Abstract
There is growing evidence that some members of cytochrome P450 enzymes contribute to regulation of normal prenatal development. CYP epoxygenases (CYP2C and CYP2J subfamilies) convert arachidonic acid into four regioisomeric epoxyeicosatrienoic acids (EETs), biologically active molecules involved in mitogenesis and cell signaling. Almost nothing is known about localization of their expression in tissues during human prenatal development. The spatio-temporal expression pattern of CYP2C8, CYP2C9, CYP2C19 and CYP2J2 in human embryonic/fetal intestines, liver, and kidney was investigated by immunohistochemical method. CYP epoxygenases are expressed already in early stages of development in these embryonic/fetal tissues (as early as 7th week of IUD in the intestines, 5th week of IUD in the liver, and 6th week of IUD in the kidney). In kidney, CYP epoxygenases are expressed in the metanephrogenic blastema (but not in the uninduced mesenchyme) and in the tubular system. In the intestines, diverse CYP epoxygenases distribution along crypt-villus axis could suggest role in cell differentiation. Moreover, we detected higher CYP2J2 level in these organs than in adult tissue samples.
Introduction
Cytochromes P450 (CYPs), especially CYP1, 2, 3, and 4 families, are well known as “drug metabolizers” responsible for the phase I metabolism of xenobiotics. Nevertheless, CYPs had been present millions of years before industrial created drugs and environmental pollution, and many of these genes are highly conserved across different species. Thus, it implies they have important endogenous functions. Moreover, there is some evidence to suggest that some members of CYPs are involved in the regulation of developmental processes.Citation1 The importance of CYPs during the prenatal development is supported by the fact that the homozygous deletion of NADPH-cytochrom P450 oxireductase, a primary (if not sole) electron donor for the CYPs enzymes, is lethal during organogenesis.Citation2
In this regard, the interesting group of CYP enzymes are CYP epoxygenases (CYP2C and CYP2J subfamilies). CYP2C subfamily metabolizes about 20% of clinically important drugs.Citation3 CYP2J2 also participates in the drug metabolism.Citation4 The endogenous function of these enzymes is a conversion of arachidonic acid to four regioisomeric epoxyeicosatrienoic acids (EETs), biologically active eicosanoids with many functions in organisms. They play a role in processes potentially relevant to the development, e.g., regulation of realizing of intracellular signaling molecules, protein kinase activity, cellular differentiation, and mitogenesis. Above that, EETs could be suggested as potential morphogens proposed to Local Source-Dispersed Sink model.Citation1 According to this model, a gradient of morphogen over a narrow embryonic field could be established by the synchronized action of cell producing morphogen (source) and cells degrading the morphogen (sink).Citation5 Cells in the field react to various concentration of morphogen with the specific developmental response. EETs have relatively short half-life in vivo. They are metabolized to less active dihydroxyeicosatrienoic acids (DHETs) by epoxide hydrolases and also incorporated to membrane phospoholipids.Citation6
CYP epoxygenases are expressed in various normal,Citation6,Citation7 and tumor tissues,Citation8,Citation9 as well as in developing tissues and organs.Citation3,Citation7,Citation10 The occurrence of CYP epoxygenases in tumors is responsible for inactivation of some anticancer drugs and thus they contribute to multidrug resistance in cancer patients. Moreover, EETs production inhibits apoptosis, promotes angiogenesis and increasing metastatic potential.Citation9,Citation11
The main organs involved in the absorption, distribution, metabolism, and elimination of xenobiotics are liver, intestines, and kidney. The development of these organs is summarized in . The expression pattern of CYP epoxygenases in these organs could provide not only information about the architectural development of these organs but also about development of protection mechanisms against xenobiotics.
Table 1. Brief summary of intestine, liver and kidney IUD events
Although several studies dealing with CYP epoxygenases expression in human embryonic/fetal tissues, to the best of our knowledge, this is the first complex spatio-temporal immunohistological analysis of CYP2C8, CYP2C9, CYP2C19, and CYP2J2 in human embryonic/fetal intestine, liver, and kidney in various stages of intrauterine development (IUD). Notwithstanding descriptive character of this study, it brings new and valuable information about human prenatal development.
Results
All tissue samples exhibited a cytoplasmic staining for all tested CYP epoxygenases (CYP2C8, CYP2C9, CYP2C19, and CYP2J2). CYP epoxygenases are proteins of the smooth endoplasmic reticulum. Because it is not possible to distinguish between cytosolic or endoplasmic reticulum-binded proteins by immunohistochemistry, cytoplasmic signal is in accordance with their expected localization.
