Abstract
The tissue scale deformations (≥1mm) required to form an amniote embryo are poorly understood. Here, we studied ∼400 μm-sized explant units from gastrulating quail embryos. The explants deformed in a reproducible manner when grown using a novel vitelline membrane-based culture method. Time-lapse recordings of latent embryonic motion patterns were analyzed after disk-shaped tissue explants were excised from three specific regions near the primitive streak: 1) anterolateral epiblast, 2) posterolateral epiblast, and 3) the avian organizer (Hensen's node). The explants were cultured for 8 hours—an interval equivalent to gastrulation. Both the anterolateral and the posterolateral epiblastic explants engaged in concentric radial/centrifugal tissue expansion. In sharp contrast, Hensen's node explants displayed Cartesian-like, elongated, bipolar deformations—a pattern reminiscent of axis elongation. Time-lapse analysis of explant tissue motion patterns indicated that both cellular motility and extracellular matrix fiber (tissue) remodeling take place during the observed morphogenetic deformations. As expected, treatment of tissue explants with a selective Rho-Kinase (p160ROCK) signaling inhibitor, Y27632, completely arrested all morphogenetic movements. Microsurgical experiments revealed that lateral epiblastic tissue was dispensable for the generation of an elongated midline axis— provided that an intact organizer (node) is present. Our computational analyses suggest the possibility of delineating tissue-scale morphogenetic movements at anatomically discrete locations in the embryo. Further, tissue deformation patterns, as well as the mechanical state of the tissue, require normal actomyosin function. We conclude that amniote embryos contain tissue-scale, regionalized morphogenetic motion generators, which can be assessed using our novel computational time-lapse imaging approach. These data and future studies—using explants excised from overlapping anatomical positions—will contribute to understanding the emergent tissue flow that shapes the amniote embryo.
Abbreviations
| A-P axis | = | anterior-posterior axis |
| PIV | = | particle image velocimetry |
| HH stage | = | Hamburger-Hamilton stage |
Introduction
The morphogenetic forces that shape vertebrate embryos involve both cellular-scale motility and tissue-level deformations. Investigations have shown that tissue-level events, which determine the post-gastrulation body plan by executing a series of millimeter-sized morphogenetic deformations, are prominent in warm-blooded animals.Citation1-3 Here we explore the question—To what extent, if any, are morphogenetic movements spatially regionalized at the tissue level of organization in bird embryos?
Formation of the primitive streak and Hensen's node (the avian organizer) exemplify the spatial segregation of morphogenetic movements in pre-gastrulation amniote embryos. The convergent extension movements of epiblastic cells from the caudal midline of the area pellucida are spatially localized—resulting in the formation of the incipient streak and the node.Citation4 In concert with the axially-localized convergent extension motion pattern, there is a bilaterally localized large-scale vortex-like motion pattern driven by intercalation of epiblastic cells.Citation5 These dynamic studies indicate that spatially localized movements shape the relatively uncomplicated blastodermal plate into an embryo with topologically distinct structural landmarks—a presumptive primitive streak and Hensen's node—aligned along the nascent anterior-posterior axis.
Once the primitive streak and organizer are in place, the avian embryo is molded by a series of multi-scale morphogenetic movements—most notably gastrulation.Citation6-8 In stark contrast to our knowledge of localized collective cell motion in pre-gastrulation embryos, it is unclear to what extent spatially-localized tissue motion occurs during and after gastrulation and, if so, what forces drive the motion. The close temporal coincidence of several morphogenetic events, such as establishment of right/left symmetry, axis elongation, mesendodermal invagination and head fold formation, all complicate identification of hypothetical tissue-level “compartments”—to say nothing of understanding how spatially-localized motion patterns might influence subsequent morphogenesis.
We formulated a vitelline membrane-based culture system to assess quail embryos for the presence of spatially-identifiable morphogenetic motion compartments during gastrulation. The novel approach allowed us to study “tissue-autonomous” motion patterns in explanted tissues obtained from specific regions of gastrulating quail embryos. Time-lapse analysis of explants removed from discrete anatomical positions revealed that tissue-scale motion compartments appear to exist within gastrulating amniote embryos, which conserve their motion properties in culture. Related microsurgical experiments show that midline anterior-posterior axis elongation requires the presence of nodal tissue but not tissue lateral to the primitive streak.
Results
Design of a suitable explant culture system for tissue motion analysis
Conventional 2D and 3D substrates and scaffolds were unsuitable for uncovering latent motion patterns in the explant tissue —due mainly to the fact that embryonic cells immediately emigrate and scatter. Further, suspension culture was unsuitable for time-lapse microscopy and maintaining a frame-of-reference. Early explant culture methods used epiblast tissue devoid of the vitelline membrane.Citation9 However, in our hands, removal of the vitelline membrane from the explant resulted in increased tissue adherence to the substrate and a failure to undergo tissue deformations. After various trials, we determined that excised embryonic tissues displayed robust motion patterns when cultured on a layer of vitelline membrane at the air-culture medium interface (). More importantly, the explants maintained tissue integrity, due presumably to the fact that the vitelline membrane is a natural substrate for moisture and nutrient transfer in ovo.Citation10
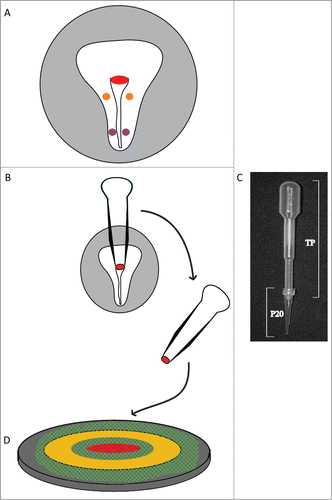
Figure 1. Diagram explaining the method used for microsurgery and tissue culture on a microscope stage of explanted HH4-stage quail embryo tissue. A) Tissue explants were derived from three A/P positions: 1) Hensen's node (red), 2) anterolateral (orange) and 3) posterolateral (purple) epiblast. The extra-embryonic area opaca is shaded in light gray. B) A modified microsurgical pipette (pictured in C) was constructed from a common polyvinyl transfer pipet (TP) and a “P20” micropipette tip. The resulting device was prefilled with embryonic PBS, the tip was embedded into the epiblastic tissue at the locations indicated, and then using gentle suction the tissue of interest was excised (Hensen's node in this illustration). D) The explant was then placed, ventral surface up, onto a piece of vitelline membrane (cross-hatched green) that was previously attached to a Whatman paper ring (yellow circle). The paper ring preparation was transferred to a hydrated bed of albumin/agar (dark gray) contained in the well of a microscope stage tissue culture plate and then subjected to time-lapse imaging.
The discoid explants from each embryo were monitored via time-lapse imaging for approximately 8 hours (HH stages 4 through 7)—an interval during which gastrulation occurs in quail embryos.Citation11 Time-lapse recording showed that explanted lateral epiblastic and Hensen's node tissues, when cultured on vitelline membranes, maintained a relatively stable 3D conformation and did not demonstrate flattening secondary to strong substrate adhesion (e.g. movie s2, supplementary information). Here, we present the results obtained using explants from a single embryo; however, similar explant culture and time-lapse analysis were performed on at least nineteen specimens (n ≥ 19). The data shown are typical of the majority of observations ().
