Abstract
The Wnt gene family, which encodes secreted growth and differentiation factors, has been implicated in kidney organogenesis. The Wnts control both ureteric bud development and signalling, but they also serve as inductive factors to regulate nephrogenesis in the mesenchcymal cells. Several of the Wnt genes are expressed in the developing kidney, and gene knock-out studies have revealed specific developmental functions for these. Consistent with this, changes in Wnt ligands and pathway components are associated with many kidney diseases, including kidney cancers, renal fibrosis, cystic kidney diseases, acute renal failure, diabetic nephropathy and ischaemic injury. It is these associations of the Wnt signalling system with kidney development and kidney diseases that form to topic of this review.
Introduction
The permanent mammalian kidney, or metanephros, is a typical organ that develops on the basic of interactions between epithelial and mesenchymal tissues. Kidney development initiates when the epithelial Wolffian duct generates the ureteric bud at its extreme caudal end. The ureteric duct then grows into the predetermined kidney mesenchyme, starts to branch and induces nephrogenesis in a sequential manner in the metanephric blastema, leading via mesenchymal epithelial transition and simple morphogenesis steps to formation of the nephrons. Following these processes the endothelial cells are required for glomerulogenesis in order to form a functional nephron.Citation1
Several studies have revealed a critical role for the Wnt family of secreted growth and differentiation factors in kidney development. Many of the Wnts are expressed in the specific tissue compartment during kidney ontogeny, and Wnt knock-outs performed on gene-targeted embryonic stem (ES) cell-derived embryos have revealed critical roles for the Wnts in certain developmental steps. Consequently, the Wnt pathway is expected to be involved in many kidney diseases.
The Wnt Signaling Pathway
Wnts are thought to signal via at least three distinct pathways. The canonical pathway involves the critical cytoplasmic protein β-catenin.Citation2 In the absence of Frizzled (Fz) receptor binding of a Wnt, cytoplasmic protein β-catenin is phosphorylated by glycogen synthase kinase 3 (GSK3) and degraded by a destruction complex composed of the adenomatous polyposis coli (APC) protein and axin. The most typical Wnt signaling occurs when a Wnt binds to a Fz and a low density lipoprotein (LRP) co-receptor, leading to the phosphorylation of dishevelled (dvl) protein in the cytoplasm. The activated dvl binds to axin in order to antagonize the action of GSK3β, thus preventing phosphorylation and ubiquitination of β-catenin. This causes unphosphorylated β-catenin to accumulate in the cytoplasm and translocate to the nucleus along with the TCF/LEF family of HMG-domain transcription factors, thus regulating the Wnt target genes. The biochemistry of the non-canonical Wnt signaling pathways is less clear at present (see the Wnt web page www.stanford.edu/∼rnusse/wntwindow.html).Citation2,Citation3
Wnt Expression and Function During Kidney Organogenesis
A number of Wnt family members are expressed in the embryonic kidney (for a more detailed discussion of their expression and function, see a recent review by Carroll et al., 2007).Citation4 Observations made so far suggest that Wnt-2b, -4, -5b, -6, -7b, -9b and -11 are expressed during kidney ontogeny.Citation5–Citation9 Wnt-6, -7b, -9b and 11 are all expressed in the branching ureteric bud during the early stages of organogenesis, while Wnt-2b and -4 are transcribed in the kidney mesenchymal cells.Citation5,Citation6 In vivo gene inactivation studies with ES cells have revealed the roles for Wnt-4, 9b and 11. The Wnts expressed in the ureteric bud may have some redundant roles, while Wnt-4 appears to be the only one of the currently characterized Wnts that is expressed in the nephron precursor cells, being important for their development to nephrons.Citation6 Of the epithelial Wnts, Wnt-11 is involved in the control of ureteric bud branching,Citation8 while Wnt-9b signaling is implicated as a ureteric bud-derived inducer of nephrogenesis.Citation9
Role of Wnt Signaling in Kidney Cancers
Given the similarities between embryonic growth control, dysregulated cell proliferation in development of cancer and the findings that Wnt signaling regulates kidney organogenesis, the Wnts were candidates for involvement in the development of kidney cancers. The adult kidney cancers, referred to collectively as renal cell carcinoma (RCC), account for around 3% of the malignancies observed in humans (reviewed in ref. Citation10). Metastases are typically observed in 25–30% of the RCC patients, 75% of these being found in the lungs or lymph nodes (36%). The survival rate for patients with metastases is close to 48% one year after diagnosis but only 9% after five years (reviewed in ref. Citation10). Because of our rather poor understanding of the promoting molecular factors, surgery is still the most efficient treatment for RCC.
