Abstract
In recent years, many genes that participate in the specification, differentiation and steroidogenesis of the interrenal organ, the teleostean homologue of the adrenal cortex, have been identified and characterized in zebrafish. In-depth studies of these genes have helped to delineate the morphogenetic steps of interrenal organ formation, as well as some of the molecular and cellular mechanisms that govern these processes. The co-development of interrenal tissue with the embryonic kidney (pronephros), surrounding endothelium and invading chromaffin cells has been analyzed, by virtue of the amenability of zebrafish embryos to a variety of genetic, developmental and histological approaches. Moreover, zebrafish embryos can be subject to molecular as well as biochemical assays for the unraveling of the transcriptional regulation program underlying interrenal development. To this end, the key mechanisms that control organogenesis and steroidogenesis of the zebrafish interrenal gland have been shown to resemble those in mammals, justifying the future utilization of zebrafish model for discovering novel genes associated with adrenal development and disease.
Introduction
The external fertilization and rapid development of the zebrafish embryo has made it suitable for studying early vertebrate development.Citation1 Due to the permeability and optic transparency of the zebrafish embryo, developing organ primordia in the whole mount specimen can be readily detected by histochemistry, immunohistochemistry or in situ hybridizations.Citation2 Various cell types that integrate to constitute specific organs, such as embryonic heart and kidney, can be labeled simultaneously by a combination of histological approaches.Citation3,Citation4 Hence, the zebrafish is particularly useful for capitulating the dynamic processes of organ formation, especially cell migration events or morphogenetic movements of organ primordia. Furthermore, the establishment of transgenic fluorescent reporter lines has allowed the tracking of specific lineages as well as dissection of cellular processes with high resolution. For example, endothelium-specific transgenic reporter lines have enabled the elucidation of mechanisms underlying vessel formation, angiogenesis and endothelial tube assembly.Citation5–Citation7
Zebrafish mutants that are generated from either forward or reverse genetic approaches have offered great opportunity for understanding the interrelationships among gene, development and disease.Citation8,Citation9 Large-scale chemical mutagenesis screens have identified numerous mutants with a wide spectrum of defects manifested in various organs, helping to elucidate specific signaling pathways leading to organogenesis such as heart and blood formation. The advent of antisense morpholino oligo knockdown approach, proven effective and specific in the zebrafish embryo, has further facilitated the developmental analysis for any candidate genes of interest.Citation10
Although the endocrine gland structures of fish and mammals are largely different, their developmental processes in organogenesis appear to share high similarities.Citation11 Also, endocrine function is well conserved between teleosts and mammals, which could be reflected by the profiles of hormones, hormone receptors as well as transcriptional regulators. Interestingly, some zebrafish mutants or morphants faithfully phenocopy human endocrine disorders such as combined pituitary hormone deficienciesCitation12 and neonatal diabetes mellitus.Citation13 Hence, they promise to provide insights into the molecular etiology of their human disease counterparts.
This short review highlights the teleostean counterpart of mammalian adrenal gland, the interrenal gland, based on recent findings in the zebrafish model. The ontogeny, molecular determinants, cell migrations and tissue-tissue interactions are outlined, in order to discuss the relevance of zebrafish interrenal organ to mammalian adrenal gland.
Molecullar and Cellular Controls of Interrenal Organogenesis
FF1b marks and determines the onset of interrenal tissue.
The mode of tissue organization for teleostean interrenal organ is distinct from that of mammalian adrenal gland.Citation14 The interrenal cells intermingle with, rather than encapsulate, the chromaffin cells which are functional equivalent of the adrenal medulla, and the assembled organ is embedded at the anterior portion of the head kidney. However, the molecular determinants for specifying steroidogenic lineages have proven to be highly conserved between teleosts and mammals. Two Ftz-F1 genes, ff1b (nr5a1a) and ff1d (nr5a1b), have been identified in zebrafish to be co-orthologues of mammalian SF1/Ad4BP (NR5A1).Citation15–Citation17 While SF1 is obligatorily required for both adrenal and gonadal development, ff1b and ff1d appear to function predominantly in interrenal tissue and gonads, respectively. ff1b is the earliest molecular marker detected in the developing interrenal tissue of zebrafish embryo.Citation16 The knockdown of Ff1b by the antisense morpholino oligo approach led to specific ablation of interrenal cells, as revealed by the loss of either functional differentiation () or interrenal-specific RNA transcripts, indicating that Ff1b is absolutely required for the initiation of interrenal primordium.