Intestines
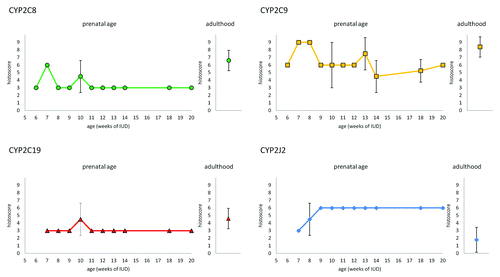
All tested CYP proteins were detected in enterocytes at all examined stages of IUD (7th – 20th week of IUD) as well as in an adults. The expression profiles (evaluated by the histoscore) of CYP2C8, CYP2C9, CYP2C19, and CYP2J2 in intestinal epithelium during prenatal period under study are shown in . Briefly, CYP2C8 expression in the cytoplasm of enterocytes increases from 7th to 16th week of IUD, when it reaches the highest level. The level of CYP2C9 in enterocytes also gradually increases but the highest level is reached in 14th week of IUD. As well as in the kidney and the liver, CYP2C19 expression was the weakest one from all examined CYPs. The expression of CYP2C19 reaches the peak of expression between 11th and 14th week of IUD. The expression of CYP2J2 remains at the same level in all the samples of various stages of IUD. CYP2C8 and CYP2C9 proteins showed stronger expression in the intervillous epithelium and then, in older embryos after crypts are developed (in 12th week of IUD), the positivity in the crypts was stronger than on the apex of villi. On the other hand, there is no difference in staining for CYP2C19 and CYP2J2 in both apices of villi and in crypts until 14th week (CYP2C19), and 16th week (CYP2J2) of IUD, respectively. After this period, crypts show loss of staining for CYP2C19 and CYP2J2 (). All studied CYPs also show weak expression in developing muscles layers and blood vessels. The mesenchyme was almost negative.
Figure 1. Expression of CYP2C8, CYP29, CYP2C19, and CYP2J2 in enterocytes of embryonic/fetal intestine during 7th-20th week of IUD (n = 29) and adult duodenal tissue samples (n = 5). Graphs show the average histoscore. The error bars represent standard deviation.
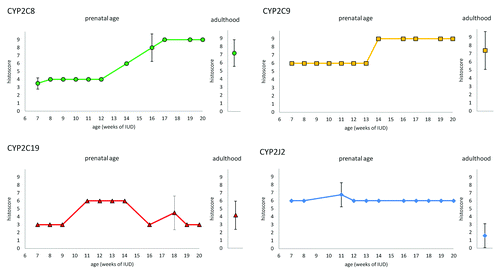
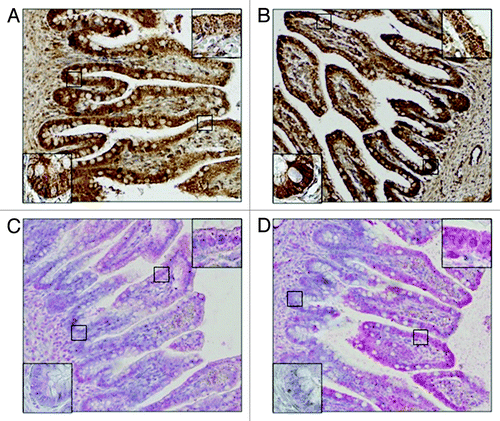
Figure 2. Differences between signal localization in fetal intestine in 16th week of IUD. CYP2C8 (A) and CYP2C9 (B) in enterocytes show stronger positivity in crypts than in apex of villi. On the other hand, CYP2C19 (C) and CYP2J2 (D) show stronger positivity in apex of villi, area of crypts is almost negative.
We detected CYP2C8, CYP2C9, and CYP2C19 expression in all tested duodenal tissue samples from adults. CYP2J2 expression was detected in 3 samples, 2 samples were negative. Generally in the adult duodenal tissue samples, the signal intensity in cytoplasm in apical parts of villi was equal to that in the crypts. Intensity of immunostaining varies between individuals. The average histoscore value (n = 5) is shown together with the embryonic/fetal expression profiles in . Interestingly, CYP2J2 expression was lower in the adult tissues than in the embryonic/fetal tissues.