Several studies have raised the possibility that the Wnt signaling pathway may be implicated in RCC. One link may be provided by hypoxia-inducible protein-2 (HIG2), a recently identified marker of RCC detected early in its onset, especially in the case of clear cell RCC and papillary cell carcinomas.Citation11 A gain of HIG2 function stimulates cell proliferation, which suggests an oncogenic potential for this molecule. HIG2 binds to the extracellular domain of the Wnt receptor Fzd10, leading to induction of certain genes typically associated with enhanced Wnt signaling.Citation11 In addition to this, the β-catenin/Tcf4 complex binds to the promoter of the HIG2 gene, which suggests that the HIG2 itself may be a target of Wnt-mediated regulation. Given these associations, it has been speculated that a humanized anti-HIG2 monoclonal antibody may provide a potential therapy for RCC via the modulation of Wnt signaling.Citation11
Apart from the indirect association of Wnt signaling with RCC, changes in the Wnt Frizzled receptors have also been implicated in this form of cancer, since Fzd5 and Fzd8 gene expression is increased, possibly promoting susceptibility of the RCC cells to the Wnt ligands expressed by them.Citation12 Changes in Wnt signaling may also occur via lowered expression of the secreted antagonists of Wnt ligands, such as the frizzled-related proteins (FRPs). The levels of secretion of frizzled-related protein 1 (sFRP1) are indeed reduced in RCC.Citation13
This reduced sFRP1 expression correlates especially well with the clear renal cell carcinoma (cRCC) type of RCC,Citation14 and levels of sFRP1 mRNA have been found to be reduced in human cRCC samples taken at different stages of the disease. The reduction in sFRP1 is still more striking at the protein level, since sFRP1 protein was lost in more than 70% of the samples analyzed, this not being the case in normal samples.Citation15 The reason for the reduction in sFRP1 expression may lie in epigenetic changes in the sFRP2 gene, since the promoter of sFRP1 was methylated in 8/10 of the patients' samples analyzed in the study, apparently inactivating the sFRP-1 gene.Citation15 Consistent with the reduction in sFRP-1 expression, the typical Wnt target genes are in turn upregulated in these cRCCs, including cyclin D1, c-myc, VEGF and fibronectin, which were also reduced in the presence of excess sFRP1. The function of sFRP-1 may normally be to inhibit cell proliferation, but reduced sFRP-1 expression may result in enhanced cell proliferation.Citation15 It is also striking that injected sFRP1 significantly reduces tumor size in the mouse model for RCC and can even abolish the tumors completely in some cases. These findings together suggest that changes in Wnt antagonists are critical for the development of RCC.