Pronephros vs. interrenal tissue: Parallel morphogenetic movements under differential endothelium-derived controls.
Temporal and spatial analyses revealed that early interrenal tissues arise as bilateral clusters of non-steroidogenic cells within the pronephric primordia (), while both pronephros and interrenal tissue are derived from intermediate mesoderm.Citation4,Citation18 The interrenal tissues then migrate out of the pronephric fields, before the initiation of steroidogenesis. After separation, the interrenal and pronephric tissues stay in close proximity to one another throughout development, and both undergo central migration followed by fusion. Temporally, the convergence of bilateral interrenal primordia well precedes the central assembly of pronephros. Interestingly, both pronephros and interrenal tissue receive endothelium-derived signals, albeit via different mechanisms, as guidance cues for central migration.Citation19,Citation20
The central migration of pronephric primordia, starting at around 30 hours post-fertilization (hpf), is temporally correlated with the angiogenesis of paired glomeruli.Citation4,Citation18,Citation20 In the meantime, the developing interrenal cells are tightly associated with endothelium (). This central fusion of glomeruli is disrupted upon the perturbations of blood flow, either genetically or pharmaceutically, implying a role of hemodynamic force for glomerular morphogenesis.Citation19 Indeed, the endothelial cells (ECs) invading pronephros, by sprouting of dorsal aorta, are regulated by blood circulation to express matrix-metalloproteinase-2, which in turn mediates the assembly of bilateral glomeruli at midline. However, hemodynamic force does not contribute to the central fusion of bilateral interrenal primordia, despite the close association of pronephros and interrenal tissue in the early embryo.Citation20 In fact, the interrenal migration and fusion is normal even as axial vessel assembly or angiogenesis is severely compromised. However, interestingly, the interrenal central convergence is disrupted in the endothelium-free mutant cloche (clo; and H), and can be rescued while endothelium near interrenal area is partially restored by the forced expression of the Scl transcription factor.Citation20 This argues for a role of endothelial signaling in guiding interrenal migration, and provides one of the few pieces of evidence that beyond the vascular functions in supporting cell growth, tissue maintenance and endocrine secretion, endothelium also plays a morphogenetic role in endocrine development.
Molecular interplays within interrenal primordium.
The expression of ff1b is spatially closely associated with those of transcription factor genes wilm'stumor(wt)1 and dax1 (nr0b1).Citation18,Citation21 ff1b-expressing interrenal primordia originate within the wt1-expressing pronephric field, while wt1 expression is essential for the development of both pronephric and interrenal tissues.Citation18 Well after the specification stage, dax1 gene is transiently expressed at interrenal tissue, and is required for the expression of steroidogenic genes cyp11a and star.Citation21 While SF1 is known to interact with WT1 and DAX1 in the mammalian systems,Citation22–Citation24 it is tempting to hypothesize that similar molecular interplays might occur in the early zebrafish embryo. Alternative splicing of WT1 results in −KTS and +KTS isoforms which differ in protein properties as well as developmental roles.Citation25–Citation27 In mammals, only the −KTS form of WT1 is able to bind to and transactivate Sf1 promoter,Citation28 or to interact physically with SF1 to promote MIS expression.Citation22 Splice variants with and without the KTS tripeptide-encoding sequence have been found for both wt1a and wt1b, the two WT1 homologues identified in zebrafish.Citation29 However, the biochemical properties of these wt1 variants, in terms of their interaction with Ff1b or regulation upon ff1b promoter, have remained unclear. DAX1 is a corepressor for several nuclear receptors involved in the steroidogenic axis, notably SF1.Citation30 DAX1 negatively regulates the transcriptional activities of SF1,Citation23,Citation24 as well as antagonizing the synergistic action between SF1 and heterodimeric partners such as WT1.Citation22 While LXXLL-related motifs are necessary for the corepressing activities of DAX1,Citation31 the zebrafish and tilapia dax1 sequences lack the first and second of four LXXLL motifs seen in mammals.Citation21 It remains unclear whether these teleostean Dax1 proteins could exert similar corepressing activities as their mammalian counterparts.