Liver
The expression profiles (evaluated by histoscore) of CYP2C8, CYP2C9, CYP2C19, and CYP2J2 in hepatocytes during the 5th – 20th week of IUD are shown in . The positive reaction for CYP2C8, CYP2C9 and CYP2J2 is present in embryonic/fetal livers as soon as 5th week of IUD. Positivity of CYP2C19 appears one week later. The signal intensity of CYP2C8 increases in the 7th week of IUD and in later stages it remains unchanged. Similarly, CYP2C9 expression increases in the 6th week of IUD. CYP2J2 expression varies between weak (histoscore 3) and moderate (histoscore 6) staining intensity among individuals. The weak positivity of CYP epoxygenases were also found in some blood cells.
Figure 3. Expression of CYP2C8, CYP29, CYP2C19, and CYP2J2 in hepatocytes of embryonic/fetal liver during 5th-20th week of IUD (n = 23) and adult tissue samples (n = 5). Graphs show the average histoscore. The error bars represent standard deviation.
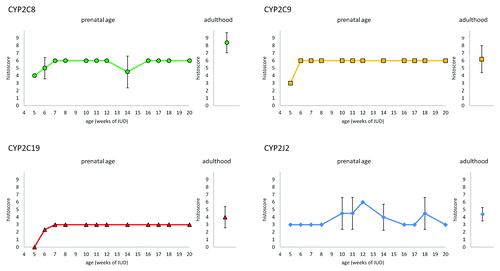
All the samples of adult liver were positive for all tested proteins. Generally, not all hepatocytes were positive, especially in samples stained for CYP2C19 and CYP2J2 the ratio of positive cells varied from 30% to 60%. As in case of duodenal tissue samples, the intensity of staining varied between individuals. Average histoscore value (n = 5) is shown together with the embryonic/fetal expression profiles in .
Kidney
We registered the positive immunoreaction of all four tested CYP epoxygenases in all examined embryonic/fetal tissue samples (6th–20th week of IUD) as well as in the adult organ, predominantly in the tubular system (). We evaluated the expression profiles for the proximal tubuli and other types of tubuli (loops of Henle, distal tubuli, and collecting ducts) separately. The expression profiles (evaluated by histoscore) of CYP2C8, CYP2C9, CYP2C19, and CYP2J2 during prenatal period are shown in (proximal tubuli) and (other types of tubuli). The staining of CYP2C8 in the cytoplasm of the proximal tubuli cells increases in 7th week of IUD and then remains unchanged. CYP2C8 expression in other types of tubuli showed weak staining except for the increase in 7th week of IUD. CYP2C9 protein was the predominant type of CYP epoxygenase in the tubular system. In the proximal tubuli, its expression from 7th week of IUD is higher than in adult tissue samples. Similarly to CYP2C8, the expression of CYP2C9 in other types of tubuli was medium except for the elevation in weeks 7 and 8. CYP2C19 expression was weak in all types of tubuli. CYP2J2 expression showed slight increase in the 10th week in whole tubular system. Its expression level between 10th–20th week of IUD is higher than in the adult tissue samples.
Figure 4. Embryonic kidney in 7th week of IUD. The proximal tubuli shows stronger staining than other type of tubuli. The metanephrogenic blastema shows weak positivity whereas uninduced mesenchyme is negative.
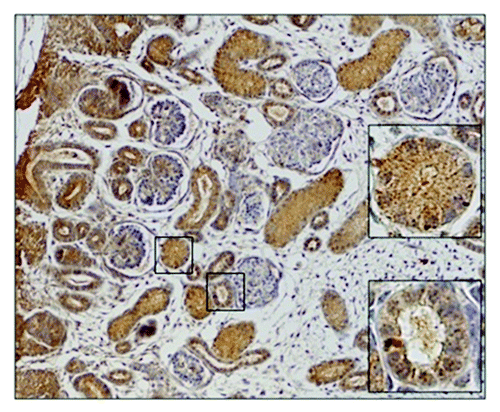
Figure 5. Expression of CYP2C8, CYP29, CYP2C19, and CYP2J2 in the proximal tubuli of embryonic/fetal kidney during 6th-20th week of IUD (n = 25) and adult tissue samples (n = 5). Graphs show the average histoscore. The error bars represent standard deviation.
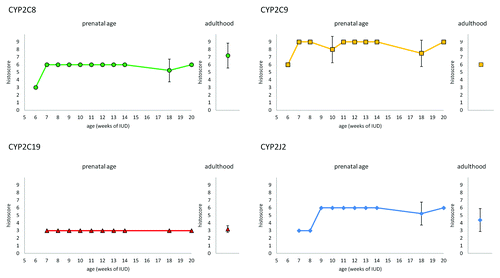
Figure 6. Expression of CYP2C8, CYP29, CYP2C19, and CYP2J2 in other tubuli (loops of Henle, distal tubuli, and collecting ducts) of embryonic/fetal kidney during 6th-20thweek of IUD (n = 25) and adult tissue samples (n = 5). Graphs show the average histoscore. The error bars represent standard deviation.