In addition to the factors that modulate Wnt signaling at the receptor level, the cytoplasmic Wnt pathway components may also be involved in the development of RCC. This suggestion is based on the observations that compound loss-of-function mutants of p53 and Apc lead to related phenotypes typical of RCC.Citation16 The kidney cells of Ah Cre+ Apcfl/flp53-/- mice, for example, had more pronounced nuclear staining for β-catenin than controls, suggesting promoted canonical Wnt signaling. Hence loss of synergy between p53 and the Wnt signaling component Apc protein appears to be connected with the development of RCC in the rodent model.Citation16
In addition to the role of the Wnts in the positive control of cell proliferation in RCC, Wnt signaling may contribute to the development of the disease by influencing apoptosis.Citation17 These properties of the Wnt pathway may be mediated in part by the splicing isoforms of the TCF, since the lack of exon 15 in one human TCF is associated with the probability of survival in RCC patients. These splice forms may function by controlling the expression of proteins involved in apoptosis. The presence of the specific isoforms of TCF in RCC, for example, is associated with reduced levels of expression of the anti-apoptotic factors Bcl-2 and Bcl-xL and the pro-apoptotic factor Bak.Citation17 These findings should be modelled in mice in order to obtain a better view of the roles of specific isoforms of TCFs in the generation of RCC. Collectively, the findings of various studies have implicated that changes in the Wnt signaling pathway are associated with the development of RCCs at several levels.
Significance of the Wnt Signaling Pathway for the Development of Wilms' Tumor
There is conclusive evidence that Wnt signaling not only influences kidney cancer development in the adult but is also involved in the prognosis for the foetal kidney tumors, namely Wilms' tumor. Also known as nephroplastoma, this is a cancer that is typically observed in children, 80% of diagnosed patients being younger than five years (reviewed in ref. Citation18). Like RCCs, Wilms' tumor can generate metastases to the lungs (85%), lymph nodes and liver. The Wilms' tumor protein (WT) I mutations lying behind some Wilms' tumor cases can lead to other diseases such as the WAGR and Drash syndromes, while a mutation in another WT protein, WT2, lies behind the Beckwith-Wiedemann syndrome (reviewed in ref. Citation18). Even though the role of WT proteins in Wilms' tumor is well established, the mutations in these do not explain the whole array of observed kidney tumor cases, and additional mutations, e.g., in chromosomes 1 and 12, are likely to be involved (reviewed in ref. Citation18).
The association of the Wnt pathway with Wilms' tumors has become clear in recent years. Typically mutations that change the coding region for β-catenin have been detected,Citation19 and a mutation in the β-catenin gene leading to stabilization of the protein is a typical finding in 75% of the Wilms' tumor cases analyzed involving a WT1 mutation.Citation20 Associations with the human CTNNB1 gene mutations that also encode β-catenin and with WT1 mutations also lie behind rhabdomyogenesis.Citation21 Since β-catenin mutations are typically associated with Wilms' tumors, screening for these mutations could lead to an earlier diagnosis in Wilms' tumor patients.Citation22
In addition to mutations in the WT proteins, the “Wilms' tumor gene in the X chromosome” (WTX) undergoes mutation in approximately 30% of Wilms' tumors.Citation23 Mutations in WTX, including ones in the WT-I and β-catenin genes, are typically detected in sporadic Wilms' tumors.Citation23 The familiar Wilms' tumor mutation represents only 1% of cases.
The function of the WTX protein is also connected with the canonical Wnt β-catenin signaling.Citation24 In the HEK293T cells, for example, WTX interacts both with the wild-type of β-catenin and with the stabilized form, and it can negatively regulate β-catenin, apparently by inducing its degradation.Citation24
Wilms' tumorigenesis may also involve enhanced expression of the Wnt ligands, since the WT-1 protein acts as a transcription factor connected with the regulation of Wnt-4 gene expression.Citation25 Out of the four splicing isoforms that are expressed during mouse kidney development,Citation26 the most common mutations associated with the Denys-Drash syndrome occur in the zinc-finger region of the WT1-C isoform protein, affecting DNA binding activity. This mutant WT1-C interacts with the normal WT1 sites in a dominant-negative fashionCitation27 and reduces the expression of the Wnt-4 gene in model cells.Citation25 The mode of action of WT-I in controlling the Wnt-4 gene, given that both are critical regulators of nephrogenesis during embryogenesis,Citation28 remains to be analyzed in detail.