The homeodomain protein Prox1 has however been confirmed as a novel Ff1b corepressor, and expressed at the developing zebrafish interrenal tissue.Citation32 Both prox1 and dax1 appear to function downstream of ff1b in interrenal development, and to be implied in steroidogenesis.Citation21,Citation32 While dax1 is only transiently expressed at around 32 hpf, prox1 expression at interrenal tissue starts from 28 hpf and persists up to three days post-fertilization (dpf). Both prox1 and dax1 morphants display reduced numbers of steroidogenic cells. Although no similar coregulation of SF-1 by mammalian Prox1 has been reported, Prox1 was interestingly found to corepress another Ftz-F1 protein Liver Receptor Homolog-1 (LRH-1; NR5A2), and thus suppress LRH-1-mediated activation of cholesterol 7-α-hydroxylase gene.Citation33,Citation34 Both mammalian and zebrafish Prox1 proteins are interacting physically with Ftz-F1s via LXXLL-related motifs. These studies indicate that the corepressor function of Prox1 upon Ftz-F1 appears to be conserved across the vertebrate species, yet diversified in terms of the profile of target genes regulated.
Interrenal steroidogenesis.
The interrenal tissue is the major site of steroidogenesis in most teleosts, as is the adrenal cortex in mammals.Citation14,Citation35 In zebrafish, many steroidogenic genes have been cloned and identified, including side chain cleavage enzyme cyp11a1 (p540scc)Citation36,Citation37 and steroidogenic acute regulatory protein (star),Citation38 both are known to mediate the rate-limiting steps of steroidogenesis. Cyp11a1 catalyzes the first step of adrenal steroidogenesis, while StAR regulates cholesterol shuttling across the mitochondrial membrane and the acute steroid production. Both cyp11a1 and star are expressed at embryonic interrenal tissue, starting from approximately 24 hpf, shortly after the specification and central convergence of interrenal primordia.Citation39 However, the functional differentiation of interrenal tissue, marked by the chromogenic detection of 3-beta-Hydroxysteroid dehydrogenase (3β-Hsd) enzymatic activity, is not initiated until about 28 hpf. The expressions of steroidogenic genes are abolished upon the knockdown of Ff1b by antisense morpholino injection, implying their direct transcriptional regulation by Ff1b protein.Citation16,Citation18 In addition, the transcripts of cyp11a and star are down-regulated at the interrenal tissue of dax1 morphant, while the enzymatic activity of 3β-Hsd is reduced in prox1 morphant.Citation21,Citation32 Collectively, the interrenal steoridogenesis in zebrafish embryo might be mediated by a combinatorial action of transcriptional regulators.
The gene expressions associated with pituitary control of interrenal steroidogenesis, including those of proopiomelanocortin (pomc)Citation40,Citation41 and acth receptor (mc2r),Citation39,Citation42 also commence early at 18 and 32 hpf respectively. In mammals, ACTH is the major pituitary regulator derived from POMC to regulate adrenal glucocorticoid synthesis and secretion. However, in contrast to the early onset of pomc and mc2r gene expressions, the hormonal control of interrenal steroidogenesis does not occur until larval stage.Citation39 In either mc2r morphant or mutants lacking pituitary corticotrophs, no perturbation of interrenal size or steroidogenesis is evident until 5 dpf, when the developing fish reaches larval stage. This indicates a developmental transition, from autonomous pituitary-independent phase, to pituitary-dependant phase of steroidogenesis and interrenal growth. These results in zebrafish are consistent with adrenal development in Pomc-null mice, where adrenal glands are normal in morphology at birth and develop adrenal hypoplasia only postnatally.Citation43,Citation44
Integration of interrenal with chromaffin cells: Mechanisms unclear.
