi.org/10.1016/j.ydbio.2005.04.029; PMID: 15936751
- Hoffmann AD, Peterson MA, Friedland-Little JM, Anderson SA, Moskowitz IP. sonic hedgehog is required in pulmonary endoderm for atrial septation. Development 2009; 136:1761 - 70; http://dx.doi.org/10.1242/dev.034157; PMID: 19369393
- Dyer LA, Kirby ML. Sonic hedgehog maintains proliferation in secondary heart field progenitors and is required for normal arterial pole formation. Dev Biol 2009; 330:305 - 17; http://dx.doi.org/10.1016/j.ydbio.2009.03.028; PMID: 19361493
- Lin L, Bu L, Cai CL, Zhang X, Evans S. Isl1 is upstream of sonic hedgehog in a pathway required for cardiac morphogenesis. Dev Biol 2006; 295:756 - 63; http://dx.doi.org/10.1016/j.ydbio.2006.03.053; PMID: 16687132
- Goddeeris MM, Rho S, Petiet A, Davenport CL, Johnson GA, Meyers EN, Klingensmith J. Intracardiac septation requires hedgehog-dependent cellular contributions from outside the heart. Development 2008; 135:1887 - 95; http://dx.doi.org/10.1242/dev.016147; PMID: 18441277
- Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res 2009; 19:71 - 88; http://dx.doi.org/10.1038/cr.2008.302; PMID: 19002158
- Kubiczkova L, Sedlarikova L, Hajek R, Sevcikova S. TGF-β - an excellent servant but a bad master. J Transl Med 2012; 10:183; http://dx.doi.org/10.1186/1479-5876-10-183; PMID: 22943793
- Mohapatra B, Casey B, Li H, Ho-Dawson T, Smith L, Fernbach SD, Molinari L, Niesh SR, Jefferies JL, Craigen WJ, et al. Identification and functional characterization of NODAL rare variants in heterotaxy and isolated cardiovascular malformations. Hum Mol Genet 2009; 18:861 - 71; PMID: 19064609
- Kosaki K, Bassi MT, Kosaki R, Lewin M, Belmont J, Schauer G, Casey B. Characterization and mutation analysis of human LEFTY A and LEFTY B, homologues of murine genes implicated in left-right axis development. Am J Hum Genet 1999; 64:712 - 21; http://dx.doi.org/10.1086/302289; PMID: 10053005
- Potts JD, Runyan RB. Epithelial-mesenchymal cell transformation in the embryonic heart can be mediated, in part, by transforming growth factor beta. Dev Biol 1989; 134:392 - 401; http://dx.doi.org/10.1016/0012-1606(89)90111-5; PMID: 2744239
- Potts JD, Dagle JM, Walder JA, Weeks DL, Runyan RB. Epithelial-mesenchymal transformation of embryonic cardiac endothelial cells is inhibited by a modified antisense oligodeoxynucleotide to transforming growth factor beta 3. Proc Natl Acad Sci U S A 1991; 88:1516 - 20; http://dx.doi.org/10.1073/pnas.88.4.1516; PMID: 1996351
- Boyer AS, Ayerinskas II, Vincent EB, McKinney LA, Weeks DL, Runyan RB. TGFbeta2 and TGFbeta3 have separate and sequential activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev Biol 1999; 208:530 - 45; http://dx.doi.org/10.1006/dbio.1999.9211; PMID: 10191064
- Bartram U, Molin DG, Wisse LJ, Mohamad A, Sanford LP, Doetschman T, Speer CP, Poelmann RE, Gittenberger-de Groot AC. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-beta(2)-knockout mice. Circulation 2001; 103:2745 - 52; http://dx.doi.org/10.1161/01.CIR.103.22.2745; PMID: 11390347
- Azhar M, Runyan RB, Gard C, Sanford LP, Miller ML, Andringa A, Pawlowski S, Rajan S, Doetschman T. Ligand-specific function of transforming growth factor beta in epithelial-mesenchymal transition in heart development. Dev Dyn 2009; 238:431 - 42; http://dx.doi.org/10.1002/dvdy.21854; PMID: 19161227
- Wang J, Nagy A, Larsson J, Dudas M, Sucov HM, Kaartinen V. Defective ALK5 signaling in the neural crest leads to increased postmigratory neural crest cell apoptosis and severe outflow tract defects. BMC Dev Biol 2006; 6:51; http://dx.doi.org/10.1186/1471-213X-6-51; PMID: 17078885
- Wurdak H, Ittner LM, Lang KS, Leveen P, Suter U, Fischer JA, Karlsson S, Born W, Sommer L. Inactivation of TGFbeta signaling in neural crest stem cells leads to multiple defects reminiscent of DiGeorge syndrome. Genes Dev 2005; 19:530 - 5; http://dx.doi.org/10.1101/gad.317405; PMID: 15741317
- Choudhary B, Ito Y, Makita T, Sasaki T, Chai Y, Sucov HM. Cardiovascular malformations with normal smooth muscle differentiation in neural crest-specific type II TGFbeta receptor (Tgfbr2) mutant mice. Dev Biol 2006; 289:420 - 9; http://dx.doi.org/10.1016/j.ydbio.2005.11.008; PMID: 16332365
