Abstract
Cutaneous scarring is a major source of morbidity and current therapies to mitigate scar formation remain ineffective. Although wound fibrosis and inflammation are highly linked, only recently have mechanical forces been implicated in these pathways. Our group has developed a topical polymer device that significantly reduces post-injury scar formation via the manipulation of mechanical forces. Here we extend these studies to examine the genomewide transcriptional effects of mechanomodulation during scar formation using a validated large animal model, the red Duroc pig. We demonstrate that mechanical loading of incisional wounds upregulates expression of genes associated with inflammatory and fibrotic pathways, and that device-mediated offloading of these wounds reverses these effects. Validation studies are needed to clarify the clinical significanceof these findings.
Introduction
Scarring represents a major clinical burden in the United States, where more than 150 000 scar revision surgeries are performed each year.Citation1 In addition to aesthetic considerations, pathological over-scarring is also associated with severe functional deficits and can result in considerable psychosocial stress.Citation2 Scar formation represents the body’s natural response to cutaneous injury, which has evolved to prioritize early and aggressive closure to avoid infection and other life threatening complications of wounds.Citation3 Nevertheless, developing methods to attenuate this fibrotic response and achieve a more regenerative healing process remains an area of active biomedical research.
One promising example of the potential for human cutaneous wounds to heal without scar is seen in human fetal surgery, where scarless healing occurs following injury during the first two trimesters.Citation4 Similar “breakpoints” between scarless and scarring wound healing have been demonstrated in small and large animal models, lending considerable insight into our understanding of the underlying physiological mechanisms.Citation5 However, translating what has been learned about scarless fetal repair into strategies to reduce scarring in children and adults remains a significant and unmet medical need.
Inflammation has long been believed to play a role in scar formation; however, therapies targeting inflammation alone have proven ineffective at reducing scarring.Citation6 A better understanding of the mechanisms underlying inflammation is required to develop rational therapies to block scarring and promote regeneration. There is increasing recognition that mechanical forces regulate inflammation and fibrosis across multiple organ systems.Citation7-Citation10 In skin, it has also been postulated that mechanical forces produce fibrosis through activation of inflammatory pathways. In prior work, we developed a dynamic polymer device to control the mechanical environment of healing wounds. We have recently demonstrated that attenuation of mechanical forces significantly decreased incisional scar formation in a large animal (red Duroc pig) model and a phase 1 human trial.Citation11 However, the transcriptional pathways underlying these effects remained unclear. Here, we applied genome-wide microarray analysis to investigate the molecular pathways modulated by mechanical force during the healing of incisional wounds in the red Duroc pig to better understand how these pathways activate fibrosis in vivo.
Results
Full-thickness incisional wounds were created on the dorsum of a purebred red Duroc pig and closed with nylon sutures which were removed on post-injury day four. Wounds were subjected to either natural skin stress, elevated stress, or reduced stress (i.e., “stress-shielded”) for eight weeks (). Microarray analysis was performed on tissue harvested from all wounds at 8 wk post-injury. Significance analysis of microarrays (SAM) was applied to compare wounds that healed under elevated stress and those subjected to natural stress. Using a fold change cutoff of 2 and a false discovery rate (FDR) threshold of 5%, 89 genes were significantly upregulated and 51 downregulated by mechanical stress in the context of a healing wound (Tables S1 and 2). A similar analysis was performed comparing wounds that healed under stress-shielded conditions; 194 genes were significantly upregulated, with 29 downregulated with stress reduction (Tables S3 and 4).
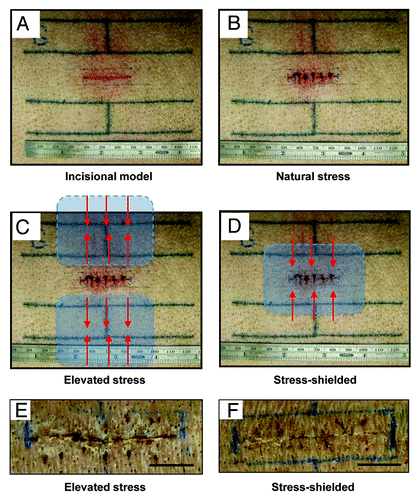
Figure 1. Incisional model in the red Duroc pig. (A) Tattoo lines to mark incision. (B) Creation of full thickness 3 cm incision on post-injury day 0. Wounds were immediately closed with nylon sutures. “Natural stress” wounds were allowed to heal without any modulation of the mechanical environment. (C) Schematic of device positioning for the “elevated stress” wound conditions. The “para” positioning of the devices results in elevated mechanical forces (red arrows) as the polymer device contracts parallel to the long axis of the wound. (D) In the “stress-shielded” wounds, incisions were mechanically offloaded by placing the device overlying the wound. As the device contracts parallel to the long axis of the wound, adjacent mechanical forces are offloaded (red arrows). Photographs at eight weeks post-injury of incisional wounds that healed under elevated stress (E) vs. stress-shielded (F) conditions. Histologic validation of scar properties has been previously published.Citation28 Scale bar = 1 cm.