Table 1. Summary of explant tissue behaviors observed in vitelline-membrane culture system
Regional tissue elongation and expansion in the gastrulating avian embryo
During avian gastrulation, the midline tissue occupied by the primitive streak elongates even as the streak is regressing, while at the same time mesendodermal cell invagination, at the streak, is in progress.Citation12 The anterior-posterior (A-P) axis elongation of the midline tissue (red boxed region in ) occurs as the result of cell intercalation movements.Citation13,14 (see movie s1, supplemental information).
Figure 2. Early stages of avian gastrulation are characterized by morphogenetic movements that drive axis elongation. Panels A–F depict avian gastrulation (HH stages 4-7). The embryo undergoes a remarkable series of morphogenetic changes that underlie axis elongation. The red boxed region includes the midline axial structures that undergo tissue-scale elongation movements, indicated by a progressive reduction of width and increase in length, profiles A through F. In contrast to the elongating midline, the width of the lateral tissue (green brackets) remains relatively constant during gastrulation. Tracking of a small cluster of yolk particles (black arrow) serves as a reference point, which demonstrates the relative extension of tissue along the midline compared with the lateral tissue. Supplementary movie 1 accompanies . Mag bar = 100 μm.
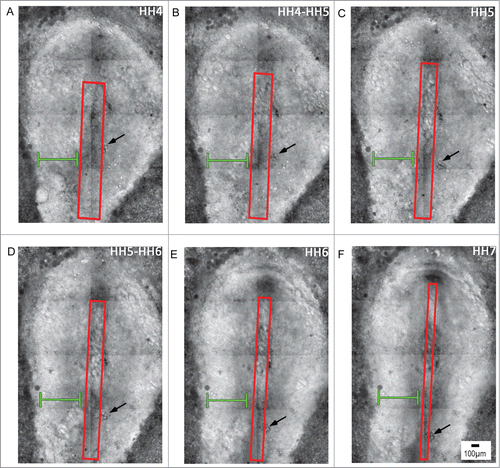
Simple inspection with a stereomicroscope shows that the tissue lateral to the primitive streak, i.e., lateral epiblast, maintains a relatively constant “width” or distance from the edge of the extraembryonic area opaca during gastrulation, as indicated by the green bar in . In contrast, the tissue at the immediate axial midline lengthens and narrows significantly in the avian gastrula (See red boxed region-of-interest, ).
Tissue length change at the embryonic midline versus stasis in lateral embryonic tissue is not due to differential rates of cell division between the two regions, which are negligible.Citation5 Instead, persistence of constant width in the lateral region versus elongation in the nearby medial region suggests that localized physical forces are deforming the embryo. We hypothesized that local or internal tissue forces (churning) drive the observed morphogenetic deformation(s). To simplify the analysis of the latent regionalized motion, we excised tissue from domains we suspected were involved in the localized motion patterns. This approach, we hypothesized, would allow a method of isolating latent morphogenetic movements of the lateral epiblast from motion patterns at the midline/primitive streak.
Avian embryonic explants continue characteristic morphogenetic movements in isolation
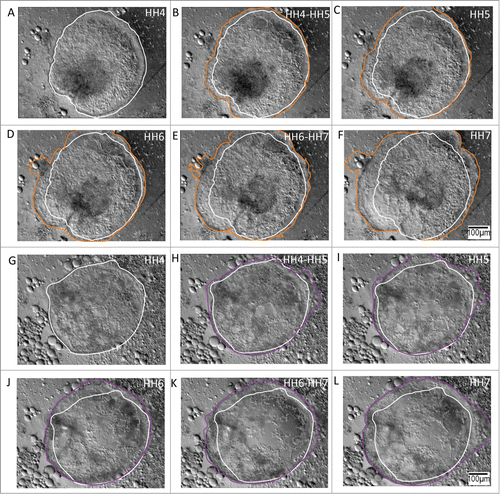
To unmask the latent morphogenetic movements from the lateral epiblast, we excised tissue from both the anterior and posterior regions 20-30 μm lateral to the streak (A). The lateral tissue excision areas were given “anterior” and “posterior” identifiers based on their relative positions in the embryo with respect to an imaginary plane passing transversely through the midpoint of the HH4 primitive streak. Once free from the embryo, the anterolateral epiblastic tissue underwent uniform concentric expansion during the time-interval corresponding to gastrulation of quail embryos in ovo (, A-F; also see movie s2, supplemental information). Similar non-polarized tissue expansion was revealed in the posterolateral epiblastic explants (, G-L; also see movie s3, supplemental information). These observations suggested that lateral epiblastic tissues, from both anterior and posterior regions, potentially function as non-polarized morphogenetic movement generators.
Figure 3. Anterolateral and posterolateral epiblastic tissue explants demonstrate unpolarized (centrifugal) expansion during morphogenesis of the avian gastrula. A – F Tissue explants from the anterolateral epiblast undergo circumferential expansion during gastrulation. The initial perimeter tracing (white) is from HH4 embryonic tissue (A), and has been retained in all the profiles to denote the initial condition of the expanding tissue border. Tracings of the expanding front (orange) through HH7 (F) demonstrate the nearly concentric topology of the anterolateral tissue, suggesting its potential as an unpolarized morphogenetic movement generator. Supplementary movie 2 accompanies , A – F. Mag bar = 100 μm. G – L Tissue explants from the posterolateral epiblast also undergo centrifugal expansion during gastrulation. The initial perimeter tracing (white) is from HH4 embryonic tissue (G) and has been highlighted in all the profiles to denote the initial state of the expanding tissue front. Tracings of the expanding edge (purple) through HH stage 7 (L) demonstrates that the posterolateral tissue, similar to the anterolateral tissue, expands nearly concentrically during the stages (HH4 – HH7) that correspond to gastrulation of the early embryo. Supplementary movie 3 accompanies , (G – L). Mag bar = 100 μm.
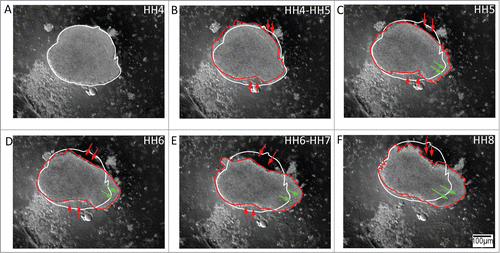
In a contrast to the uniform expansion of the lateral tissue, the Hensen's node tissue, from the embryonic midline, engaged in autonomous elongation (, A-F; also see movie s4, supplementary information). The discoid explant excised from the node typically remodeled into a bipolar elongated structure. The collective tissue motion was reminiscent of notochord elongation in the whole embryo, which is driven by convergence and extension patterns, at mutually orthogonal axes.Citation15,16 Thus, Hensen's node tissue—in isolation—demonstrated the potential to function as a polarized morphogenetic movement generator.
Figure 4. Tissue explants from Hensen's node (the avian organizer) demonstrate polarized expansion in culture, which is reminiscent of axis elongation. (A – F) The nearly circular initial perimeter (white trace) of the organizer explants from HH5 embryos fails to persist at the expanding tissue front through HH7. In contrast to anterolateral and posterolateral tissue explants, the node explants undergo a polarized extension reminiscent of notochord elongation in the intact embryo (red trace). Red and green arrows denote the convergence and extension boundaries of the tissue, respectively; thus, demonstrating that the Hensen's node tissue has the potential to act as a polarized morphogenetic movement generator. Supplementary movie 4 accompanies . Mag bar = 100 μm.
The role of extracellular matrix dynamics and cell motility in isolated morphogenetic movements
After verification that the lateral epiblastic explants and the Hensen's node explants engaged in autonomous and distinct tissue-scale morphogenetic deformations, we next examined whether extracellular matrix dynamics and cellular motility exhibit deformations or motion patterns—events that might suggest a mechanical basis for understanding the observed morphogenetic movements.