- Galvin KM, Donovan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MA Jr., et al. A role for smad6 in development and homeostasis of the cardiovascular system. Nat Genet 2000; 24:171 - 4; http://dx.doi.org/10.1038/72835; PMID: 10655064
- Todorovic V, Frendewey D, Gutstein DE, Chen Y, Freyer L, Finnegan E, Liu F, Murphy A, Valenzuela D, Yancopoulos G, et al. Long form of latent TGF-beta binding protein 1 (Ltbp1L) is essential for cardiac outflow tract septation and remodeling. Development 2007; 134:3723 - 32; http://dx.doi.org/10.1242/dev.008599; PMID: 17804598
- Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development 2005; 132:5601 - 11; http://dx.doi.org/10.1242/dev.02156; PMID: 16314491
- Liu W, Selever J, Wang D, Lu MF, Moses KA, Schwartz RJ, Martin JF. Bmp4 signaling is required for outflow-tract septation and branchial-arch artery remodeling. Proc Natl Acad Sci U S A 2004; 101:4489 - 94; http://dx.doi.org/10.1073/pnas.0308466101; PMID: 15070745
- Tan HL, Glen E, Töpf A, Hall D, O’Sullivan JJ, Sneddon L, Wren C, Avery P, Lewis RJ, ten Dijke P, et al. Nonsynonymous variants in the SMAD6 gene predispose to congenital cardiovascular malformation. Hum Mutat 2012; 33:720 - 7; http://dx.doi.org/10.1002/humu.22030; PMID: 22275001
- Zaidi S, Choi M, Wakimoto H, Ma L, Jiang J, Overton JD, Romano-Adesman A, Bjornson RD, Breitbart RE, Brown KK, et al. De novo mutations in histone-modifying genes in congenital heart disease. Nature 2013; 498:220 - 3; http://dx.doi.org/10.1038/nature12141; PMID: 23665959
- Hirokawa N, Tanaka Y, Okada Y, Takeda S. Nodal flow and the generation of left-right asymmetry. Cell 2006; 125:33 - 45; http://dx.doi.org/10.1016/j.cell.2006.03.002; PMID: 16615888
- Shinohara K, Kawasumi A, Takamatsu A, Yoshiba S, Botilde Y, Motoyama N, Reith W, Durand B, Shiratori H, Hamada H. Two rotating cilia in the node cavity are sufficient to break left-right symmetry in the mouse embryo. Nat Commun 2012; 3:622; http://dx.doi.org/10.1038/ncomms1624; PMID: 22233632
- Nonaka S, Shiratori H, Saijoh Y, Hamada H. Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature 2002; 418:96 - 9; http://dx.doi.org/10.1038/nature00849; PMID: 12097914
- Supp DM, Brueckner M, Kuehn MR, Witte DP, Lowe LA, McGrath J, Corrales J, Potter SS. Targeted deletion of the ATP binding domain of left-right dynein confirms its role in specifying development of left-right asymmetries. Development 1999; 126:5495 - 504; PMID: 10556073
- Stevens J, Ermakov A, Braganca J, Hilton H, Underhill P, Bhattacharya S, Brown NA, Norris DP. Analysis of the asymmetrically expressed Ablim1 locus reveals existence of a lateral plate Nodal-independent left sided signal and an early, left-right independent role for nodal flow. BMC Dev Biol 2010; 10:54; http://dx.doi.org/10.1186/1471-213X-10-54; PMID: 20487527
- Shiratori H, Sakuma R, Watanabe M, Hashiguchi H, Mochida K, Sakai Y, Nishino J, Saijoh Y, Whitman M, Hamada H. Two-step regulation of left-right asymmetric expression of Pitx2: initiation by nodal signaling and maintenance by Nkx2. Mol Cell 2001; 7:137 - 49; http://dx.doi.org/10.1016/S1097-2765(01)00162-9; PMID: 11172719
- Shiratori H, Hamada H. The left-right axis in the mouse: from origin to morphology. Development 2006; 133:2095 - 104; http://dx.doi.org/10.1242/dev.02384; PMID: 16672339
- Tanaka Y, Okada Y, Hirokawa N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature 2005; 435:172 - 7; http://dx.doi.org/10.1038/nature03494; PMID: 15889083
- Yoshiba S, Shiratori H, Kuo IY, Kawasumi A, Shinohara K, Nonaka S, Asai Y, Sasaki G, Belo JA, Sasaki H, et al. Cilia at the node of mouse embryos sense fluid flow for left-right determination via Pkd2. Science 2012; 338:226 - 31; http://dx.doi.org/10.1126/science.1222538; PMID: 22983710
- Takao D, Nemoto T, Abe T, Kiyonari H, Kajiura-Kobayashi H, Shiratori H, Nonaka S. Asymmetric distribution of dynamic calcium signals in the node of mouse embryo during left-right axis formation. Dev Biol 2013; 376:23 - 30; http://dx.doi.org/10.1016/j.ydbio.2013.01.018; PMID: 23357539
- Boskovski MT, Yuan S, Pedersen NB, Goth CK, Makova S, Clausen H, Brueckner M, Khokha MK. The heterotaxy gene GALNT11 glycosylates Notch to orchestrate cilia type and laterality. Nature 2013; Forthcoming http://dx.doi.org/10.1038/nature12723; PMID: 24226769