Gene expression data for elevated stress wounds were clustered hierarchically (). Canonical pathways whose expression was significantly upregulated in elevated stress wounds was determined using Ingenuity Pathway Analysis (IPA; ). Additionally, analysis of downregulated genes identified pathways likely to be suppressed in these wounds (). Increased expression of the complement system, insulin-like growth factor (IGF)-1 signaling, and acute phase response signaling was observed in elevated stress wounds, whereas pathways associated with tissue homeostasis were downregulated. Network maps were constructed for the top pathways upregulated in elevated stress wounds, and these included connective tissue disorders and cell death / survival ().
Figure 2. Microarray analysis of elevated stress wounds. (A) Hierarchical clustering of regulated genes from wounds under elevated stress vs. natural stress. Individual genes are clustering according to the dendrogram on the left, and samples correspond to columns in the heatmap on the right (n = 2 per group). Yellow and blue indicate up- and downregulation, respectively. (B) Canonical pathways significantly enriched for among genes whose expression was significantly upregulated in elevated stress wounds compared with natural stress wounds. (C) Canonical pathways significantly enriched for among genes whose expression was significantly downregulated in elevated stress wounds compared with natural stress wounds.
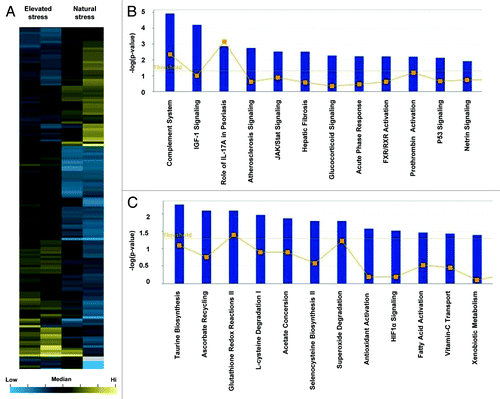
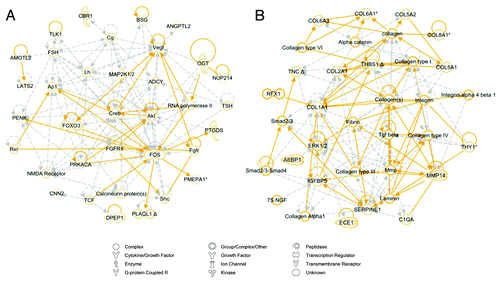
Figure 3. Network analysis of elevated stress wounds. Top two scoring Ingenuity Pathway Analysis (IPA)-constructed transcriptome networks based genes that were significantly upregulated in elevated stress wounds compared with natural stress wounds. Panel (A) represents the IPA gene expression network whose top function is “Cell Death and Survival,” and (B) corresponds to “Connective Tissue Disorders.” Direct relationships are indicated by solid lines (yellow), and dashed lines (gray) represent indirect relationships.
Gene expression data for stress-shielded wounds were similarly clustered (). Canonical pathways enriched for genes whose expression was significantly upregulated in stress-shielded wounds was determined using IPA (). Pathways associated with downregulated genes were also identified (). Notably, leukocyte extravasation and hypoxia-inducible factor (HIF)-1a signaling demonstrated decreased expression in stress-shielded wounds. By contrast, the top targets upregulated in stress-shielded wounds were not associated with inflammation or skin fibrosis. A network map was constructed for the top pathway associated with genes downregulated in stress-shielded wounds, which included connective tissue disorders and inflammatory disease ().
Figure 4. Microarray analysis of stress-shielded wounds. (A) Hierarchical clustering of regulated genes from wounds under stress-shielding or natural stress. Individual genes are clustering according to the dendrogram on the left, and samples correspond to columns in the heatmap on the right (n = 2 per group). Yellow and blue indicate up- and downregulation, respectively. (B) Canonical pathways significantly enriched for among genes whose expression was significantly upregulated in stress-shielded wounds compared with natural stress wounds. (C) Canonical pathways significantly enriched for among genes whose expression was significantly downregulated in stress-shielded wounds compared with natural stress wounds.