In renal corpuscles, we found weak positivity for all tested CYPs in the cells of the outer layer of Bowman’s capsule. CYP epoxygenases staining was barely detectable in glomeruli. We also registered weak or medium positivity of all tested CYP epoxygenases in the metanephric blastema and the expression level was constant through tested period of IUD. On the contrary, uninduced mesenchyme of tested samples was negative.
We detected CYP2C8, CYP2C9, CYP2C19, and CYP2J2 expression in all tested kidney tissue samples from adults. As in case of duodenal and liver tissue samples, the intensity of staining varied between individuals. The average histoscore value (n = 5) for the proximal tubuli is shown together with the embryonic/fetal expression profiles in , for other types of tubuli is shown in . Generally, our adult samples showed stronger expression of CYP2C8 and CYP2J2 in proximal tubuli than in other parts of the tubular system. On the other hand, CYP2C9 and CYP2C19 showed stronger intensity of staining in other types of tubuli than in the proximal tubuli. Similarly to the intestine, CYP2J2 expression in the adult kidney samples was lower in both proximal tubuli and other tubuli in comparison to the embryonic/fetal tissue samples.
Discussion and Conclusion
This study demonstrates the changes in the spatio-temporal expression pattern of CYP epoxygenases during human prenatal life. We detected all examined CYP proteins, namely CYP2C8, CYP2C9, CYP2C19, and CYP2J2, in embryonic/fetal intestines, liver, and kidney as well as in the adult tissue samples.
CYP epoxygenases convert arachidonic acid (AA) to epoxyeicosatrienoic acids (EETs), biologically active eicosanoids. AA is polyunsaturated n-6 fatty acid. Mammalian cells cannot synthetize n-6 (as well as n-3) fatty acids de novo. Thus, all of the n-6 and n-3 fatty acids accumulated by the fetus must be derived from mother organism by placental transfer. AA is derived from the essential dietary linoleic acid, which is formed in plants. The fetal concentration of AA is higher than in the maternal plasma which suggests its important role in the developing organism. The transport of fatty acids across the placenta is provided by membrane bound and cytosolic fatty acid binding proteins (FABPs). FABPs favor n-6 fatty acids over non-essential fatty acids and AA over their precursor linoleic acid.Citation12,Citation13 EETs, the metabolites of AA produced by CYP epoxygenases, are very interesting molecules. They have a lot of biological functions. Overall, they have cytoprotective character. EETs play role in the stimulation of mitogenesis, gene regulation and inhibition of apoptosis.Citation11 They also participate in the regulation of ion transport in kidney and smooth muscles, angiogenesis and have anti-inflammatory effect.Citation14 The opinion that they also could be considered as potential morphogens under Local Source-Dispersed Sink model exist.Citation1 Unfortunately, nowadays no studies bringing evidence that EETs act as morphogens in model organisms are available.
In embryonic/fetal intestines, the expression of CYP2C8 and CYP2C9 increases during IUD and in 16th week (CYP2C8) and 14th week (CYP2C9) of IUD reaches the level comparable to the expression level in the adults. Interestingly, the expression of CYP2J2 in the embryonic/fetal tissue is higher than in the adult tissue and remains unchanged during all tested prenatal period. Recently, presence of CYP2J2 transcripts in the developing intestines was confirmed also by Gaedigk.Citation10
The ontogeny of small intestine includes three successive phases: morphogenesis and cell proliferation, cell differentiation, and cellular and functional maturation. The organogenesis of the intestine is completed around 13th week of IUD.Citation15 CYP2C8 and CYP2C9 proteins are strongly expressed in the intervillous area of epithelium and then, after the crypts of Lieberkühn are developed (12th week of IUD), in the crypts. On the other hand, the expression pattern of CYP2C19 and CYP2J2 are changed during the 4th month of IUD. At first, CYP2C19 and CYP2J2 expression are equal along crypt-villus axis. Later, the expression of CYP2C19 in 14th week of IUD and CYP2J2 in 16th week of IUD disappears in the crypts. Although there are some discrepancies in opinions of timing of embryonic/fetal organs function, in this time the organ begins work—secretion of bile acid, intestinal enzymes, and peristaltic movement are detectable. Fetus also starts to swallow amnionic fluid (which is absorbed by epithelium and recycled by kidney).