In zebrafish, the parallel development of interrenal steroidogenic and chromaffin cells resembles organ formation of adrenal gland in mammals. Chromaffin cells, detected by the expression of Dopamine β-hydroxylase (Dβh) which converts dopamine to noradrenaline, are dispersed as several clusters in the zebrafish interrenal region at 2 dpf, which then converge to midline by 3 dpf and stay in intimate contact with steroidogenic cells throughout the subsequent stages ().Citation16,Citation39 Similar to the mammalian adrenal medulla, the chromaffin component of the interrenal organ is originated from the neural crest through sequential steps of differentiation.Citation45 In the zebrafish embryo, the trunk neural crest gives rise to dorsal root ganglion sensory neurons and autonomic sympathetic neurons. Sympathetic neurons from cervical ganglion subsequently express adrenergic differentiation markers dβh and tyrosine hydroxylase, and some migrate to the interrenal region to become endocrine chromaffin cells. In vertebrates, the specification and diversification of neural crest-derived lineages require a highly regulated network of signaling molecules and transcription factors, with the factor(s) determining the final differentiation of chromaffin cells remaining unclear.Citation46,Citation47 While reverse genetics to this end could not solve the question concerning how endocrine vs. neural sympathoadrenal cells develop, forward genetic approach using zebrafish might help to identify the unknown signal(s) that instruct migration and maturation of chromaffin cells.
Concluding Remarks
Since the identification of the ff1b gene, subsequent studies in various groups combined to demonstrate that interrenal development in the zebrafish shares many conserved molecular and developmental mechanisms with higher vertebrates. Moreover, various morphants and genetic mutants can be successfully used to manipulate molecular, cellular and hormonal signalings, for dissection of the regulatory network that directs interrenal organogenesis. The availability of specific markers for steroidogenic cells will allow chemical mutagenesis screens for the discovery of novel genes associated with interrenal disorders, such as interrenal hypoplasia. Therefore the zebrafish interrenal model is promising to provide insights on the interplay among gene, development and disease of mammalian adrenal gland, and even a platform for pharmaceutical screenings targeting adrenal functions.
Figures and Tables
Figure 1 The detection of steroidogenic interrenal tissue by whole-mount chromogenic 3β-Hsd enzymatic activity assay in wild-type control (upper panel) and ff1b antisense morpholino (ff1bMO) injected (lower panel) embryos. The embryos were treated with 0.003% phenylthiourea from 12 hpf onwards to prevent pigmentation. Dorso-lateral views of embryos at 2 dpf are shown with anterior oriented to the right. The steroidogenic interrenal cells are completely ablated in ff1b morphant. Red arrowhead indicates interrenal tissue (IR), lying above yolk sac in the mid-trunk region.
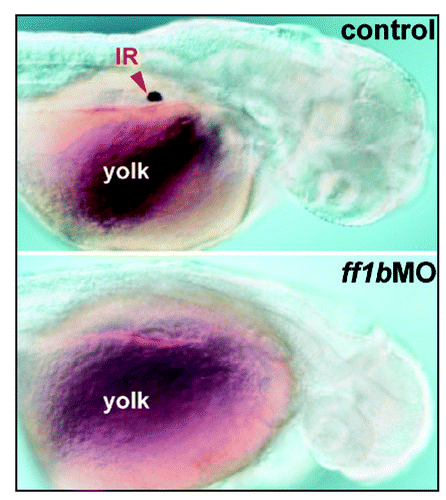
Figure 2 Early morphogenetic processes in the interrenal development of zebrafish. The parallel migrations of interrenal tissue, pronephros and chromaffin cells in this diagram are depicted based on the results of references Citation18, Citation20 and Citation39. The panels represent dorsal views of embryos, at the indicated stages, oriented with anterior to the top. Through the sequential stages of interrenal specification, migration, steroidogenesis and chromaffin cell invasion, an assembled interrenal organ is evident by 3 dpf. NC, notochord; 2S and 3S, the second and third somite, respectively.