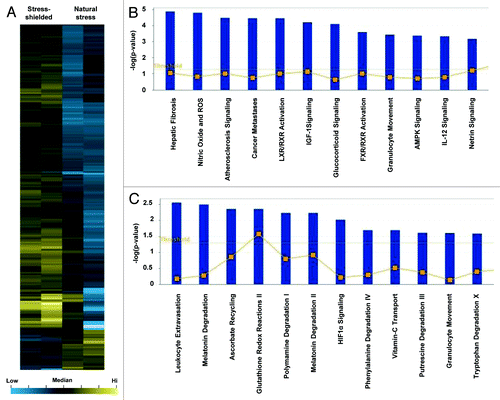
Figure 5. Network analysis of stress-shielded wounds. Top scoring Ingenuity Pathway Analysis (IPA)-constructed transcriptome network based on genes that were significantly downregulated in stress-shielded wounds compared with natural stress wounds, corresponding to the IPA gene expression network whose top functions consist of “Connective Tissue Disorders” and “Inflammatory Disease.” Direct relationships are indicated by solid lines (blue), and dashed lines (gray) represent indirect relationships.
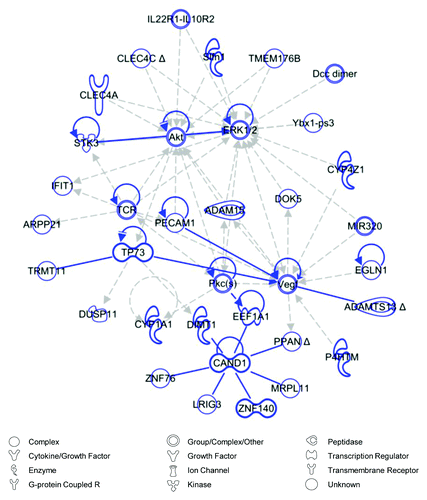
Given that genes associated with connective tissue disorders and inflammation were upregulated in elevated stress wounds and downregulated in stress-shielded wounds, we hypothesized that there may be a common mechanism linking these opposing forms of mechanomodulation. To explore this, we used IPA to merge top networks associated with each condition ( and ) to generate a super-network including all genes associated with either pathway (). The central element linking these networks was extracellular-related kinase (ERK)1/2, an intracellular signaling node which has previously been associated with inflammation and scarring.Citation8 Further, when we artificially grew our network using the IPA pathway growth tool, which employs known relationships based on the Ingenuity Knowledge Base, we found that focal adhesion kinase (FAK) assumed a central position among the genes associated with both pathways. These data suggest a molecular link between inflammation and fibrosis that can be mechanically regulated via FAK and ERK1/2.
Figure 6. Merged network analysis. The top scoring network generated from genes significantly downregulated by stress-shielding (blue) and the second top scoring network from genes significantly upregulated under elevated stress (yellow) were merged using Ingenuity Pathway Analysis (IPA), creating a super-network centered on ERK1/2. This network was expanded with the IPA pathway growth tool using direct and indirect relationships to add 30 additional molecules (gray) based on the Ingenuity Knowledge Base. Direct relationships are indicated by solid lines, and dashed lines represent indirect relationships. Indirect relationships arising from network growth have been removed for visual clarity.
Discussion
Fibroproliferative diseases represent a major cause of morbidity and mortality in the developed world.Citation12 Although some organs such as the liver maintain a level of regenerative capacity beyond embryonic development, human skin inevitably heals with varying degrees of scar formation that can severely impact functional outcomes.Citation3 Clinical experience and recent experimental studies have recently implicated mechanical force as playing a major role in post-injury inflammation and fibrosis.Citation7,Citation13-Citation15 Pathways involved in mechanotransduction (the conversion of physical cues to biochemical signals) have been increasingly elucidated on the molecular, cellular, and organ levels and are thought to drive repair processes throughout life.Citation16
Based on the recognition that mechanical forces may induce fibrotic repair, strategies to offload mechanical forces have been developed to mitigate pathologic tissue responses in various organ systems. For example, low tidal volume ventilation has become the standard of care to prevent barotrauma, inflammation, and subsequent pulmonary fibrosis.Citation17 Similarly, ventricular assist devices are used to mechanically offload the failing heart and prevent pathologic hypertrophic remodeling,Citation18 and mechanical shear stresses have even been proposed as a target to reduce atherosclerotic disease.Citation19 In the context of wound healing, mechanical offloading has been applied to mitigate scar formation in large animals and humans.Citation11 These studies and many others strongly suggest that attenuation of mechanical forces may block inflammation and prevent fibrotic healing.