Distribution of CYP epoxygenases expression could lead to diverse concentration of EETs regioisomers along crypt-villus axis. Each CYP epoxygenase produces several regioisomers, in which one form usually predominates. In the crypt area of the intestines is the strong expression of CYP2C8 and CYP2C9. These enzymes produce mainly 14,15-EET and 11,12-EET.Citation16 In tumor cell lines it was proved, that these regioisomers are stronger potential stimulators of proliferation than 8,9-EET. The proliferation occurs through increased phosphorylation of ERK1/2 and PI3K.Citation11 Production of 14,15- and 11,12-regioisomers in apex part of villi is presumably lower because of expression pattern of CYP epoxygenases. It has been shown that inactivation of ERK1/2 and PI3K pathway is crucial for differentiation intestinal epithelium cells.Citation17,Citation18 On the other hand, CYP2C19 is main producer of 8,9-EET and CYP2J2 is less regioselective epoxygenase which produce all four regioisomers. Besides the effect on proliferation, it has been shown that CYP2J2 in the intestine influences vascular tone and probably plays a role in releasing intestine neuropeptides, control of motility and modulation of transport of intestinal fluids and electrolytes.Citation19 The exact role of CYP epoxygenases or their products (EETs) in intestinal development still remains unclear.
The liver serves a broad range of metabolic functions, including processing of ingested nutrients, detoxification of a variety of different compounds and maintenance of physiological metabolites. Moreover, the liver serves as the homeopoietic organ during embryonic/fetal development. In the embryonic/fetal liver, the positive reaction for CYP2C8, CYP2C9, and CYP2J2 is detected very early during development, as soon as 5th week of IUD. The expression of CYP2C19 starts one week later. The expression of CYP2C in the fetal liver was investigated by several authors by qPCR and western blot.Citation3,Citation7,Citation20 A previous study found CYP2C8, CYP2C9 and CYP2C18 mRNAs in fetal liver in the age ranging from 16 to 40 wk of IUD but failed in detection of CYP2C proteins by western blot and enzyme activity.Citation20 More recently, Bieche et al.Citation7 also detected CYP2C8, CYP2C9, CYP2C18 mRNAs in fetal liver from 22th to 44th weeks of IUD. CYP2C9 mRNA was main isoform. Moreover, they proved the presence of CYP2C19 mRNA. At protein level, increasing trend of CYP2C9 content was detected in fetal liver from 8th to 24th of IUD. CYP2C19 protein was also found but at a constant level. The enzyme activity of these proteins was consistent with protein level.Citation3 Although we have examined embryonic/fetal liver in approximately same stages of IUD, CYP2C9, and CYP2C19 expression remains unchanged. The increasing CYP2C9 expression occurs only between 5th and 6th week of IUD. This discrepancy could be caused by different sensitivity of used techniques of detection. Expression of CYP2J2 in fetal liver was studied by Gaedikg et al.Citation10 They demonstrated the presence of both, CYP2J2 mRNA and protein. Their results showed strong interindividual variability at protein level, but not at mRNA level. Despite the fact that several studies provide information about presence of CYP epoxygenases in liver during human prenatal development, our understanding of their function is still elusive.
The expression of examined CYP epoxygenases in metanephros (definitive kidney) was detected as soon as in 6th week of IUD. We detected positive staining of all CYP epoxygenases in the metanephric blastema, a unique group of cells predetermined to form the nephrons. On the other hand, the uninduced mesenchyme was negative. Besides the expression in the metanephric blastema, CYP epoxygenases were predominantly expressed in the tubular system in the embryonic as well as in the adult tissue samples. CYP2C9 was the predominant isoform of CYP epoxygenases in the whole tubular system.
Interestingly, the expression of CYP2J2 in fetal kidney from 10th to 20th weeks of IUD is higher than in the adult tissue. The elevated expression of CYP2J2 in the tubular system occurs approximately at the commencement of renal ultrafiltration. It is well known that EETs have specific function in kidney. They regulate blood flow in glomeruli, release of renin, stimulate proliferation of renal epithelium (14,15-EET is the most potent one), and ion transport.Citation14 Other potential explanation of higher expression of CYP2J2 in embryonic/fetal kidney could be related to matrix metalloproteinases (MMPs) activity. It has been shown in developing kidneys of MMP-9 deficient mice, that MMP-9 is a major regulator of nephron number, because it protects nephrons from apoptosis during kidney development.Citation21 In tumor cells, it has been proved that CYP2J2 producing EETs increases their metastatic potential by increasing MMP-9 expression and activity.Citation9 At mRNA level, the expression of CYP2J2 in fetal kidneys was confirmed by Gaedigk et al.Citation8 Mouse ortholog of CYP2J enzyme, Cyp2J5, is also developmentally regulated during kidney development.Citation22 Despite information mentioned above, our knowledge of CYP epoxygenases and their products function in kidney development is far from complete.