- Norris D. Breaking the left-right axis: do nodal parcels pass a signal to the left?. Bioessays 2005; 27:991 - 4; http://dx.doi.org/10.1002/bies.20309; PMID: 16163731
- Takeda S, Yonekawa Y, Tanaka Y, Okada Y, Nonaka S, Hirokawa N. Left-right asymmetry and kinesin superfamily protein KIF3A: new insights in determination of laterality and mesoderm induction by kif3A-/- mice analysis. J Cell Biol 1999; 145:825 - 36; http://dx.doi.org/10.1083/jcb.145.4.825; PMID: 10330409
- Supp DM, Witte DP, Potter SS, Brueckner M. Mutation of an axonemal dynein affects left-right asymmetry in inversus viscerum mice. Nature 1997; 389:963 - 6; http://dx.doi.org/10.1038/40140; PMID: 9353118
- Sutherland MJ, Wang S, Quinn ME, Haaning A, Ware SM. Zic3 is required in the migrating primitive streak for node morphogenesis and left-right patterning. Hum Mol Genet 2013; 22:1913 - 23; http://dx.doi.org/10.1093/hmg/ddt001; PMID: 23303524
- Brody SL, Yan XH, Wuerffel MK, Song SK, Shapiro SD. Ciliogenesis and left-right axis defects in forkhead factor HFH-4-null mice. Am J Respir Cell Mol Biol 2000; 23:45 - 51; http://dx.doi.org/10.1165/ajrcmb.23.1.4070; PMID: 10873152
- Bonnafe E, Touka M, AitLounis A, Baas D, Barras E, Ucla C, Moreau A, Flamant F, Dubruille R, Couble P, et al. The transcription factor RFX3 directs nodal cilium development and left-right asymmetry specification. Mol Cell Biol 2004; 24:4417 - 27; http://dx.doi.org/10.1128/MCB.24.10.4417-4427.2004; PMID: 15121860
- Belmont JW, Mohapatra B, Towbin JA, Ware SM. Molecular genetics of heterotaxy syndromes. Curr Opin Cardiol 2004; 19:216 - 20; http://dx.doi.org/10.1097/00001573-200405000-00005; PMID: 15096953
- Nakhleh N, Francis R, Giese RA, Tian X, Li Y, Zariwala MA, Yagi H, Khalifa O, Kureshi S, Chatterjee B, et al. High prevalence of respiratory ciliary dysfunction in congenital heart disease patients with heterotaxy. Circulation 2012; 125:2232 - 42; http://dx.doi.org/10.1161/CIRCULATIONAHA.111.079780; PMID: 22499950
- Kennedy MP, Omran H, Leigh MW, Dell S, Morgan L, Molina PL, Robinson BV, Minnix SL, Olbrich H, Severin T, et al. Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation 2007; 115:2814 - 21; http://dx.doi.org/10.1161/CIRCULATIONAHA.106.649038; PMID: 17515466
- Silversides CK, Lionel AC, Costain G, Merico D, Migita O, Liu B, Yuen T, Rickaby J, Thiruvahindrapuram B, Marshall CR, et al. Rare copy number variations in adults with tetralogy of Fallot implicate novel risk gene pathways. PLoS Genet 2012; 8:e1002843; http://dx.doi.org/10.1371/journal.pgen.1002843; PMID: 22912587
- Tan SY, Rosenthal J, Zhao XQ, Francis RJ, Chatterjee B, Sabol SL, Linask KL, Bracero L, Connelly PS, Daniels MP, et al. Heterotaxy and complex structural heart defects in a mutant mouse model of primary ciliary dyskinesia. J Clin Invest 2007; 117:3742 - 52; PMID: 18037990
- Rash JE, Shay JW, Biesele JJ. Cilia in cardiac differentiation. J Ultrastruct Res 1969; 29:470 - 84; http://dx.doi.org/10.1016/S0022-5320(69)90067-7; PMID: 5365371
- Myklebust R, Engedal H, Saetersdal TS, Ulstein M. Primary 9 + 0 cilia in the embryonic and the adult human heart. Anat Embryol (Berl) 1977; 151:127 - 39; http://dx.doi.org/10.1007/BF00297476; PMID: 920963
- Bystrevskaya VB, Lichkun VV, Krushinsky AV, Smirnov VN. Centriole modification in human aortic endothelial cells. J Struct Biol 1992; 109:1 - 12; http://dx.doi.org/10.1016/1047-8477(92)90061-E; PMID: 1286005
- Iomini C, Tejada K, Mo W, Vaananen H, Piperno G. Primary cilia of human endothelial cells disassemble under laminar shear stress. J Cell Biol 2004; 164:811 - 7; http://dx.doi.org/10.1083/jcb.200312133; PMID: 15024030
- Slough J, Cooney L, Brueckner M. Monocilia in the embryonic mouse heart suggest a direct role for cilia in cardiac morphogenesis. Dev Dyn 2008; 237:2304 - 14; http://dx.doi.org/10.1002/dvdy.21669; PMID: 18729223
- Van der Heiden K, Groenendijk BC, Hierck BP, Hogers B, Koerten HK, Mommaas AM, Gittenberger-de Groot AC, Poelmann RE. Monocilia on chicken embryonic endocardium in low shear stress areas. Dev Dyn 2006; 235:19 - 28; http://dx.doi.org/10.1002/dvdy.20557; PMID: 16145662