Tissue lateral to the avian primitive streak is particularly rich in extracellular matrix (ECM) fibers, notably, fibronectin.Citation17 Recent investigations in the avian gastrula have confirmed fibronectin-associated tissue deformations along the regions lateral to the primitive streak.Citation18,19 Accordingly, anterolateral and posterolateral tissue explants were obtained from fibronectin-rich locations lateral to the primitive streak. We visualized fibronectin matrix deformations using fluorescently conjugated antibodies,Citation3,Citation18 and observed that the fibronectin ECM filaments were highly dynamic during the circumferential expansion of the lateral tissue explants in culture (, A-F; also see movie s5, supplemental information). At the present level of resolution (1 μm) we were able to identify two morphologically distinct fibronectin networks within the moving tissue: 1) a relatively dense core network at the explant center, and 2) a fluid-like front of fibronectin filaments at the explant's periphery. The fibronectin deformations persisted through the duration of explant expansion in the lateral tissue. Fibronectin fibrils are not self-propelled, thus their motion pattern reflects tissue dynamics.
Figure 5. Both extracellular matrix (ECM) scaffold motion and cell autonomous motion characterize tissue-level morphogenetic movements in cultured explants. (A – F) Representative anterolateral tissue explant obtained from an embryo injected with fluorescent (Cy3) fibronectin antibody. Since fibronectin filaments are not self-propelled the fluorescence profiles demonstrate tissue dynamics as judged by passive ECM motion. In this way the anisotropic morphology of the expanding tissue could be distinguished by a relatively dense fibronectin network at the explant's core, yellow boundary trace, surrounded by a relatively sparse fluid-like boundary traced in blue. The time-lapse series demonstrates a circumferentially expanding fluid-like front, in which the peripheral expansion is accompanied by deformation of the ECM rich core region. Green arrows mark bundles of fibronectin that appear to extend between the dense ECM and sparse ECM regions. Supplementary movie 5 accompanies (A – F). Mag bar = 100 μm. (G – J) Representative Hensen's node tissue explant obtained from a transgenic quail bearing a ubiquitous H2B-mcherry nuclear marker (Lansford et al. manuscript in preparation). The time-lapse frames demonstrate collective cellular motion within the explants. The time-trace cell motility analysis (K) shows the cell populations at the circular explant periphery (blue/purple) driving the polarized tissue extension (red/orange), thus reaching the elongating ends of the explant (yellow/white) during the time period that corresponds to HH stages 4 through 8. Meanwhile, displacement vector analysis of a subpopulation of cells in the nodal explant (L) shows cellular convergence and extension motion patterns. Extension at the antero-posterior edges of the nodal tissue is particularly evident. Supplementary movie 6 accompanies (G – J). Heat-map depicts the elapsed time course. Mag bar = 100 μm.
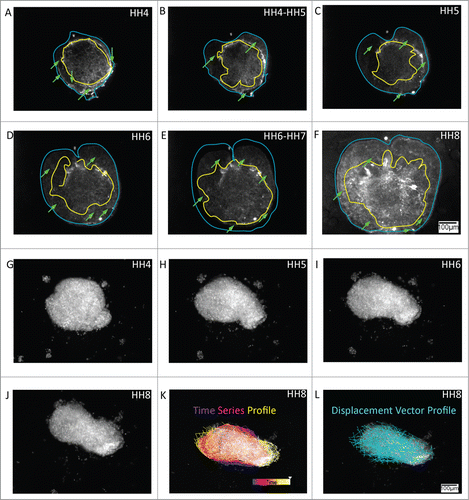
Hensen's node tissue, along the embryonic midline, is a cell-enriched region of the early avian embryo,Citation20-22 and comprises the source tissue of all midline cells in the avian embryo.Citation23 Due to the cell-dominant composition of the nodal tissue, we assessed the motility characteristics of the cells within elongating nodal explants. Explant tissues were obtained from transgenic quail embryos, Tg(pgk:H2B-ChFP)qCitation1, generated as described,Citation24 which express cherry red fluorescence in all cell nuclei (Dr. R. Lansford, Children's Hosp., LA; manuscript in preparation). Cell tracking analysis revealed that coordinated cellular motility accompanied tissue remodeling in nodal explants—as the explant morphology changed from a discoid to an elongated rod-like structure in culture (, G-J; also see movie s6, supplementary information). The autonomous tissue-scale remodeling of the organizer explant in culture was reminiscent of notochord elongation in whole intact embryos; a process thought to be driven primarily by the convergent extension motion of the midline cell clusters.Citation22 Together, the time-lapse analyses confirmed a dynamic role for both the ECM and cellular constituents in isolated morphogenetic movements of explant tissues.
Assessing the role of localized morphogenetic movements in the generation of axial structures
A remarkable characteristic of early embryos is morphogenetic self-regulation–an ability to restore the normal course of embryogenesis after tissue injury/insult.Citation5 Previous results from avian gastrulae have indicated regulation of axial form.Citation25 The identification of tissue motion domains in lateral tissue versus Hensen's node tissue suggests possible force-based mechanisms for driving morphological changes in whole gastrulae.Citation1,Citation26
To explore our hypothesis that locally-acting tissue-scale movements drive axial morphogenesis, we employed microsurgical experiments. We reasoned that mechanically decoupling one domain from the surrounding embryonic tissue offered the possibility of isolating the hypothetical variable (existence of a local mechanical force generator). Accordingly, mechanical disruption was accomplished by surgically removing a disk-shaped tissue mass from either the lateral tissue or excision of the Hensen's node (HH4). Time-lapse recording of whole embryos subjected to removal of lateral tissue showed that axial morphogenesis continued, albeit with a compromised morphology. Our results showed that anterior-posterior axis elongation proceeded even in the absence of lateral epiblast expansion (, A-C; also see movie s7, supplementary information). Axial structures formed at the embryonic anterior-posterior axis even as the lateral void (wound) created by tissue removal progressively expanded with developmental age. Thus, despite the presence of a massive wound, axis elongation continued.
Figure 6. Loss of lateral and nodal tissue regions compromises the morphogenetic movements during the generation of axial structures. A – C HH4 embryos allowed to develop after the removal of posterolateral epiblastic tissue (purple trace) generate extended axial structures (green and yellow profiles) despite significant expansion of the wounded area. Whereas, embryos deprived of nodal tissue fail to form recognizable axial structures (6D – 6F, red trace). Supplementary movies 7 & 8 accompany (A – C) and 6(D – F) respectively. Mag bar = 100 μm.
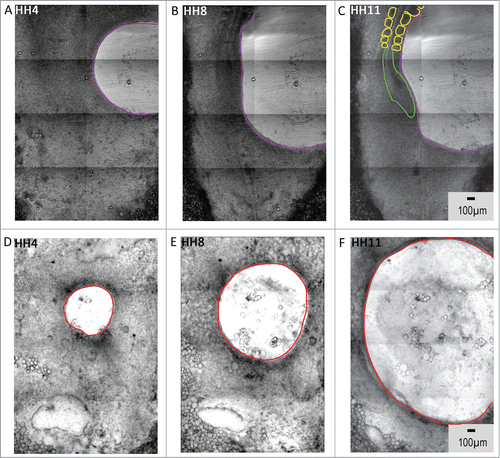
When the nodal tissue was excised, however, we observed striking malformations at the midline axis. In the absence of Hensen's node tissue, axial structures fail to form, resulting in an embryo with severe developmental anomalies (, D-F; also see movie s8, supplementary information). The area voided by nodal tissue extraction (wound) widened over time, resulting in an embryo devoid of axial and paraxial structural landmarks. Thus, in the absence of a “polarized force generator”, the tissue “void” immediately adjacent to the excised nodal tissue did not close or shrink; instead the opening expanded, and the elongation of the anterior-posterior axis was abrogated.