Besides the metabolism of arachidonic acid, CYP epoxygenases also participate in the drug metabolism and the increase of their expression level in tumors could contribute to multidrug resistance in cancer patients by drug inactivation.Citation23 Their expression in tumors was confirmed by several authors.Citation8,Citation9,Citation24 CYP2C enzymes metabolize about 20% of clinically important drugs,Citation3 for example anticancer drugs such as paclitaxel, bexarotene, cyclophosphamide, ifosfamide, imatinib mesylate, idarubicin, tamoxifen, and tretionin.Citation25 Although xenobiotic metabolism by CYPs presents a certain type of cell protection against harmful molecules, their expression during the prenatal life could be suggested as a mechanism for developmental toxicity of drugs and environmental pollutants. Alterations in their strict spatio-temporal pattern of expression have fatal consequences for developing organism.Citation1 For example, it has been described recently that changes in expression of CYP2C induced by environmental teratogen trichlorethylen lead to improper development of chicken heart.Citation26
Our immunohistological study brings a complex view of time-dependent expression of CYP epoxygenases in main human embryonic/fetal organs involved in drug metabolism. Although it is obvious that they play crucial role in the prenatal development, which is based on the expression profiles of these proteins in embryonic/fetal tissues, our understanding of their exact role is far from complete. The consequences of these findings for the proper human development and a mother’s nutrition during pregnancy, as well as for cancer patients warrant further investigation.
Material and Methods
Tissue samples of human embryonic/fetal intestines, liver and kidney were obtained from the archive of the Department of Histology and Embryology, Faculty of Medicine and Dentistry, Palacky University in Olomouc. It is a collection of tissue samples from clinically aborted embryos/fetuses. We have examined 29 intestines samples from the 7th to 20th week of IUD, 23 liver samples from the 5th to 20th week of IUD, and 25 kidney samples from the 6th to 20th week of IUD. All samples were presumed to be normal as no tissue were provided from cases known to have defects or adverse exposures. The distribution of samples in different age is shown in . We also studied the distribution pattern of CYP epoxygenases in samples of the adult intestine (duodenum), liver, and kidney. The number of samples and basic donor characterization (age, gender) is summarized in These samples were obtained from patients who underwent biopsies for medical examination and their histological findings were normal. The tissue samples were fixed in formalin or methacarn and embedded in paraffin. The use of all samples has been approved by the Ethics Committee of the University Hospital and the Faculty of Medicine and Dentistry, Palacky University in Olomouc.
Table 2. Number of embryonic/fetal tissue samples in different ages of intrauterine development
Table 3. Number of used adult tissue samples and the basic characterization of donors (age, gender)
Proteins of interest were detected in 4 µm thick paraffin sections by immunohistochemistry. These rabbit polyclonal antibodies were used: CYP2C8 (Proteintech), CYP2C9 (Abgent), CYP2C19 (NovusBiologicals), and CYP2J2 (Abcam). Western blot and immunohistochemistry evaluation data for these antibodies are provided by manufacturers. To specify appropriate dilution for the primary antibodies, we stained positive control tissues by variety of concentrations of the antibodies. As positive control, we used human liver tissue samples for CYP2C8 and CYP2C9, human liver carcinoma for CYP2C19 and human myocardial tissue for CYP2J2. According to this procedure, dilution 1:50 was used for immunostaining of our samples for all antibodies which were diluted in Dako REALTM Antibody Diluent (DAKO). As a negative control, primary antibody was substituted by Tris buffer. Positive and negative controls were present in each immunostaining of the samples to confirm staining process.
Slides were deparaffinized and hydrated by passage through a series of xylen, ethanol, and distilled water washes. To unmask antigens, heat antigen retrieval was performed for all samples. For blocking non-specific background staining, we incubated the samples with Protein Block (DAKO) for 30 min in the room temperature. Then, all samples were incubated with primary antibody for 1 h in the room temperature. The detection of CYP2C8 and CYP2C9 protein was performed by EnVisionTM Detection System, Peroxidase/DAB, Rabbit/Mouse (DAKO) and of CYP2C19 and CYP2J2 was done by the detection kit EnVisionTM G|2 System/AP, Rabbit/Mouse-Permanent Red (DAKO). Tris buffer (pH 7,6) was used for washing between different steps. Nuclei of all samples were counterstained with hematoxylin and washed in tap water. Then, the samples were dehydrated and coverslipped.