- Groenendijk BC, Hierck BP, Gittenberger-De Groot AC, Poelmann RE. Development-related changes in the expression of shear stress responsive genes KLF-2, ET-1, and NOS-3 in the developing cardiovascular system of chicken embryos. Dev Dyn 2004; 230:57 - 68; http://dx.doi.org/10.1002/dvdy.20029; PMID: 15108309
- Egorova AD, van der Heiden K, Poelmann RE, Hierck BP. Primary cilia as biomechanical sensors in regulating endothelial function. Differentiation 2012; 83:S56 - 61; http://dx.doi.org/10.1016/j.diff.2011.11.007; PMID: 22169885
- Samsa LA, Yang B, Liu J. Embryonic cardiac chamber maturation: Trabeculation, conduction, and cardiomyocyte proliferation. Am J Med Genet C Semin Med Genet 2013; 163C:157 - 68; http://dx.doi.org/10.1002/ajmg.c.31366; PMID: 23720419
- Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 2008; 117:1161 - 71; http://dx.doi.org/10.1161/CIRCULATIONAHA.107.710111; PMID: 18285569
- Willaredt MA, Gorgas K, Gardner HA, Tucker KL. Multiple essential roles for primary cilia in heart development. Cilia 2012; 1:23; http://dx.doi.org/10.1186/2046-2530-1-23; PMID: 23351706
- Deretic D. Crosstalk of Arf and Rab GTPases en route to cilia. Small GTPases 2013; 4:70 - 7; http://dx.doi.org/10.4161/sgtp.24396; PMID: 23567335
- Hsiao YC, Tuz K, Ferland RJ. Trafficking in and to the primary cilium. Cilia 2012; 1:4; http://dx.doi.org/10.1186/2046-2530-1-4; PMID: 23351793
- Benmerah A. The ciliary pocket. Curr Opin Cell Biol 2013; 25:78 - 84; http://dx.doi.org/10.1016/j.ceb.2012.10.011; PMID: 23153502
- Kaplan OI, Doroquez DB, Cevik S, Bowie RV, Clarke L, Sanders AA, Kida K, Rappoport JZ, Sengupta P, Blacque OE. Endocytosis genes facilitate protein and membrane transport in C. elegans sensory cilia. Curr Biol 2012; 22:451 - 60; http://dx.doi.org/10.1016/j.cub.2012.01.060; PMID: 22342749
- Follit JA, San Agustin JT, Xu F, Jonassen JA, Samtani R, Lo CW, Pazour GJ. The Golgin GMAP210/TRIP11 anchors IFT20 to the Golgi complex. PLoS Genet 2008; 4:e1000315; http://dx.doi.org/10.1371/journal.pgen.1000315; PMID: 19112494
- Wu G, Markowitz GS, Li L, D’Agati VD, Factor SM, Geng L, Tibara S, Tuchman J, Cai Y, Park JH, et al. Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat Genet 2000; 24:75 - 8; http://dx.doi.org/10.1038/71724; PMID: 10615132
- Collin GB, Marshall JD, King BL, Milan G, Maffei P, Jagger DJ, Naggert JK. The Alström syndrome protein, ALMS1, interacts with α-actinin and components of the endosome recycling pathway. PLoS One 2012; 7:e37925; http://dx.doi.org/10.1371/journal.pone.0037925; PMID: 22693585
- Chen Y, Wu B, Xu L, Li H, Xia J, Yin W, Li Z, Shi D, Li S, Lin S, et al. A SNX10/V-ATPase pathway regulates ciliogenesis in vitro and in vivo. Cell Res 2012; 22:333 - 45; http://dx.doi.org/10.1038/cr.2011.134; PMID: 21844891
- Egorova AD, Khedoe PP, Goumans MJ, Yoder BK, Nauli SM, ten Dijke P, Poelmann RE, Hierck BP. Lack of primary cilia primes shear-induced endothelial-to-mesenchymal transition. Circ Res 2011; 108:1093 - 101; http://dx.doi.org/10.1161/CIRCRESAHA.110.231860; PMID: 21393577
- Reiter JF, Blacque OE, Leroux MR. The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep 2012; 13:608 - 18; http://dx.doi.org/10.1038/embor.2012.73; PMID: 22653444
- Nozawa YI, Lin C, Chuang PT. Hedgehog signaling from the primary cilium to the nucleus: an emerging picture of ciliary localization, trafficking and transduction. Curr Opin Genet Dev 2013; 23:429 - 37; http://dx.doi.org/10.1016/j.gde.2013.04.008; PMID: 23725801
- Keady BT, Samtani R, Tobita K, Tsuchya M, San Agustin JT, Follit JA, Jonassen JA, Subramanian R, Lo CW, Pazour GJ. IFT25 links the signal-dependent movement of Hedgehog components to intraflagellar transport. Dev Cell 2012; 22:940 - 51; http://dx.doi.org/10.1016/j.devcel.2012.04.009; PMID: 22595669
- Ruiz-Perez VL, Blair HJ, Rodriguez-Andres ME, Blanco MJ, Wilson A, Liu YN, Miles C, Peters H, Goodship JA. Evc is a positive mediator of Ihh-regulated bone growth that localises at the base of chondrocyte cilia. Development 2007; 134:2903 - 12; http://dx.doi.org/10.1242/dev.007542; PMID: 17660199
- Ruiz-Perez VL, Goodship JA. Ellis-van Creveld syndrome and Weyers acrodental dysostosis are caused by cilia-mediated diminished response to hedgehog ligands. Am J Med Genet C Semin Med Genet 2009; 151C:341 - 51; http://dx.doi.org/10.1002/ajmg.c.30226; PMID: 19876929