These microsurgical data are consistent with the hypothesis that emergent bio-mechanical force generators, acting near the primitive streak, are required for narrowing and elongation of midline axis tissue in avian embryos. These results also showed that the morphogenetic movements generated by the lateral tissue, though not required for axial elongation, per se, are nevertheless critical for maintenance of normal embryonic topology during gastrulation.
Localized morphogenetic movements in isolated tissue explants are dependent on cytoskeletal effectors of cell motility
Tissue deformation and morphogenetic changes are primarily driven by cell shape changes and cell motility.Citation27,28 The cellular-scale dynamics that underlie tissue morphogenetic changes are the direct result of cytoskeletal effector signaling.Citation29,30 The predominant effector pathway, which is conserved in multiple cell lineages, employs Rho-associated protein kinase to drive actomyosin contraction.Citation31 In an effort to confirm which cytoskeletal signaling effectors enable the motive forces driving explant movement patterns, we used an inhibitor of Rho-associated protein kinase (p160 ROCK) signaling.Citation32
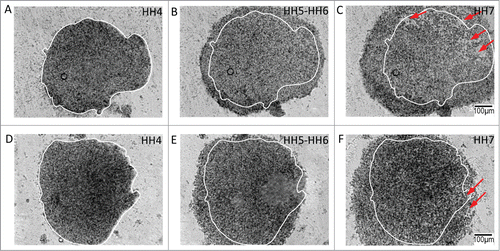
Culturing the explants in a medium containing p160 ROCK inhibitor Y27632 completely inhibited the morphogenetic motion patterns in both lateral epiblastic (, A-C; also see movie s9) and nodal explants (, D-F; also see movie s10). In the presence of the inhibitor, tissue explants failed to sustain flow at the boundary resulting in the inhibition of isolated morphogenetic tissue behavior(s) seen in untreated explants. These findings confirmed that morphogenetic deformations in both lateral and nodal explants depend, at the molecular-scale, on actin polymerization and actomyosin contraction.
Figure 7. Tissue-level kinematics of both lateral and nodal explants are dependent on intact rho-associated protein kinase (p160ROCK) signaling. Tissue explants fail to demonstrate morphogenetic movements when cultured in the presence of Y-27632, an inhibitor of ROCK signaling. The interference with actin polymerization and actomyosin contraction affects both lateral tissue and nodal explant morphogenetic movements. Circumferential expansion in the posterolateral explants (A – C, initial perimeter tracing in white) is diminished and is accompanied by a loss of tissue integrity and tearing (red arrows). The tissue expansion appears to be driven by loss of cell-cell contact and overall tissue disintegration. The treated explants from Hensen's node (D – F) fail to undergo their characteristic convergent extension movements, and also manifest a loss of tissue integrity (red arrows). Indeed, treatment of lateral and nodal explants results in termination of morphogenesis – compare the similar appearance of the final time points. Supplementary movies 9 & 10 accompany (A – C) and 7(D – F) respectively. Mag bar = 100 μm.
Tissue flow-field analysis of explants and embryos
Previous efforts to quantify cell and tissue-level motion patterns made use of particle image velocimetry (PIV).Citation33-35 The PIV algorithm is a correlation-based technique for computing velocity field maps of moving objects–in this case, collective cell and ECM filament motion.Citation3,Citation18,Citation19,Citation36 Mathematical analysis of biological motion, using PIV, allowed a rigorous, mathematically unbiased, search for underlying morphogenetic motion patterns.
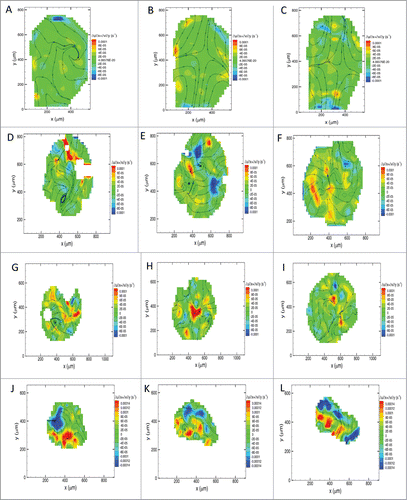
Tissue flow-field maps were calculated for both the intact embryos and the tissue explants. The PIV data were consistent with our observations of the morphogenetic motion patterns in the time-lapse recordings. In particular, axial elongation was observed as distinct patterns of localized positive divergence in the whole embryos (, A-C). We anticipated, however, that the flow patterns we observed in lateral explants would not mimic the PIV flow plots observed in intact embryos (in vivo). Our prediction was confirmed. The lateral epiblastic flow patterns observed in whole embryos were not replicated in cognate explants. However, the PIV maps did show positive divergence values and radially diverging streamlines over the majority of the periphery of both anterolateral (, D-F) and posterolateral tissue plugs in culture (, G-I).
Figure 8. Tissue flow-field analysis of morphogenetic movements in avian gastrula. Tissue flow-field analysis showing the velocity stream lines overlaid on divergence contour maps in the whole embryo at a representative time-lapse transition during the early (A), intermediate (B) and late (C) gastrula stages. Note the predominant divergent forces driving anteroposterior elongation of the embryo (B & C). Similar morphogenetic maps are shown for the anterolateral (D – F), posterolateral (G – I), and node (J – L) tissue explants. Foci of positive divergence (red, orange and yellow) propel expansion of lateral tissue at the periphery (F & I). Foci of convergence (blue) and divergence (red, orange and yellow) elongate the nodal explant (J – L). The white-out regions are areas where no reliable vectors could be computed. Zero values for divergence are indicated by dashed lines over the contour maps. Positive values for divergence on the heat maps signify locally expanding tissue flow within explants.
While not a replica of in vivo motion, these data establish that centrifugal tissue flow is observed when lateral explants are freed from the constraints of surrounding tissues. Thus, at the very least, the PIV data suggest that tissue grade centrifugal flow, in lateral explants, is not a trivial observation. It is possible that intrinsic tissue motion becomes fully unconstrained when explants are cultured in the vitelline membrane-based system— whereas if a lateral “motion compartment” (explant) had not been removed, its emergent motion patterns would have been constrained and molded by the prevailing biomechanical state of surrounding blastodermal tissue. Assuming our conjecture is correct, it follows that if left unperturbed (in situ), the tissue domain in question would have generated a latent morphogenetic force.
In the case of explanted nodal tissue, PIV analysis showed convergent extension streamlines (polarized positive divergence map), which were entirely consistent with the observed time-lapse movies of normal embryonic nodal tissue. The velocity field plots confirmed that the morphogenetic elongation movements observed in culture (, J-L) were computationally consistent with the results from whole embryos (, A-C). Thus, the motion pattern of isolated nodal tissue is a useful model of tissue flow in ovo.
In summary, our vitelline membrane-based explant tissue flow-field maps conformed to, or were consistent with, observations of the morphogenetic motion patterns that typify normal amniote embryogenesis.