The link between mechanotransduction and inflammation has previously been studied at the transcriptional level. Rat cardiac fibroblasts subjected to mechanical stress in vitro exhibited alterations in gene expression programs related to extracellular matrix formation and cytoskeletal components.Citation20 In experimental ventilator-induced lung injury models, researchers found mechanical upregulation of immune, inflammatory, proliferation, and matrix pathways.Citation21 Our group recently utilized genome-wide microarray analysis to investigate the role of mechanical tension on incisional wound healing in mice and identified regulation of inflammatory, chemokine, and matrix components during stress-induced scar formation.Citation7 However, these small animal models do not completely replicate human wound healing. The red Duroc pig is considered the best model for cutaneous healing in humans, and we previously demonstrated that mechanical forces regulate scar formation using this model.Citation11
In this study, we found that elevated mechanical forces during incisional wound repair in the red Duroc pig activated gene transcription pathways linked to inflammation and matrix production, similar to our earlier findings using a murine model. Specifically, we demonstrated that numerous collagen (COL1A1, COL2A1, COL5A1, COL5A2, COL6A1, COL6A3, COL8A1), as well as matrix metalloproteinase (MMP) and insulin-like growth factor binding protein (IGFBP), genes were significantly upregulated in elevated stress wounds. These findings are also consistent with the consensus results of previously published microarray data for hypertrophic scarring in human tissue, in which collagen genes in particular are strongly implicated.Citation22 Not surprisingly, we found that blocking mechanical forces using a stress shielding device resulted in transcriptional downregulation of markers associated with inflammatory disease and connective tissue disorders. These findings suggest that functional pathways central to tissue remodeling are capable of being mechanically modulated in a large animal known to heal similar to human healing.
Furthermore, when the network maps involving elevated stress and stress-shielding were merged using pathway analysis tools, the intracellular targets FAK and ERK1/2 emerged as common mediators of these pathways. We have previously shown that both FAK and ERK play a primary role in scar mechanotransduction in mice.Citation8 These intracellular targets are linked to cell-surface integrin signaling and act as major mechanosensors during wound repair.Citation12,Citation23-Citation26 Further, FAK and ERK have been closely linked to canonical pro-fibrotic pathways including transforming growth factor β and α smooth muscle actin. As such, the FAK-ERK mechanotransduction pathway may be a valuable target for future clinical research in scar blockade via molecular or genetic pharmacologic approaches.
The results presented in this study are based on evaluation of gene expression using DNA microarray. While this is a highly effective tool for exploratory analysis of complex tissue and organ-level pathways, it is important that follow-up studies validate specific transcriptional findings through qPCR. Furthermore, as the correlation between gene expression and protein expression can be as low as 40%,Citation27 it is also critical that confirmatory studies measure these changes at a protein level. We have previously used immunohistochemistry and protein quantitation to demonstrate inflammatory changes in this model,Citation11 but specific validation of the FAK-ERK axis remains an ongoing effort.
In aggregate, these findings support the notion that physical forces act via cellular mechanotransduction pathways to control wound repair. Specifically, intracellular transcriptional pathways linked to FAK and ERK appear conserved across mammalian species (mice and pigs) and function to activate pro-fibrotic pathways in the setting of high mechanical stress. These central mediators regulate a wide range of both extracellular (matrix production, cytokine secretion) and intracellular (proliferation, survival) programs to drive pathologic scar formation in humans. Targeting of these mechanotransduction pathways via a device or molecular strategies may direct wound repair toward regeneration and away from fibrosis.
Materials and Methods
Pig model
Studies were performed on an adult purebred red Duroc swine in accordance with Stanford University animal guidelines, as previously published.Citation11 General inhalational anesthesia and sedation were provided by veterinary staff. Full-thickness incisional wounds measuring three centimeters in length were created on the pig dorsum and closed with 4–0 nylon sutures (). Sterile occlusive dressings were applied and sutures were removed on post-injury day four.