Prior to evaluation, the samples were coded to minimize observer bias. The expression of CYPs in embryonic intestines, liver, and kidney was evaluated by histoscore, i. e. the intensity of the signal multiplied by the ratio of positive cells. The evaluation of signal intensity was following: 0 = no signal, 1 = weak signal, 2 = moderate signal, 3 = strong signal. The ratio of positive cells was 0 up to 5%, 1 up to 30%, 2 up to 60% and 3 for more than 60%. Therefore, the maximal histoscore is 9. The whole sample collection was evaluated twice in different time. The average histoscore value for samples in the same stage of IUD was calculated and values are displayed in charts which demonstrate age-dependent differences in the expression of CYP epoxygenases during IUD and average histoscore of adult samples for comparison.
| Abbreviations: | ||
| CYP | = | cytochrome P450 |
| EET | = | epoxyeicosatrienoic acid |
| DHET | = | dihydroxyeicosatrienoic acid |
| IUD | = | intrauterine development |
| AA | = | arachidonic acid |
| FABP | = | fatty acid binding protein |
| ERK | = | extracellular signal-regulated kinase |
| PI3K | = | phosphoinositide 3-kinase |
| qPCR | = | quantitative polymerase chain reaction |
| mRNA | = | messenger ribonucleic acid |
| MMP | = | matrix metalloproteinase |
Additional material
Download Zip (106 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Eva Kasparkova and Eva Nezhybova for excellent technical support. This study was supported by LF_2013_006.
References
- Stoilov I. Cytochrome P450s: coupling development and environment. Trends Genet 2001; 17:629 - 32; http://dx.doi.org/10.1016/S0168-9525(01)02444-1; PMID: 11672862
- Shen AL, O’Leary KA, Kasper CB. Association of multiple developmental defects and embryonic lethality with loss of microsomal NADPH-cytochrome P450 oxidoreductase. J Biol Chem 2002; 277:6536 - 41; http://dx.doi.org/10.1074/jbc.M111408200; PMID: 11742006
- Koukouritaki SB, Manro JR, Marsh SA, Stevens JC, Rettie AE, McCarver DG, Hines RN. Developmental expression of human hepatic CYP2C9 and CYP2C19. J Pharmacol Exp Ther 2004; 308:965 - 74; http://dx.doi.org/10.1124/jpet.103.060137; PMID: 14634042
- Lee CA, Neul D, Clouser-Roche A, Dalvie D, Wester MR, Jiang Y, Jones JP 3rd, Freiwald S, Zientek M, Totah RA. Identification of novel substrates for human cytochrome P450 2J2. Drug Metab Dispos 2010; 38:347 - 56; http://dx.doi.org/10.1124/dmd.109.030270; PMID: 19923256
- Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol 1969; 25:1 - 47; http://dx.doi.org/10.1016/S0022-5193(69)80016-0; PMID: 4390734
- Enayetallah AE, French RA, Thibodeau MS, Grant DF. Distribution of soluble epoxide hydrolase and of cytochrome P450 2C8, 2C9, and 2J2 in human tissues. J Histochem Cytochem 2004; 52:447 - 54; http://dx.doi.org/10.1177/002215540405200403; PMID: 15033996
- Bièche I, Narjoz C, Asselah T, Vacher S, Marcellin P, Lidereau R, Beaune P, de Waziers I. Reverse transcriptase-PCR quantification of mRNA levels from cytochrome (CYP)1, CYP2 and CYP3 families in 22 different human tissues. Pharmacogenet Genomics 2007; 17:731 - 42; http://dx.doi.org/10.1097/FPC.0b013e32810f2e58; PMID: 17700362
- Enayetallah AE, French RA, Grant DF. Distribution of soluble epoxide hydrolase, cytochrome P450 2C8, 2C9 and 2J2 in human malignant neoplasms. J Mol Histol 2006; 37:133 - 41; http://dx.doi.org/10.1007/s10735-006-9050-9; PMID: 16957870
- Jiang JG, Ning YG, Chen C, Ma D, Liu ZJ, Yang S, Zhou J, Xiao X, Zhang XA, Edin ML, et al. Cytochrome p450 epoxygenase promotes human cancer metastasis. Cancer Res 2007; 67:6665 - 74; http://dx.doi.org/10.1158/0008-5472.CAN-06-3643; PMID: 17638876