Discussion
We used quail embryos to identify early morphogenetic motion patterns following the formation of the amniote primitive streak. Discoidal-shaped embryonic explants were cultured under conditions that allowed time-lapse imaging and recording of morphogenetic tissue deformations in isolation. By imaging tissues excised from various anatomical regions, we were able to analyze the degree to which specific regions of early embryos engage in patterned motion. Our results demonstrate that: a) the tissue excised from lateral regions of the embryo (bilaterally adjacent to the primitive streak) undergo radial/concentric expansion that is otherwise latent in the intact embryo, b) the tissue excised from Hensen's node undergoes an extension behavior reminiscent of axis elongation in amniotes, c) the extracellular matrix fibers and cells display highly-correlated tissue-scale morphogenetic motion patterns, d) the regionally compartmentalized morphogenetic movements appear to exert differential roles during the generation of midline axial form, and e) the tissue-scale movements depend on actin polymerization and actomyosin contraction.
Localization of morphogenetic movements in tissue explants
Our observations in whole quail embryos showed persistence of a relatively constant width of paraxial tissue (adjacent to the embryonic axis) during the time that the AP axis is narrowing and lengthening along mutually orthogonal planes. Persistence of constant paraxial width in the presence of constant axial lengthening in the gastrula (HH4-HH7) suggests the presence of a dynamic equilibrium.
In contrast to the constant width of the paraxial tissue, it is obvious that growing embryos lengthen their midline axis — a process driven by primitive streak regression and axial elongation. The boundary between the highly deformable midline tissue and its immediately adjacent paraxial epiblastic tissue layer is reminiscent of that between a moving plane (boundary) and a layer of fluid subject to viscous flow by the impulsive motion of the boundary as defined by Stokes’ first problem.Citation37 Analogous to Stokes’ first problem, the tissue-flow/elongation along the midline of gastrulae is most likely to ‘drag’ the paraxial epiblastic tissue medially, i.e., toward the midline. It is possible that the viscous ‘drag’ on the lateral tissue results as a consequence of diffusion of momentum emanating from the narrowing midline epiblast. This bilateral ‘drag’ adjacent to the streak would lead to the narrowing of mediolateral (paraxial) tissue width. However, as embryologists have known for over a century, paraxial tissue undergoes little if any narrowing, with a constant width persisting throughout gastrulation.
Persistence of “width-equilibrium” is possible only if radially-directed compensatory morphogenetic “churning” – on the part of lateral epiblastic tissue – counteracts the loss of paraxial tissue width due to midline flow. It is likely that tissues are expanding, via a ‘Goldilocks effect’, which compensates for tissue narrowing at the immediate midline axis.
Homeostasis of “lateral width equilibrium” requires just the right amount of motion providing just the right amount of “new” tissue. Too much additional tissue and the width increases; too much churning and pushing and the lateral tissue would bend or warp. Both sides of the axis must supply just the right amount of each compensatory activity—otherwise the future vertebral axis would deviate from normal. Parenthetically it is worth noting that the primary vascular cells are beginning to emerge from lateral tissue during the later developmental stages studied here (HH7-8). Subsequent formation of normal aortae and capillary networks requires “width-equilibrium” of lateral tissue.
Whatever the biomechanical mechanism, lateral tissue must not become narrower as it is lengthening. Lateral tissue must in some way compensate for the ‘narrowing’ of the midline tissue. We note that differential rates in cell division-within various epiblastic tissue regions - fail to account for the length disparities between the paraxial and midline axial tissue.Citation5 Hence, our hypothesis evoked forces driving latent morphogenetic movements within the lateral epiblast. These movements were hypothesized to be latent in the intact gastrula in the sense that subtle localized tissue motion within the lateral epiblast is masked by the proximity of relatively high magnitude morphogenetic deformations such as axis elongation and mesendodermal ingression.
Our task, therefore, was to identify latent morphogenetic movements capable of maintaining a constant width of paraxial tissue. Here we chose to explore our latent mechanism hypothesis using tissue explantation; a system that has the advantage of isolating a tissue plug from the surrounding large-scale tissue motion/forces, in situ. In order to unmask latent morphogenetic movements, we devised a culture system in which the excised tissue disks were able to deform in an autonomous manner, devoid of strong adhesion to a planar substrate or a 3D scaffold. Accordingly, the tissue explants were placed on hydrated vitelline membranes (an interface to which the embryonic tissue is naturally exposed). The rationale being that by isolating the explants from nearby movements and strong adhesive forces we could analyze inherent/emergent tissue dynamics and possibly reveal spatially localized latent morphogenetic potential. In previous work analyzing tissue deformations in avian gastrula, the donor tissues were implanted into a host embryo – a paradigm that is subject to inductive interactions and the biophysical properties of surrounding host tissue.Citation38 Our method, however, precludes such inductive interactions, thus allowing us to investigate directly whether specific embryonic tissues possess self-emergent morphogenetic motion potential.
Time-lapse recordings and image analysis revealed an unpolarized centrifugal expansion of the epiblastic tissue that could potentially maintain a constant mediolateral width of the lateral epiblast during gastrulation. Indeed, the motion of the fibronectin network was morphologically distinct within the lateral explants, self-organizing into a relatively stable dense core, with high fluorescence intensity and coarser filaments, which was juxtaposed to a more fluid-like expansion front at the periphery. Formation of a fibronectin-rich core driving ECM dynamics during in vivo tissue deformation has been demonstrated earlier in avian embryos.Citation3,Citation19 It was also noted that the fibronectin matrix network in the gastrulating embryo is also texturally distinct at tissue-scales comparable to the explant sizes used in this study.Citation17 It is reasonable to postulate that structurally and functionally distinct ECM networks characterize specific anatomical ‘hotspots’, thus providing the obligatory material properties to enable ‘morphogenetic’ tissue-scale deformations during gastrulation. One such ECM network has been previously ascribed to the formation of the primitive streak in the pre-gastrulating avian embryo.Citation19 Interestingly, the earliest experiments on chicken embryonic explant tissue performed by Spratt on ‘topographically disarranged blastoderms’ concluded the presence of a ‘growth center’ that gave rise to ‘fountain like movements’ that are reminiscent of the tissue deformations observed within lateral epiblastic explants in our study.Citation39
Our time-lapse analysis showed that the avian organizer explant, from the Hensen's node, is capable of undergoing an autonomous polarized elongation, reminiscent of axis elongation in the whole embryo. This finding suggests that Hensen's node is capable of functioning as a polarized morphogenetic movement generator. To analyze the morphogenetic potential of this cell-rich explant, we tracked nuclei from transgenic quail organizer explants. The time traces revealed the polarized motion of cells at the periphery of the explant toward the elongating fronts of the tissue. The displacement vector analysis, meanwhile, confirmed the mutually orthogonal displacement of cells from various neighborhoods within the explant, reminiscent of the convergent extension movements described for gastrulating vertebrate embryos.Citation6-8 Interestingly, the capacity for autonomous planar shaping movements in epidermal (neural plate) isolates, similar to those observed in our study, was reported to persist as late as HH6 in chick embryos.Citation40 Early studies also showed that Hensen's node maintained the capacity to generate notochord even after tissue separation in ∼35% of cases.Citation41 Collectively, our experimental biology revealed the existence of localized morphogenetic movements well after placement in culture—the observed motion patterns appeared to be driven by both ECM fiber (tissue) dynamics and individual cell motility.