The different mechanical conditions in this incisional wound model have been previously described.Citation11 Briefly, natural stress incisions were allowed to heal without the application of any topical polymer device. Stress-shielded incisions had the polymer device placed immediately after closure, providing approximately 20% compressive strain. Elevated stress incisions had two polymer devices placed on each side of the wound, pulling across the wound to augment mechanical tension across the incision.
The polymer devices were replaced on day four and weekly thereafter for up to eight weeks. A custom-designed pig jackets was used to protect the wounds and stress-shielding devices. After euthanasia at eight weeks post-injury, the wounds were harvested for tissue analysis. Full thickness skin samples measuring 3 mm by 3 mm by 3 mm were harvested from separate incisional wounds that were allowed to heal under natural, elevated stress, and stress-shielded conditions (n = 3 for each mechanical condition).
Microarray analysis and statistics
Total RNA was extracted from skin samples using TRIzol Reagent (Invitrogen Life Technologies) according to the manufacturer’s instructions. Samples were loaded onto the GeneChip Porcine Genome Array (Affymetrix, Inc.) which contains 23 937 probe sets to interrogate 20 201 genes. Data were normalized using Genespring’s robust multi-array average (RMA) algorithm. Probes with intensity values in the bottom 20% of all samples were removed. Significance analysis of microarrays (SAM) was performed between wounds healed under induced tension and those subject to natural tension using a fold change cutoff of 2 and a false discovery rate (FDR) threshold of 5%. Hierarchical clustering of gene expression data for each group of wounds was performed using MATLAB (Mathworks). Canonical pathway calculations and network analyses were performed using Ingenuity Pathway Analysis (IPA, Ingenuity Systems).
Additional material
Download Zip (162.9 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Ms. Yujin Park for assistance with histologic processing. This work was funded by Department of Defense (Armed Forces Institute of Regenerative Medicine #W81XWH-08–2-0032) and the National Library of Medicine (#LM0077033).
References
- American Society of Plastic Surgeons. 2012 Report of the 2011 Statistics National Clearinghouse of Plastic Surgery Statistics.
- Young VL, Hutchison J. Insights into patient and clinician concerns about scar appearance: semiquantitative structured surveys. Plast Reconstr Surg 2009; 124:256 - 65; http://dx.doi.org/10.1097/PRS.0b013e3181a80747; PMID: 19568089
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008; 453:314 - 21; http://dx.doi.org/10.1038/nature07039; PMID: 18480812
- Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plast 2 Surg 2010; 126:1172 - 80; http://dx.doi.org/10.1097/PRS.0b013e3181eae781; PMID: 20885241
- Yannas IV, Kwan MD, Longaker MT. Early fetal healing as a model for adult organ regeneration. Tissue Eng 2007; 13:1789 - 98; http://dx.doi.org/10.1089/ten.2006.0054; PMID: 17518739
- JUVISTA EU Phase 3 Trial Results. Renovo Group PLC. 2011. Available from: http://www.renovo.com/en/news/juvista-eu-phase-3-trial-results.
- Wong VW, Paterno J, Sorkin M, Glotzbach JP, Levi K, Januszyk M, Rustad KC, Longaker MT, Gurtner GC. Mechanical force prolongs acute inflammation via T-cell-dependent pathways during scar formation. FASEB J 2011; 25:4498 - 510; http://dx.doi.org/10.1096/fj.10-178087; PMID: 21911593
- Wong VW, Rustad KC, Akaishi S, Sorkin M, Glotzbach JP, Januszyk M, Nelson ER, Levi K, Paterno J, Vial IN, et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med 2012; 18:148 - 52; http://dx.doi.org/10.1038/nm.2574; PMID: 22157678
- Drakos SG, Kfoury AG, Selzman CH, Verma DR, Nanas JN, Li DY, Stehlik J. Left ventricular assist device unloading effects on myocardial structure and function: current status of the field and call for action. Curr Opin Cardiol 2011; 26:245 - 55; http://dx.doi.org/10.1097/HCO.0b013e328345af13; PMID: 21451407