- Caparrós-Martín JA, Valencia M, Reytor E, Pacheco M, Fernandez M, Perez-Aytes A, Gean E, Lapunzina P, Peters H, Goodship JA, et al. The ciliary Evc/Evc2 complex interacts with Smo and controls Hedgehog pathway activity in chondrocytes by regulating Sufu/Gli3 dissociation and Gli3 trafficking in primary cilia. Hum Mol Genet 2013; 22:124 - 39; http://dx.doi.org/10.1093/hmg/dds409; PMID: 23026747
- Dorn KV, Hughes CE, Rohatgi R. A Smoothened-Evc2 complex transduces the Hedgehog signal at primary cilia. Dev Cell 2012; 23:823 - 35; http://dx.doi.org/10.1016/j.devcel.2012.07.004; PMID: 22981989
- Blair HJ, Tompson S, Liu YN, Campbell J, MacArthur K, Ponting CP, Ruiz-Perez VL, Goodship JA. Evc2 is a positive modulator of Hedgehog signalling that interacts with Evc at the cilia membrane and is also found in the nucleus. BMC Biol 2011; 9:14; http://dx.doi.org/10.1186/1741-7007-9-14; PMID: 21356043
- Besse L, Neti M, Anselme I, Gerhardt C, Rüther U, Laclef C, Schneider-Maunoury S. Primary cilia control telencephalic patterning and morphogenesis via Gli3 proteolytic processing. Development 2011; 138:2079 - 88; http://dx.doi.org/10.1242/dev.059808; PMID: 21490064
- Vierkotten J, Dildrop R, Peters T, Wang B, Rüther U. Ftm is a novel basal body protein of cilia involved in Shh signalling. Development 2007; 134:2569 - 77; http://dx.doi.org/10.1242/dev.003715; PMID: 17553904
- Liu L, Zhang M, Xia Z, Xu P, Chen L, Xu T. Caenorhabditis elegans ciliary protein NPHP-8, the homologue of human RPGRIP1L, is required for ciliogenesis and chemosensation. Biochem Biophys Res Commun 2011; 410:626 - 31; http://dx.doi.org/10.1016/j.bbrc.2011.06.041; PMID: 21689635
- Arts HH, Doherty D, van Beersum SE, Parisi MA, Letteboer SJ, Gorden NT, Peters TA, Märker T, Voesenek K, Kartono A, et al. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat Genet 2007; 39:882 - 8; http://dx.doi.org/10.1038/ng2069; PMID: 17558407
- Coene KL, Mans DA, Boldt K, Gloeckner CJ, van Reeuwijk J, Bolat E, Roosing S, Letteboer SJ, Peters TA, Cremers FP, et al. The ciliopathy-associated protein homologs RPGRIP1 and RPGRIP1L are linked to cilium integrity through interaction with Nek4 serine/threonine kinase. Hum Mol Genet 2011; 20:3592 - 605; http://dx.doi.org/10.1093/hmg/ddr280; PMID: 21685204
- Mahuzier A, Gaudé HM, Grampa V, Anselme I, Silbermann F, Leroux-Berger M, Delacour D, Ezan J, Montcouquiol M, Saunier S, et al. Dishevelled stabilization by the ciliopathy protein Rpgrip1l is essential for planar cell polarity. J Cell Biol 2012; 198:927 - 40; http://dx.doi.org/10.1083/jcb.201111009; PMID: 22927466
- Clement CA, Larsen LA, Christensen ST. Using nucleofection of siRNA constructs for knockdown of primary cilia in P19.CL6 cancer stem cell differentiation into cardiomyocytes. Methods Cell Biol 2009; 94:181 - 97; http://dx.doi.org/10.1016/S0091-679X(08)94009-7; PMID: 20362091
- Arthur HM, Bamforth SD. TGFβ signaling and congenital heart disease: Insights from mouse studies. Birth Defects Res A Clin Mol Teratol 2011; 91:423 - 34; http://dx.doi.org/10.1002/bdra.20794; PMID: 21538815
- Tang WB, Ling GH, Sun L, Liu FY. Smad anchor for receptor activation (SARA) in TGF-beta signaling. Front Biosci (Elite Ed) 2010; 2:857 - 60; http://dx.doi.org/10.2741/E147; PMID: 20515759
- Huang F, Chen YG. Regulation of TGF-β receptor activity. Cell Biosci 2012; 2:9; http://dx.doi.org/10.1186/2045-3701-2-9; PMID: 22420375
- Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev 2004; 15:197 - 204; http://dx.doi.org/10.1016/j.cytogfr.2004.03.007; PMID: 15207811
- Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol 2005; 15:1861 - 6; http://dx.doi.org/10.1016/j.cub.2005.09.012; PMID: 16243034
- Egeberg DL, Lethan M, Manguso R, Schneider L, Awan A, Jørgensen TS, Byskov AG, Pedersen LB, Christensen ST. Primary cilia and aberrant cell signaling in epithelial ovarian cancer. Cilia 2012; 1:15; http://dx.doi.org/10.1186/2046-2530-1-15; PMID: 23351307
- Anderson CT, Stearns T. Centriole age underlies asynchronous primary cilium growth in mammalian cells. Curr Biol 2009; 19:1498 - 502; http://dx.doi.org/10.1016/j.cub.2009.07.034; PMID: 19682908