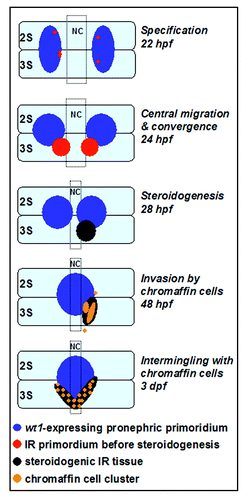
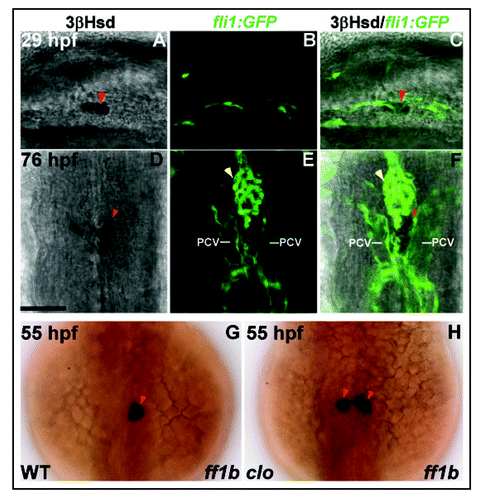
Figure 3 (A–F) Interaction of interrenal and endothelial cells as revealed in Tg(fli1:EGFP)Y1 transgenic zebrafish. Single confocal sections showing the interrenal tissues as stained by 3β-Hsd activity assay (left panel: A and D), and the neighboring ECs as labeled by the green fluorescence (middle panel: B and E), of the Tg(fli1:EGFP)Y1 embryos while staged at 29 hpf (A–C) and 76 hpf (D–F) respectively. The merged images of 3β-Hsd activity and GFP are shown in the right panel (C and F). (A–C) are lateral views with anterior oriented to the right. (D–F) are dorsal views with anterior oriented to the top. The interrenal cells are in close contact with ECs that are engaged in axial vessel assembly. PCV, posterior cardinal vein. Red arrowheads indicate interrenal tissues, while yellow arrowheads indicate kidney glomeruli. Bar, 50 µM. (G and H) ff1b expression in the clom39 mutant and its wild type sibling. Embryos of clom39 mutant (H) and its wild-type sibling (WT; G) were labeled by in situ hybridization for ff1b (dark blue) at 55 hpf. Dorsal views of embryos are shown, with anterior oriented to the top. The central migration of interrenal tissue is arrested in clom39, resulting in persistent distribution of a pair of cell clusters on either side of midline. Reproduced with permission.Citation20
Acknowledgements
The author would like to thank Dr. Woon-Khiong Chan and Dr. Chou Chai for pioneering work on zebrafish interrenal organ; Prof. Bon-chu Chung and her team for inspiring discussions. This work was supported by grants NSC94-2320-B-029-001 and NSC95-2311-B-029-008 from National Science Council (R.O.C).
References
- Grunwald DJ, Eisen JS. Headwaters of the zebrafish—Emergence of a new model vertebrate. Nat Rev Genet 2002; 3:717 - 724
- Westerfield M. The zebrafish book: Guide for the laboratory use of zebrafish (Danio rerio) 2000; 4th ed Eugene, OR Univ. of Oregon Press
- Beis D, Stainier DY. In vivo cell biology: Following the zebrafish trend. Trends Cell Biol 2006; 16:105 - 112
- Drummond I. Making a zebrafish kidney: A tale of two tubes. Trends Cell Biol 2003; 13:357 - 365
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol 2002; 248:307 - 318
- Jin SW, Beis D, Mitchell T, Chen JN, Stainier DY. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development 2005; 132:5199 - 5209
- Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 2006; 442:453 - 456
- Patton EE, Zon LI. The art and design of genetic screens: Zebrafish. Nat Rev Genet 2001; 2:956 - 966
- Shin JT, Fishman MC. From Zebrafish to human: Modular medical models. Annu Rev Genomics Hum Genet 2002; 3:311 - 340
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet 2000; 26:216 - 220
- McGonnell IM, Fowkes RC. Fishing for gene function—endocrine modelling in the zebrafish. J Endocrinol 2006; 189:425 - 439
- Nica G, Herzog W, Sonntag C, Hammerschmidt M. Zebrafish pit1 mutants lack three pituitary cell types and develop severe dwarfism. Mol Endocrinol 2004; 18:1196 - 1209