The role of localized morphogenetic movements in ovo
The morphological goal of gastrulation is the creation of future mesendoderm and the generation of a longitudinal axis. To investigate the origin of gastrulation motion we conducted experiments to determine the relative importance of “regional” movements within intact embryos. Whole-mounted embryos were subjected to precise microsurgical removal of tissue from distinct anatomical sites and then recorded using time-lapse microscopy. We hypothesized that there are critical morphogenetic ‘hot spots’, which, if removed, would seriously compromise gastrulation. The idea being that loss of a ‘tissue-motion-generator’ would provide clues as to how localized morphogenetic displacement patterns are manifested during gastrulation.
Embryos with incomplete lateral epiblastic tissue proceeded through development to form axial structures, albeit with abnormal topology. This occurred, despite the progressive expansion of the ‘wound’ area (the void created by microsurgery). The continuous expansion of the tissue void suggests that the centrifugal (lateral-ward) expansion of lateral tissue is not critical for formation of axial structures, although it is necessary for their proper alignment–moreover, the data show that intact lateral tissue is required for the persistence of constant paraxial width in the specimens. In striking contrast, embryos deprived of organizer tissue showed massive developmental anomalies. Embryos with incomplete organizer tissue failed to form axial structures, suggesting the absolute requirement of the organizer. The abnormalities juxtaposed to the ‘wounded’ area expanded to include the entire embryonic plate—thus demonstrating that nodal tissue is indispensable for axial elongation movements; i.e., for gastrulation to proceed.
The inability of gastrulae to self-regulate after the removal of tissue fragments in our study is in contrast to prior studies demonstrating the ability of lateral blastoderm isolates to reconstitute axial patterning.Citation25,Citation42 It is possible that the specimens in our study were unable to regulate axial patterning due to the size of the tissue removed, which exceeded the upper size limit (0.3 mm × 0.3 mm) for regulation in HH4 gastrulae.Citation43,44 It is also possible that our nodal explants included cells in the vicinity of the primitive streak that hold the capacity to regenerate a node. It is worth noting that care was taken to avoid nicking of the outer edge of the area opaca–a procedure that reduces tension of the blastoderm and promotes healing of the opening.Citation45
From a bio-mechanistic standpoint, it is also likely that the material properties of the nodal tissue (which is a composite of three germ layers) are significantly different from those of the significantly thinner lateral blastoderm, which may contribute to the different behaviors observed in explant culture. Remarkably, experimental observations from the mouse gastrula suggest that the forces generated by radial intercalation (reminiscent of the forces in our lateral epiblastic explants) tend to oppose the tendency for convergence forces to elongate the midline tissue (reminiscent of the forces in our nodal tissue explants).Citation46 Ultimately, it is possible that the lateral width of the avian gastrula is set by striking a balance between local cell-cell cohesion forces in the lateral epiblast and global tensional forces across the entire epithelium.
Molecular drivers of local morphogenetic movements
Our explant experiments demonstrated that the morphogenetic movements observed at the local tissue-scale are dependent on intact Rho-associated protein kinase (p160ROCK) signaling. This dependency was location invariant, i.e. explants from both the lateral epiblastic tissue and the node failed to sustain cohesive morphogenetic movements in the presence of an inhibitor of ROCK signaling. Both actin polymerization and actomyosoin contraction depend on intact ROCK signaling,Citation32,Citation47 and potentially drive the motion patterns observed in explant culture. In fact, during Xenopus gastrulation, polarized morphogenetic movements are driven in a similar manner by cortical actomyosin remodeling.Citation48,49 Interestingly, the cytoskeletal forces from within the cells also influence signaling pathways that mediate localized assembly of extracellular matrix.Citation50 These results, taken together, suggest that it is possible that both the cellular and extracellular (matrix) forces/properties driving local morphogenetic movements are recruited by a common upstream signal.
Global versus local morphogenetic movements
Our explant cultures provide some clues to the anisotropy of tissue-scale morphogenesis observed in the gastrula, i.e. the maintenance of constant width in the mediolateral tissue compared to the elongation of the anteroposterior (midline) tissue. The analyses of localized morphogenetic movements in the explants revealed the presence of centrifugal (expansion) motion on the part of the lateral epiblastic tissue. We speculate that the localized centrifugal movements are the latent components of large-scale morphogenetic motion patterns spanning the amniote gastrula. It is possible that the net positive divergence in tissue flow resulting from localized circumferential expansion movements offset the otherwise narrowing mediolateral tissue. The tendency to narrow is caused by stretching due to frictional coupling with adjacent midline axial tissue as it extends. Thus, as discussed above, there is a “Goldilocks effect” such that expansion of lateral tissue compensates for narrowing of more medial tissue.
The study of morphogenetic movements in tissue explants raises an intriguing question – Are embryo tissue flows produced by the global morphogenetic movements we recorded in the explants? Our results preclude a conclusive answer to this critical question. We contend, however, that our data indicate that whole-embryo morphogenetic movement patterns are the result of multiple overlapping tissue-scale motion generators. Morphogenesis, like a hurricane, is a complex motion system and not a simple linear combination of the localized movements, which are individually isolable (i.e., explants). To investigate this biological complexity, our studies are aimed at formulating a comprehensive multi-scale morphogenetic map that includes motion pattern analysis in regions of overlapping tissue fields. Given sufficient details of tissue motion patterns in gastrulae, correlation analysis may allow more robust conclusions regarding whether large-scale morphogenetic movements are the cumulative result of regionalized tissue-scale deformations. Ideally, this problem will also require information regarding how the biophysical properties of the tissue (ECM and cells) are changing during gastrulation. Bénazéraf and colleaguesCitation20 provided evidence that an anterior-to-posterior “hardening” or increase in viscosity of pre-somitic mesoderm directly creates tissue motion fluctuations in the amniote embryo. More recently, Sehring and colleaguesCitation51 observed a correlation between discrete local contractions at the individual cell equator and pushing forces at the cell poles during notochord elongation in the simple chordate Ciona intestinalis. In other words, we propose that regionalized motion patterns, in concert with changes in tissue material properties, lead to the large-scale deformations that shape amniote embryos. The aim of our study was to begin to identify latent motion patterns present in the embryos of warm-blooded animals.
Conclusions
Our results demonstrate that motion within individual regions of the early avian embryo can be studied in isolation from the rest of the embryo. The data suggest that the large-scale morphogenetic movements, which sculpt the amniote body plan during gastrulation, may be the result of complex interactions between regionalized tissue deformations that occur throughout the embryonic plate. While these local deformations are latent in the intact embryo—we, none-the-less surmise it is exactly these latent morphogenetic movements that drive the shaping of the amniote body plan. One approach to understand the forces that shape the embryo is to identify and understand latent tissue-level motion patterns, using time-lapse analysis of explanted tissue and whole embryos.
Materials and Methods
Quail embryo preparation
Quail embryos (Coturnix coturnix japonica, Ozark Egg Co., Stover, MO) were incubated for 21h at 37°C in a humidified chamber to reach HH stage 4.Citation52 Embryos were then dissected from the egg, mounted on filter paper rings, and placed ventral side up on egg white agar culture medium.Citation53,54
Explant extraction and culture
We used Hamburger-Hamilton stage 4 (HH4) quail embryos to study the morphogenetic potential of distinct regions of the gastrulating avian embryo. Epiblastic tissue was obtained from regions lateral to the primitive streak at two locations along the AP axis. Explants from the Hensen's node were also obtained. Disk-shaped tissues approximately 300-500 μm in diameter were excised using a pipette tip. The explants were transferred onto vitelline membranes, which were harvested from stage-matched eggs and secured on paper rings. Specimens were incubated in Petri culture dishes on the microscope stage for time-lapse imaging ().