- Jacoby M, Cox JJ, Gayral S, Hampshire DJ, Ayub M, Blockmans M, Pernot E, Kisseleva MV, Compère P, Schiffmann SN, et al. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat Genet 2009; 41:1027 - 31; http://dx.doi.org/10.1038/ng.427; PMID: 19668215
- Danilov AI, Gomes-Leal W, Ahlenius H, Kokaia Z, Carlemalm E, Lindvall O. Ultrastructural and antigenic properties of neural stem cells and their progeny in adult rat subventricular zone. Glia 2009; 57:136 - 52; http://dx.doi.org/10.1002/glia.20741; PMID: 18709646
- Awan A, Oliveri RS, Jensen PL, Christensen ST, Andersen CY. Immunoflourescence and mRNA analysis of human embryonic stem cells (hESCs) grown under feeder-free conditions. Methods Mol Biol 2010; 584:195 - 210; PMID: 19907979
- Schneider L, Cammer M, Lehman J, Nielsen SK, Guerra CF, Veland IR, Stock C, Hoffmann EK, Yoder BK, Schwab A, et al. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiol Biochem 2010; 25:279 - 92; http://dx.doi.org/10.1159/000276562; PMID: 20110689
- Clement DL, Mally S, Stock C, Lethan M, Satir P, Schwab A, Pedersen SF, Christensen ST. PDGFRα signaling in the primary cilium regulates NHE1-dependent fibroblast migration via coordinated differential activity of MEK1/2-ERK1/2-p90RSK and AKT signaling pathways. J Cell Sci 2013; 126:953 - 65; http://dx.doi.org/10.1242/jcs.116426; PMID: 23264740
- Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 1999; 79:1283 - 316; PMID: 10508235
- Bax NA, Lie-Venema H, Vicente-Steijn R, Bleyl SB, Van Den Akker NM, Maas S, Poelmann RE, Gittenberger-de Groot AC. Platelet-derived growth factor is involved in the differentiation of second heart field-derived cardiac structures in chicken embryos. Dev Dyn 2009; 238:2658 - 69; http://dx.doi.org/10.1002/dvdy.22073; PMID: 19705434
- Kang J, Gu Y, Li P, Johnson BL, Sucov HM, Thomas PS. PDGF-A as an epicardial mitogen during heart development. Dev Dyn 2008; 237:692 - 701; http://dx.doi.org/10.1002/dvdy.21469; PMID: 18297729
- Van Den Akker NM, Lie-Venema H, Maas S, Eralp I, DeRuiter MC, Poelmann RE, Gittenberger-De Groot AC. Platelet-derived growth factors in the developing avian heart and maturating coronary vasculature. Dev Dyn 2005; 233:1579 - 88; http://dx.doi.org/10.1002/dvdy.20476; PMID: 15973731
- Richarte AM, Mead HB, Tallquist MD. Cooperation between the PDGF receptors in cardiac neural crest cell migration. Dev Biol 2007; 306:785 - 96; http://dx.doi.org/10.1016/j.ydbio.2007.04.023; PMID: 17499702
- Smith CL, Baek ST, Sung CY, Tallquist MD. Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circ Res 2011; 108:e15 - 26; http://dx.doi.org/10.1161/CIRCRESAHA.110.235531; PMID: 21512159
- Price RL, Haley ST, Bullard TA, Goldsmith EC, Simpson DG, Thielen TE, Yost MJ, Terracio L. Effects of platelet-derived growth factor-AA and -BB on embryonic cardiac development. Anat Rec A Discov Mol Cell Evol Biol 2003; 272:424 - 33; PMID: 12704700
- Bax NA, Bleyl SB, Gallini R, Wisse LJ, Hunter J, Van Oorschot AA, Mahtab EA, Lie-Venema H, Goumans MJ, Betsholtz C, et al. Cardiac malformations in Pdgfralpha mutant embryos are associated with increased expression of WT1 and Nkx2.5 in the second heart field. Dev Dyn 2010; 239:2307 - 17; http://dx.doi.org/10.1002/dvdy.22363; PMID: 20658695
- Tallquist MD, Soriano P. Cell autonomous requirement for PDGFRalpha in populations of cranial and cardiac neural crest cells. Development 2003; 130:507 - 18; http://dx.doi.org/10.1242/dev.00241; PMID: 12490557
- Schatteman GC, Motley ST, Effmann EL, Bowen-Pope DF. Platelet-derived growth factor receptor alpha subunit deleted Patch mouse exhibits severe cardiovascular dysmorphogenesis. Teratology 1995; 51:351 - 66; http://dx.doi.org/10.1002/tera.1420510602; PMID: 7502236
- Henderson DJ, Phillips HM, Chaudhry B. Vang-like 2 and noncanonical Wnt signaling in outflow tract development. Trends Cardiovasc Med 2006; 16:38 - 45; http://dx.doi.org/10.1016/j.tcm.2005.11.005; PMID: 16473760
- Moeller H, Jenny A, Schaeffer HJ, Schwarz-Romond T, Mlodzik M, Hammerschmidt M, Birchmeier W. Diversin regulates heart formation and gastrulation movements in development. Proc Natl Acad Sci U S A 2006; 103:15900 - 5; http://dx.doi.org/10.1073/pnas.0603808103; PMID: 17032765
- Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, Greer J, Kardos N, Wang J, Sussman DJ, et al. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet 2008; 4:e1000259; http://dx.doi.org/10.1371/journal.pgen.1000259; PMID: 19008950
- Gessert S, Kühl M. The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circ Res 2010; 107:186 - 99; http://dx.doi.org/10.1161/CIRCRESAHA.110.221531; PMID: 20651295
- Dawson K, Aflaki M, Nattel S. Role of the Wnt-Frizzled system in cardiac pathophysiology: a rapidly developing, poorly understood area with enormous potential. J Physiol 2013; 591:1409 - 32; http://dx.doi.org/10.1113/jphysiol.2012.235382; PMID: 23207593
- High FA, Epstein JA. The multifaceted role of Notch in cardiac development and disease. Nat Rev Genet 2008; 9:49 - 61; http://dx.doi.org/10.1038/nrg2279; PMID: 18071321
- Kestler HA, Kühl M. From individual Wnt pathways towards a Wnt signalling network. Philos Trans R Soc Lond B Biol Sci 2008; 363:1333 - 47; http://dx.doi.org/10.1098/rstb.2007.2251; PMID: 18192173
- Gao C, Chen YG. Dishevelled: The hub of Wnt signaling. Cell Signal 2010; 22:717 - 27; http://dx.doi.org/10.1016/j.cellsig.2009.11.021; PMID: 20006983
- Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev 2011; 25:201 - 13; http://dx.doi.org/10.1101/gad.2008011; PMID: 21289065
- Zilber Y, Babayeva S, Seo JH, Liu JJ, Mootin S, Torban E. The PCP effector Fuzzy controls cilial assembly and signaling by recruiting Rab8 and Dishevelled to the primary cilium. Mol Biol Cell 2013; 24:555 - 65; http://dx.doi.org/10.1091/mbc.E12-06-0437; PMID: 23303251
- Veland IR, Awan A, Pedersen LB, Yoder BK, Christensen ST. Primary cilia and signaling pathways in mammalian development, health and disease. Nephron Physiol 2009; 111:39 - 53; http://dx.doi.org/10.1159/000208212; PMID: 19276629
- Veland IR, Montjean R, Eley L, Pedersen LB, Schwab A, Goodship J, Kristiansen K, Pedersen SF, Saunier S, Christensen ST. Inversin/Nephrocystin-2 is required for fibroblast polarity and directional cell migration. PLoS One 2013; 8:e60193; http://dx.doi.org/10.1371/journal.pone.0060193; PMID: 23593172
- Jones C, Chen P. Primary cilia in planar cell polarity regulation of the inner ear. Curr Top Dev Biol 2008; 85:197 - 224; http://dx.doi.org/10.1016/S0070-2153(08)00808-9; PMID: 19147007
- Ocbina PJ, Tuson M, Anderson KV. Primary cilia are not required for normal canonical Wnt signaling in the mouse embryo. PLoS One 2009; 4:e6839; http://dx.doi.org/10.1371/journal.pone.0006839; PMID: 19718259
- Borovina A, Ciruna B. IFT88 plays a cilia- and PCP-independent role in controlling oriented cell divisions during vertebrate embryonic development. Cell Rep 2013; 5:37 - 43; http://dx.doi.org/10.1016/j.celrep.2013.08.043; PMID: 24095732
- Phillips HM, Rhee HJ, Murdoch JN, Hildreth V, Peat JD, Anderson RH, Copp AJ, Chaudhry B, Henderson DJ. Disruption of planar cell polarity signaling results in congenital heart defects and cardiomyopathy attributable to early cardiomyocyte disorganization. Circ Res 2007; 101:137 - 45; http://dx.doi.org/10.1161/CIRCRESAHA.106.142406; PMID: 17556662
- Davis EE, Katsanis N. Cell polarization defects in early heart development. Circ Res 2007; 101:122 - 4; http://dx.doi.org/10.1161/CIRCRESAHA.107.157446; PMID: 17641235
- Jonassen JA, San Agustin J, Follit JA, Pazour GJ. Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J Cell Biol 2008; 183:377 - 84; http://dx.doi.org/10.1083/jcb.200808137; PMID: 18981227
- Borgal L, Habbig S, Hatzold J, Liebau MC, Dafinger C, Sacarea I, Hammerschmidt M, Benzing T, Schermer B. The ciliary protein nephrocystin-4 translocates the canonical Wnt regulator Jade-1 to the nucleus to negatively regulate β-catenin signaling. J Biol Chem 2012; 287:25370 - 80; http://dx.doi.org/10.1074/jbc.M112.385658; PMID: 22654112
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Krönig C, Schermer B, Benzing T, Cabello OA, Jenny A, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet 2005; 37:537 - 43; http://dx.doi.org/10.1038/ng1552; PMID: 15852005
- Bellavia S, Dahan K, Terryn S, Cosyns JP, Devuyst O, Pirson Y. A homozygous mutation in INVS causing juvenile nephronophthisis with abnormal reactivity of the Wnt/beta-catenin pathway. Nephrol Dial Transplant 2010; 25:4097 - 102; http://dx.doi.org/10.1093/ndt/gfq519; PMID: 20798123