- Lin JW, Biankin AV, Horb ME, Ghosh B, Prasad NB, Yee NS, Pack MA, Leach SD. Differential requirement for ptf1a in endocrine and exocrine lineages of developing zebrafish pancreas. Dev Biol 2004; 274:491 - 503
- Grassi Milano E, Basari F, Chimenti C. Adrenocortical and adrenomedullary homologs in eight species of adult and developing teleosts: Morphology, histology, and immunohistochemistry. Gen Comp Endocrinol 1997; 108:483 - 496
- Kuo MW, Postlethwait J, Lee WC, Lou SW, Chan WK, Chung BC. Gene duplication, gene loss and evolution of expression domains in the vertebrate nuclear receptor NR5A (Ftz-F1) family. Biochem J 2005; 389:19 - 26
- Chai C, Liu YW, Chan WK. Ff1b is required for the development of steroidogenic component of the zebrafish interrenal organ. Dev Biol 2003; 260:226 - 244
- von Hofsten J, Larsson A, Olsson PE. Novel steroidogenic factor-1 homolog (ff1d) is coexpressed with anti-Mullerian hormone (AMH) in zebrafish. Dev Dyn 2005; 233:595 - 604
- Hsu HJ, Lin G, Chung BC. Parallel early development of zebrafish interrenal glands and pronephros: Differential control by wt1 and ff1b. Development 2003; 130:2107 - 2116
- Serluca FC, Drummond IA, Fishman MC. Endothelial signaling in kidney morphogenesis: A role for hemodynamic forces. Curr Biol 2002; 12:492 - 497
- Liu YW, Guo L. Endothelium is required for the promotion of interrenal morphogenetic movement during early zebrafish development. Dev Biol 2006; 297:44 - 58
- Zhao Y, Yang Z, Phelan JK, Wheeler DA, Lin S, McCabe ER. Zebrafish dax1 is required for development of the interrenal organ, the adrenal cortex equivalent. Mol Endocrinol 2006; 20:2630 - 2640
- Nachtigal MW, Hirokawa Y, Enyeart-VanHouten DL, Flanagan JN, Hammer GD, Ingraham HA. Wilms' tumor 1 and Dax-1 modulate the orphan nuclear receptor SF-1 in sex-specific gene expression. Cell 1998; 93:445 - 454
- Ito M, Yu R, Jameson JL. DAX-1 inhibits SF-1-mediated transactivation via a carboxy-terminal domain that is deleted in adrenal hypoplasia congenita. Mol Cell Biol 1997; 17:1476 - 1483
- Crawford PA, Dorn C, Sadovsky Y, Milbrandt J. Nuclear receptor DAX-1 recruits nuclear receptor corepressor N-CoR to steroidogenic factor 1. Mol Cell Biol 1998; 18:2949 - 2956
- Haber DA, Sohn RL, Buckler AJ, Pelletier J, Call KM, Housman DE. Alternative splicing and genomic structure of the Wilms tumor gene WT1. Proc Natl Acad Sci USA 1991; 88:9618 - 9622
- Englert C. WT1—more than a transcription factor?. Trends Biochem Sci 1998; 23:389 - 393
- Hammes A, Guo JK, Lutsch G, Leheste JR, Landrock D, Ziegler U, Gubler MC, Schedl A. Two splice variants of the Wilms' tumor 1 gene have distinct functions during sex determination and nephron formation. Cell 2001; 106:319 - 329
- Wilhelm D, Englert C. The Wilms tumor suppressor WT1 regulates early gonad development by activation of Sf1. Genes Dev 2002; 16:1839 - 1851
- Bollig F, Mehringer R, Perner B, Hartung C, Schafer M, Schartl M, Volff JN, Winkler C, Englert C. Identification and comparative expression analysis of a second wt1 gene in zebrafish. Dev Dyn 2006; 235:554 - 561