The ventral surfaces of the embryos were kept moist using embryonic phosphate buffered saline (E-PBS). A P20 pipette-tip attached to the cut end of a transfer-pipette (Samco Scientific Inc., Cat# 231, San Fernando, CA) was used to excise tissue punches from the embryos. After securing the P20 tip to the cut-end of the transfer pipette, the tip was lubricated by pipetting silicone (Sigmacote, Sigma, St. Louis, MO) and then washed three times in E-PBS to retain a thin coat of silicone in the P20 tip. The lubrication step was essential to avoid tissue adherence to the P20 tip during explant punching and transfer. After locating the region to be punched on the ventral surface of the embryo, suction on the transfer pipette (pre-filled with ∼100 μl of E-PBS) was used to extract the embryonic tissue. The tissue was transferred, by gentle pressure, to a fresh vitelline membrane (pre-washed in E-PBS to clear yolk) previously mounted on a filter paper ring and placed on a 35-mm plate. ROCK signaling inhibitor experiments were performed on explants cultured on egg white agar culture medium with 3 μM of Y27632 (Sigma, St. Louis, MO) added in the medium.Citation55
Generation of transgenic quails
Hensen's node explants from transgenic quails were imaged for cell motility analyses. The protocol for generation of transgenic quails is described in detail elsewhere.Citation56-58
In vivo immunofluorescence labeling of extracellular matrix
Monoclonal antibody directed against fibronectin (B3D6, DSHB, Iowa City, IA) was directly conjugated to AlexaFluor 555 (Molecular Probes) according to the manufacturer's instructions. The antibody was injected (5-25 nl boluses) bilaterally into the regions all along the length of the primitive streak using a PLI-100 (Harvard instruments) microinjector.Citation59 Microinjections were performed 60 min prior to the dissection of lateral epiblastic explants to allow for antibody diffusion and antigen binding.
Digital wide-field time-lapse microscopy
Embryos and tissue punches were cultured ventral side up on a semisolid mixture of agar/albumen (egg whites), modified after the method of New,Citation59,60 and incubated in a custom-built temperature- and humidity-controlled chamber mounted on a microscope stage.Citation18,Citation56,Citation61 Briefly, using custom-written software, a computer-controlled wide field (10X objective) epifluorescent microscope (Leica DMR) workstation, equipped with motorized stage and cooled digital camera (Qlmaging Retiga 1300), was used to acquire 12-bit grayscale intensity images at multiple xy locations (fields) and focal planes (z-stacks). Images were uploaded to a storage server (Xserv RAID, Apple, Inc.) for archiving and subsequent processing. To reduce a 3-D tiled image data set to a single focused plane, custom-written processing software automatically merged adjacent fields (i.e. mosaic-ing), and then collapsed the merged focal planes using either a maximum local contrast algorithm or maximum through-plane grayscale intensity (i.e. z-projection), as is often done for confocal image sets. The local contrast method was used for brightfield or DIC images, and z-projection was used for fluorescent images. The resulting collapsed frames were then registered, using relatively fixed landmarks on tissue, thus correcting for any drift in the x-y plane that may have occurred during an experiment, due to the nature of the ring culture method.
Flow-field analysis of morphogenetic movements
The images were histogram equalized in Adobe Photoshop CS5 and velocity vectors were calculated by DaVis software (LaVision). To describe the relative motion of the various regions across the explant tissue, successive images were correlated by PIV to obtain the displacement field. The size of the interrogation windows was 64 × 64 pixelsCitation2 and the vector spacing was 16 pixels (75% overlap between the windows). The in-plane velocity of various regions in the flow map at a given time instant was derived from the displacement fields.
Supplemental_Material.zip
Download Zip (29.6 MB)Acknowledgments
We would like to thank Dr. Brian R. Potetz for helpful discussions with the manuscript. We would also like to thank Mr. Alan Petersen for support with computational hardware. This work was supported by NIH HL085694 (BJR), and the G. Harold & Leila Y. Mathers Charitable Foundation (CDL, BJR).
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- Filla MB, Czirok A, Zamir EA, Little CD, Cheuvront TJ, Rongish BJ. Dynamic imaging of cell, extracellular matrix, and tissue movements during avian vertebral axis patterning. Birth defects research Part C, Embryo today : reviews 2004; 72:267-76.
- Wyczalkowski MA, Chen Z, Filas BA, Varner VD, Taber LA. Computational models for mechanics of morphogenesis. Birth defects research Part C, Embryo today : reviews 2012; 96:132-52.
- Aleksandrova A, Czirok A, Szabo A, Filla MB, Hossain MJ, Whelan PF, Lansford R, Rongish BJ. Convective tissue movements play a major role in avian endocardial morphogenesis. Developmental biology 2012; 363:348-61.
- Lawson A, Schoenwolf GC. Cell populations and morphogenetic movements underlying formation of the avian primitive streak and organizer. Genesis (New York, NY : 2000) 2001; 29:188-95.
- Voiculescu O, Bertocchini F, Wolpert L, Keller RE, Stern CD. The amniote primitive streak is defined by epithelial cell intercalation before gastrulation. Nature 2007; 449:1049-52.
- Schoenwolf GC, Smith JL. Gastrulation and early mesodermal patterning in vertebrates. Methods in molecular biology (Clifton, NJ) 2000; 135:113-25.
- Keller R, Davidson LA, Shook DR. How we are shaped: the biomechanics of gastrulation. Differentiation; research in biological diversity 2003; 71:171-205.
- Solnica-Krezel L, Sepich DS. Gastrulation: making and shaping germ layers. Annual review of cell and developmental biology 2012; 28:687-717.
- Spratt NT, Jr. Development in vitro of the early chick blastoderm explanted on yolk and albumen extract saline-agar substrata. The Journal of experimental zoology 1947; 106:345-65.
- Bellairs R, Bromham DR, Wylie CC. The influence of the area opaca on the development of the young chick embryo. Journal of embryology and experimental morphology 1967; 17:195-212.
- Cui C, Lansford R, Filla MB, Little CD, Cheuvront TJ, Rongish BJ. Electroporation and EGFP labeling of gastrulating quail embryos. Developmental dynamics : an official publication of the American Association of Anatomists 2006; 235:2802-10.
- Patten BM. The early embryology of the chick. Philadelphia: P. Blakiston's Son & Co., 1920.
- Keller R. Mechanisms of elongation in embryogenesis. Development 2006; 133:2291-302.
- Adams DS, Keller R, Koehl MA. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development 1990; 110:115-30.
- Healy KH, Schoenwolf GC, Darnell DK. Cell interactions underlying notochord induction and formation in the chick embryo. Developmental dynamics : an official publication of the American Association of Anatomists 2001; 222:165-77.
- Catala M, Teillet MA, Le Douarin NM. Organization and development of the tail bud analyzed with the quail-chick chimaera system. Mechanisms of development 1995; 51:51-65.
- Loganathan R, Potetz BR, Rongish BJ, Little CD. Spatial anisotropies and temporal fluctuations in extracellular matrix network texture during early embryogenesis. PloS one 2012; 7:e38266.
- Zamir EA, Czirok A, Cui C, Little CD, Rongish BJ. Mesodermal cell displacements during avian gastrulation are due to both individual cell-autonomous and convective tissue movements. Proceedings of the National Academy of Sciences of the United States of America 2006; 103:19806-11.
- Zamir EA, Rongish BJ, Little CD. The ECM moves during primitive streak formation–computation of ECM versus cellular motion. PLoS biology 2008; 6:e247.