- Gaedigk A, Baker DW, Totah RA, Gaedigk R, Pearce RE, Vyhlidal CA, Zeldin DC, Leeder JS. Variability of CYP2J2 expression in human fetal tissues. J Pharmacol Exp Ther 2006; 319:523 - 32; http://dx.doi.org/10.1124/jpet.106.109215; PMID: 16868033
- Shen GF, Jiang JG, Fu XN, Wang DW. Promotive effects of epoxyeicosatrienoic acids (EETs) on the proliferation of tumour cells. Ai Zheng 2008; 27:390 - 5
- Haggarty P. Placental regulation of fatty acid delivery and its effect on fetal growth--a review. Placenta 2002; 23:Suppl A S28 - 38; http://dx.doi.org/10.1053/plac.2002.0791; PMID: 11978057
- Innis SM. Essential fatty acid transfer and fetal development. Placenta 2005; 26:Suppl A S70 - 5; http://dx.doi.org/10.1016/j.placenta.2005.01.005; PMID: 15837071
- Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J Lipid Res 2000; 41:163 - 81; PMID: 10681399
- Montgomery RK, Mulberg AE, Grand RJ. Development of the human gastrointestinal tract: twenty years of progress. Gastroenterology 1999; 116:702 - 31; http://dx.doi.org/10.1016/S0016-5085(99)70193-9; PMID: 10029630
- Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem 2001; 276:36059 - 62; http://dx.doi.org/10.1074/jbc.R100030200; PMID: 11451964
- Aliaga JC, Deschênes C, Beaulieu JF, Calvo EL, Rivard N. Requirement of the MAP kinase cascade for cell cycle progression and differentiation of human intestinal cells. Am J Physiol 1999; 277:G631 - 41; PMID: 10484389
- Laprise P, Chailler P, Houde M, Beaulieu JF, Boucher MJ, Rivard N. Phosphatidylinositol 3-kinase controls human intestinal epithelial cell differentiation by promoting adherens junction assembly and p38 MAPK activation. J Biol Chem 2002; 277:8226 - 34; http://dx.doi.org/10.1074/jbc.M110235200; PMID: 11756422
- Zeldin DC, Foley J, Goldsworthy SM, Cook ME, Boyle JE, Ma J, Moomaw CR, Tomer KB, Steenbergen C, Wu S. CYP2J subfamily cytochrome P450s in the gastrointestinal tract: expression, localization, and potential functional significance. Mol Pharmacol 1997; 51:931 - 43; PMID: 9187259
- Treluyer JM, Gueret G, Cheron G, Sonnier M, Cresteil T. Developmental expression of CYP2C and CYP2C-dependent activities in the human liver: in-vivo/in-vitro correlation and inducibility. Pharmacogenetics 1997; 7:441 - 52; http://dx.doi.org/10.1097/00008571-199712000-00002; PMID: 9429229
- Arnould C, Lelièvre-Pégorier M, Ronco P, Lelongt B. MMP9 limits apoptosis and stimulates branching morphogenesis during kidney development. J Am Soc Nephrol 2009; 20:2171 - 80; http://dx.doi.org/10.1681/ASN.2009030312; PMID: 19713309
- Ma J, Qu W, Scarborough PE, Tomer KB, Moomaw CR, Maronpot R, Davis LS, Breyer MD, Zeldin DC. Molecular cloning, enzymatic characterization, developmental expression, and cellular localization of a mouse cytochrome P450 highly expressed in kidney. J Biol Chem 1999; 274:17777 - 88; http://dx.doi.org/10.1074/jbc.274.25.17777; PMID: 10364221
- Gillet JP, Gottesman MM. Mechanisms of multidrug resistance in cancer. In: Zhou J, editor. Multidrug resistance in cancer (Methods in molecular biology. Vol. 596). New York: Humana Press; c2010. p.47-76.
- Jiang JG, Chen CL, Card JW, Yang S, Chen JX, Fu XN, Ning YG, Xiao X, Zeldin DC, Wang DW. Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res 2005; 65:4707 - 15; http://dx.doi.org/10.1158/0008-5472.CAN-04-4173; PMID: 15930289
- Scripture CD, Sparreboom A, Figg WD. Modulation of cytochrome P450 activity: implications for cancer therapy. Lancet Oncol 2005; 6:780 - 9; http://dx.doi.org/10.1016/S1470-2045(05)70388-0; PMID: 16198984
- Makwana O, Ahles L, Lencinas A, Selmin OI, Runyan RB. Low-dose trichloroethylene alters cytochrome P450-2C subfamily expression in the developing chick heart. Cardiovasc Toxicol 2013; 13:77 - 84; http://dx.doi.org/10.1007/s12012-012-9180-0; PMID: 22855351