- Pelosi P, Rocco PR. Effects of mechanical ventilation on the extracellular matrix. Intensive Care Med 2008; 34:631 - 9; http://dx.doi.org/10.1007/s00134-007-0964-9; PMID: 18264691
- Gurtner GC, Dauskardt RH, Wong VW, Bhatt KA, Wu K, Vial IN, Padois K, Korman JM, Longaker MT. Improving cutaneous scar formation by controlling the mechanical environment: large animal and phase I studies. Ann Surg 2011; 254:217 - 25; http://dx.doi.org/10.1097/SLA.0b013e318220b159; PMID: 21606834
- Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest 2007; 117:524 - 9; http://dx.doi.org/10.1172/JCI31487; PMID: 17332879
- Carver W, Goldsmith EC. Regulation of tissue fibrosis by the biomechanical environment. Biomed Res Int 2013; 2013:101979; http://dx.doi.org/10.1155/2013/101979; PMID: 23781495
- Ogawa R. Mechanobiology of scarring. Wound Repair Regen 2011; 19:Suppl 1 s2 - 9; http://dx.doi.org/10.1111/j.1524-475X.2011.00707.x; PMID: 21793962
- Chen W, Frangogiannis NG. Fibroblasts in post-infarction inflammation and cardiac repair. Biochim Biophys Acta 2013; 1833:945 - 53; http://dx.doi.org/10.1016/j.bbamcr.2012.08.023; PMID: 22982064
- Wong VW, Akaishi S, Longaker MT, Gurtner GC. Pushing back: wound mechanotransduction in repair and regeneration. J Invest Dermatol 2011; 131:2186 - 96; http://dx.doi.org/10.1038/jid.2011.212; PMID: 21776006
- Villar J, Cabrera NE, Valladares F, Casula M, Flores C, Blanch L, Quilez ME, Santana-Rodríguez N, Kacmarek RM, Slutsky AS. Activation of the Wnt/β-catenin signaling pathway by mechanical ventilation is associated with ventilator-induced pulmonary fibrosis in healthy lungs. PLoS One 2011; 6:e23914; http://dx.doi.org/10.1371/journal.pone.0023914; PMID: 21935365
- Drakos SG, Kfoury AG, Hammond EH, Reid BB, Revelo MP, Rasmusson BY, Whitehead KJ, Salama ME, Selzman CH, Stehlik J, et al. Impact of mechanical unloading on microvasculature and associated central remodeling features of the failing human heart. J Am Coll Cardiol 2010; 56:382 - 91; http://dx.doi.org/10.1016/j.jacc.2010.04.019; PMID: 20650360
- Lee MY, Wu CM, Yu KH, Chu CS, Lee KT, Sheu SH, Lai WT. Association between wall shear stress and carotid atherosclerosis in patients with never treated essential hypertension. Am J Hypertens 2009; 22:705 - 10; http://dx.doi.org/10.1038/ajh.2009.77; PMID: 19407806
- Boerma M, van der Wees CG, Vrieling H, Svensson JP, Wondergem J, van der Laarse A, Mullenders LH, van Zeeland AA. Microarray analysis of gene expression profiles of cardiac myocytes and fibroblasts after mechanical stress, ionising or ultraviolet radiation. BMC Genomics 2005; 6:6; http://dx.doi.org/10.1186/1471-2164-6-6; PMID: 15656902
- Wurfel MM. Microarray-based analysis of ventilator-induced lung injury. Proc Am Thorac Soc 2007; 4:77 - 84; http://dx.doi.org/10.1513/pats.200608-149JG; PMID: 17202295
- Huang C, Nie F, Qin Z, Li B, Zhao X. A snapshot of gene expression signatures generated using microarray datasets associated with excessive scarring. Am J Dermatopathol 2013; 35:64 - 73; http://dx.doi.org/10.1097/DAD.0b013e31825ba13f; PMID: 22785331
- Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci 2003; 116:1409 - 16; http://dx.doi.org/10.1242/jcs.00373; PMID: 12640026
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 2008; 214:199 - 210; http://dx.doi.org/10.1002/path.2277; PMID: 18161745
- Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann Med 2003; 35:564 - 77; http://dx.doi.org/10.1080/07853890310016333; PMID: 14708967
- Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol 2009; 10:63 - 73; http://dx.doi.org/10.1038/nrm2597; PMID: 19197333
- Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 2012; 13:227 - 32; PMID: 22411467
- Ashinoff RL, Cetrulo CL Jr., Galiano RD, Dobryansky M, Bhatt KA, Ceradini DJ, Michaels J 5th, McCarthy JG, Gurtner GC. Bone morphogenic protein-2 gene therapy for mandibular distraction osteogenesis. Ann Plast Surg 2004; 52:585 - 90, discussion 591; http://dx.doi.org/10.1097/01.sap.0000123023.28874.1e; PMID: 15166991