- Benazeraf B, Francois P, Baker RE, Denans N, Little CD, Pourquie O. A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature 2010; 466:248-52.
- Gros J, Feistel K, Viebahn C, Blum M, Tabin CJ. Cell movements at Hensen's node establish left/right asymmetric gene expression in the chick. Science (New York, NY) 2009; 324:941-4.
- Solnica-Krezel L. Conserved patterns of cell movements during vertebrate gastrulation. Current biology : CB 2005; 15:R213-28.
- Charrier JB, Catala M, Lapointe F, Le-Douarin N, Teillet MA. Cellular dynamics and molecular control of the development of organizer-derived cells in quail-chick chimeras. The International journal of developmental biology 2005; 49:181-91.
- Sato Y, Lansford R. Transgenesis and imaging in birds, and available transgenic reporter lines. Development, growth & differentiation 2013; 55:406-21.
- Yuan S, Schoenwolf GC. Reconstitution of the organizer is both sufficient and required to re-establish a fully patterned body plan in avian embryos. Development 1999; 126:2461-73.
- Rupp PA, Filla MB, Cui C, Little CD. Chapter 5. Avian embryos a model for the study of primary vascular assembly in warm-blooded animals. Methods in enzymology 2008; 445:107-23.
- Guillot C, Lecuit T. Mechanics of epithelial tissue homeostasis and morphogenesis. Science (New York, NY) 2013; 340:1185-9.
- Holmes WR, Edelstein-Keshet L. A comparison of computational models for eukaryotic cell shape and motility. PLoS computational biology 2012; 8:e1002793.
- Levayer R, Lecuit T. Biomechanical regulation of contractility: spatial control and dynamics. Trends in cell biology 2012; 22:61-81.
- Mason FM, Martin AC. Tuning cell shape change with contractile ratchets. Current opinion in genetics & development 2011; 21:671-9.
- Hall A. Rho family GTPases. Biochemical Society transactions 2012; 40:1378-82.
- Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 2011; 472:51-6.
- Levayer R, Lecuit T. Oscillation and polarity of E-cadherin asymmetries control actomyosin flow patterns during morphogenesis. Developmental cell 2013; 26:162-75.
- Czirok A, Zamir EA, Filla MB, Little CD, Rongish BJ. Extracellular matrix macroassembly dynamics in early vertebrate embryos. Current topics in developmental biology 2006; 73:237-58.
- Zamir EA, Czirok A, Rongish BJ, Little CD. A digital image-based method for computational tissue fate mapping during early avian morphogenesis. Annals of biomedical engineering 2005; 33:854-65.
- Czirok A, Varga K, Mehes E, Szabo A. Collective cell streams in epithelial monolayers depend on cell adhesion. New journal of physics 2013; 15.
- Schlichting H GK. Boundary Layer Theory. Berlin: Springer, 2000.
- Storey KG, Selleck MA, Stern CD. Neural induction and regionalisation by different subpopulations of cells in Hensen's node. Development 1995; 121:417-28.
- Spratt NT, Jr., Haas H. Integrative mechanisms in development of the early chick blastoderm. II. Role of morphogenetic movements and regenerative growth in synthetic and topographically disarranged blastoderms. The Journal of experimental zoology 1961; 147:57-93.
- Moury JD, Schoenwolf GC. Cooperative model of epithelial shaping and bending during avian neurulation: autonomous movements of the neural plate, autonomous movements of the epidermis, and interactions in the neural plate/epidermis transition zone. Developmental dynamics : an official publication of the American Association of Anatomists 1995; 204:323-37.
- Spratt NT, Jr. Analysis of the organizer center in the early chick embryo. III. Regulative properties of the chorda and somite centers. The Journal of experimental zoology 1957; 135:319-53.
- Yuan S, Schoenwolf GC. De novo induction of the organizer and formation of the primitive streak in an experimental model of notochord reconstitution in avian embryos. Development 1998; 125:201-13.
- Grabowski CT. The induction of secondary embryos in the early chick blastoderm by grafts of Hensen's node. The American journal of anatomy 1957; 101:101-33.
- Grabowski CT. Neural induction and notochord formation by mesoderm from the node area of the early chick blastoderm. The Journal of experimental zoology 1962; 150:233-45.
- Psychoyos D, Stern CD. Restoration of the organizer after radical ablation of Hensen's node and the anterior primitive streak in the chick embryo. Development 1996; 122:3263-73.
- Yen WW, Williams M, Periasamy A, Conaway M, Burdsal C, Keller R, Lu X, Sutherland A. PTK7 is essential for polarized cell motility and convergent extension during mouse gastrulation. Development 2009; 136:2039-48.
- Kitase Y, Shuler CF. Multi-layered hypertrophied MEE formation by microtubule disruption via GEF-H1/RhoA/ROCK signaling pathway. Developmental dynamics : an official publication of the American Association of Anatomists 2012; 241:1169-82.
- Skoglund P, Rolo A, Chen X, Gumbiner BM, Keller R. Convergence and extension at gastrulation require a myosin IIB-dependent cortical actin network. Development 2008; 135:2435-44.
- Zhou J, Kim HY, Davidson LA. Actomyosin stiffens the vertebrate embryo during crucial stages of elongation and neural tube closure. Development 2009; 136:677-88.
- Skoglund P, Keller R. Integration of planar cell polarity and ECM signaling in elongation of the vertebrate body plan. Current opinion in cell biology 2010; 22:589-96.
- Sehring IM, Dong B, Denker E, Bhattachan P, Deng W, Mathiesen BT, Jiang D. An Equatorial Contractile Mechanism Drives Cell Elongation but not Cell Division. PLoS biology 2014; 12:e1001781.
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Developmental dynamics : an official publication of the American Association of Anatomists 1992; 195:231-72.
- Drake CJ, Davis LA, Little CD. Antibodies to beta 1-integrins cause alterations of aortic vasculogenesis, in vivo. Developmental dynamics : an official publication of the American Association of Anatomists 1992; 193:83-91.
- Streit A. EC culture: a method to culture early chick embryos. Methods in molecular biology (Clifton, NJ) 2008; 461:255-64.
- Svoboda KK, Moessner P, Field T, Acevedo J. ROCK inhibitor (Y27632) increases apoptosis and disrupts the actin cortical mat in embryonic avian corneal epithelium. Developmental dynamics : an official publication of the American Association of Anatomists 2004; 229:579-90.
- Sato Y, Poynter G, Huss D, Filla MB, Czirok A, Rongish BJ, Little CD, Fraser SE, Lansford R. Dynamic analysis of vascular morphogenesis using transgenic quail embryos. PloS one 2010; 5:e12674.
- Poynter G, Huss D, Lansford R. Japanese quail: an efficient animal model for the production of transgenic avians. Cold Spring Harbor protocols 2009; 2009:pdb emo112.
- Poynter G, Lansford R. Generating transgenic quail using lentiviruses. Methods in cell biology 2008; 87:281-93.
- Little CD, Drake CJ. Whole-mount immunolabeling of embryos by microinjection. Increased detection levels of extracellular and cell surface epitopes. Methods in molecular biology (Clifton, NJ) 2000; 135:183-9.
- Chapman SC, Collignon J, Schoenwolf GC, Lumsden A. Improved method for chick whole-embryo culture using a filter paper carrier. Developmental dynamics : an official publication of the American Association of Anatomists 2001; 220:284-9.
- Czirok A, Rupp PA, Rongish BJ, Little CD. Multi-field 3D scanning light microscopy of early embryogenesis. Journal of microscopy 2002; 206:209-17.