- de la Pompa JL, Epstein JA. Coordinating tissue interactions: Notch signaling in cardiac development and disease. Dev Cell 2012; 22:244 - 54; http://dx.doi.org/10.1016/j.devcel.2012.01.014; PMID: 22340493
- Timmerman LA, Grego-Bessa J, Raya A, Bertrán E, Pérez-Pomares JM, Díez J, Aranda S, Palomo S, McCormick F, Izpisúa-Belmonte JC, et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev 2004; 18:99 - 115; http://dx.doi.org/10.1101/gad.276304; PMID: 14701881
- Lage K, Møllgård K, Greenway S, Wakimoto H, Gorham JM, Workman CT, Bendsen E, Hansen NT, Rigina O, Roque FS, et al. Dissecting spatio-temporal protein networks driving human heart development and related disorders. Mol Syst Biol 2010; 6:381; http://dx.doi.org/10.1038/msb.2010.36; PMID: 20571530
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature 2005; 437:1018 - 21; http://dx.doi.org/10.1038/nature04117; PMID: 16136078
- Wong SY, Reiter JF. The primary cilium at the crossroads of mammalian hedgehog signaling. Curr Top Dev Biol 2008; 85:225 - 60; http://dx.doi.org/10.1016/S0070-2153(08)00809-0; PMID: 19147008
- Ma R, Li WP, Rundle D, Kong J, Akbarali HI, Tsiokas L. PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol Cell Biol 2005; 25:8285 - 98; http://dx.doi.org/10.1128/MCB.25.18.8285-8298.2005; PMID: 16135816
- Wu J, Du H, Wang X, Mei C, Sieck GC, Qian Q. Characterization of primary cilia in human airway smooth muscle cells. Chest 2009; 136:561 - 70; http://dx.doi.org/10.1378/chest.08-1549; PMID: 19318679
- Marshall JD, Bronson RT, Collin GB, Nordstrom AD, Maffei P, Paisey RB, Carey C, Macdermott S, Russell-Eggitt I, Shea SE, et al. New Alström syndrome phenotypes based on the evaluation of 182 cases. Arch Intern Med 2005; 165:675 - 83; http://dx.doi.org/10.1001/archinte.165.6.675; PMID: 15795345
- Hjortshøj TD, Grønskov K, Philp AR, Nishimura DY, Riise R, Sheffield VC, Rosenberg T, Brøndum-Nielsen K. Bardet-Biedl syndrome in Denmark--report of 13 novel sequence variations in six genes. Hum Mutat 2010; 31:429 - 36; http://dx.doi.org/10.1002/humu.21204; PMID: 20120035
- Moore SJ, Green JS, Fan Y, Bhogal AK, Dicks E, Fernandez BA, Stefanelli M, Murphy C, Cramer BC, Dean JC, et al. Clinical and genetic epidemiology of Bardet-Biedl syndrome in Newfoundland: a 22-year prospective, population-based, cohort study. Am J Med Genet A 2005; 132:352 - 60; http://dx.doi.org/10.1002/ajmg.a.30406; PMID: 15637713
- Beales PL, Elcioglu N, Woolf AS, Parker D, Flinter FA. New criteria for improved diagnosis of Bardet-Biedl syndrome: results of a population survey. J Med Genet 1999; 36:437 - 46; PMID: 10874630
- Harper T, Fordham LA, Wolfe HM. The fetal dandy walker complex: associated anomalies, perinatal outcome and postnatal imaging. Fetal Diagn Ther 2007; 22:277 - 81; http://dx.doi.org/10.1159/000100790; PMID: 17369695
- O’Connor MJ, Rider NL, Thomas Collins R, Hanna BD, Holmes Morton D, Strauss KA. Contemporary management of congenital malformations of the heart in infants with Ellis - van Creveld syndrome: a report of nine cases. Cardiol Young 2011; 21:145 - 52; http://dx.doi.org/10.1017/S1047951110001587; PMID: 21070693
- Karp N, Grosse-Wortmann L, Bowdin S. Severe aortic stenosis, bicuspid aortic valve and atrial septal defect in a child with Joubert Syndrome and Related Disorders (JSRD) - a case report and review of congenital heart defects reported in the human ciliopathies. Eur J Med Genet 2012; 55:605 - 10; http://dx.doi.org/10.1016/j.ejmg.2012.07.010; PMID: 22910529
- Salonen R. The Meckel syndrome: clinicopathological findings in 67 patients. Am J Med Genet 1984; 18:671 - 89; http://dx.doi.org/10.1002/ajmg.1320180414; PMID: 6486167
- Chaki M, Hoefele J, Allen SJ, Ramaswami G, Janssen S, Bergmann C, Heckenlively JR, Otto EA, Hildebrandt F. Genotype-phenotype correlation in 440 patients with NPHP-related ciliopathies. Kidney Int 2011; 80:1239 - 45; http://dx.doi.org/10.1038/ki.2011.284; PMID: 21866095
- Lin AE, Traum AZ, Sahai I, Keppler-Noreuil K, Kukolich MK, Adam MP, Westra SJ, Arts HH. Sensenbrenner syndrome (Cranioectodermal dysplasia): clinical and molecular analyses of 39 patients including two new patients. Am J Med Genet A 2013; 161A:2762 - 76; http://dx.doi.org/10.1002/ajmg.a.36265; PMID: 24123776