- Iyer AK, McCabe ER. Molecular mechanisms of DAX1 action. Mol Genet Metab 2004; 83:60 - 73
- Suzuki T, Kasahara M, Yoshioka H, Morohashi K, Umesono K. LXXLL-related motifs in Dax-1 have target specificity for the orphan nuclear receptors Ad4BP/SF-1 and LRH-1. Mol Cell Biol 2003; 23:238 - 249
- Liu YW, Gao W, The HL, Tan JH, Chan WK. Prox1 is a novel coregulator of Ff1b and is involved in the embryonic development of the zebra fish interrenal primordium. Mol Cell Biol 2003; 23:7243 - 7255
- Qin J, Gao DM, Jiang QF, Zhou Q, Kong YY, Wang Y, Xie YH. Prospero-related homeobox (Prox1) is a corepressor of human liver receptor homolog-1 and suppresses the transcription of the cholesterol 7-alpha-hydroxylase gene. Mol Endocrinol 2004; 18:2424 - 2439
- Steffensen KR, Holter E, Bavner A, Nilsson M, Pelto-Huikko M, Tomarev S, Treuter E. Functional conservation of interactions between a homeodomain cofactor and a mammalian FTZ-F1 homologue. EMBO Rep 2004; 5:613 - 619
- Keegan CE, Hammer GD. Recent insights into organogenesis of the adrenal cortex. Trends Endocrinol Metab 2002; 13:200 - 208
- Hu MC, Hsu HJ, Guo IC, Chung BC. Function of Cyp11a1 in animal models. Mol Cell Endocrinol 2004; 215:95 - 100
- Hsu HJ, Hsu NC, Hu MC, Chung BC. Steroidogenesis in zebrafish and mouse models. Mol Cell Endocrinol 2006; 248:160 - 163
- Bauer MP, Bridgham JT, Langenau DM, Johnson AL, Goetz FW. Conservation of steroidogenic acute regulatory (StAR) protein structure and expression in vertebrates. Mol Cell Endocrinol 2000; 168:119 - 125
- To TT, Hahner S, Nica G, Rohr KB, Hammerschmidt M, Winkler C, Allolio B. Pituitary-interrenal interaction in zebrafish interrenal organ development. Mol Endocrinol 2007; 21:472 - 485
- Hansen IA, To TT, Wortmann S, Burmester T, Winkler C, Meyer SR, Neuner C, Fassnacht M, Allolio B. The pro-opiomelanocortin gene of the zebrafish (Danio rerio). Biochem Biophys Res Commun 2003; 303:1121 - 1128
- Liu NA, Huang H, Yang Z, Herzog W, Hammerschmidt M, Lin S, Melmed S. Pituitary corticotroph ontogeny and regulation in transgenic zebrafish. Mol Endocrinol 2003; 17:959 - 966
- Logan DW, Bryson-Richardson RJ, Taylor MS, Currie P, Jackson IJ. Sequence characterization of teleost fish melanocortin receptors. Ann N Y Acad Sci 2003; 994:319 - 330
- Karpac J, Ostwald D, Bui S, Hunnewell P, Shankar M, Hochgeschwender U. Development, maintenance, and function of the adrenal gland in early postnatal proopiomelanocortin-null mutant mice. Endocrinology 2005; 146:2555 - 2562
- Coll AP, Challis BG, Yeo GS, Snell K, Piper SJ, Halsall D, Thresher RR, O'Rahilly S. The effects of proopiomelanocortin deficiency on murine adrenal development and responsiveness to adrenocorticotropin. Endocrinology 2004; 145:4721 - 4727
- An M, Luo R, Henion PD. Differentiation and maturation of zebrafish dorsal root and sympathetic ganglion neurons. J Comp Neurol 2002; 446:267 - 275
- Unsicker K, Huber K, Schutz G, Kalcheim C. The chromaffin cell and its development. Neurochem Res 2005; 30:921 - 925
- Huber K. The sympathoadrenal cell lineage: Specification, diversification, and new perspectives. Dev Biol 2006; 298:335 - 343