Association of the Wnt Signaling Pathway with Invasive Behavior of Renal Cells
The association of Wnt signals with ureteric bud branching during kidney development represents a situation in which an epithelial tissue is invading the mesenchymal cells as they are transforming to epithelial cells.Citation29 Given these associations, the Wnt signaling pathway may also play a role in invasion by renal cancer cells, which is critical for the development of their metastasis potential. Indeed, overexpression of dishevelled-2 (Dvl-2) and axin having only the GSK-3β binding domain increases transcription of the LEF/TCF reporter in kidney- associated transformed model cells, as expected. Interestingly, when these cells were manipulated with respect to Wnt signaling by means of GSK-3β inhibitors this promoted their invasiveness with respect to the type 1 collagen matrix in a dose-dependent manner. The ligand that may regulate cell invasiveness is Wnt-2. This suggestion is based on the findings that the HEK-293T cells that had been engineered to express the Wnt-2 protein invaded the type 1 collagen matrix more efficiently than did the controls. Cell invasiveness probably involves GSK function, since RNAi against GSK-3β almost completely prevented such Wnt-2-induced invasion.Citation30 Taken together, these observations raise the possibility that the Wnt signaling pathway may be connected with the invasive behavior of transformed cells, but precisely defined in vivo kidney cancer disease models are required in order to address this possibility in more detail.
Wnt Signaling in Kidney Diseases Involving Cystogenesis
There are indications that deregulated Wnt signaling is connected with the development of cystic kidney diseases. When a patient has a frame shift mutation in the gene for the Wnt pathway component TCF2 this will be associated with enlarged kidneys displaying cysts, reduced renal function and pancreatic hypoplasia that can already be diagnosed in utero.Citation31 These phenotypes are connected with elevated β-catenin expression, suggesting that the TCF2 frame shift mutation leads to enhanced Wnt signaling that contributes to the disease phenotypes. The mechanism that may promote cystogenesis in the kidney at the cellular level may involve not only changes in the canonical Wnt signaling but also alterations in cell adhesion and planar cell polarity in the collecting duct, which can be regulated by Wnt signaling as well in some contexts.Citation31
Some other cytoplasmic Wnt pathway components as well as TCF have been implicated in the generation of cysts in the kidney. Ksp-Cre; Apc580S/580S mice, in which Apc function is conditionally inactivated in the collecting duct epithelium, frequently die, probably due to kidney dysfunction, while some surviving Apc knock-out mice have been reported to have cysts, nephroblastoma and renal adenoma.Citation32 The cysts may originate from some portion of the nephron or collecting duct, but this remains to be defined in detail.
Cystogenesis has also been induced by the expression of dominant- positive β-catenin in the kidney,Citation33 in which case it is connected with high levels of ΔN131-β-catenin expression, as lower levels do not induce cystogenesis in the same way.Citation33 The renal cysts in this mouse model may originate from either the glomerulus, the tubules of the nephron or the collecting duct, but this remains to be analyzed by in vivo fate mapping approaches.
Changes in Wnt signaling may not necessarily be connected only with polycystic kidney disease, as studies with mouse models are also starting to reveal that this pathway may contribute to renal medullary cystic dysplasias as well. This idea is based on the finding that the level of β-catenin expression is increased in a related mouse model. It has been speculated that such changes in the Wnt signaling pathway may be connected with changes in bone morphogenetic protein (BMP) signaling, since β-catenin can form a complex with the BMP pathway component SMAD1, so that these may perhaps be jointly involved.Citation34 Taken together, the mouse disease models of renal cystogenesis that have been generated so far are expected to serve as useful tools for addressing the detailed mechanisms by which changes in Wnt signaling contribute to the generation of cystic diseases of the kidney, perhaps providing new avenues for developing novel therapies.
Connection of Wnt Signaling with Renal Fibrosis
The formation of fibrotic lesions in the kidney involves distinct transition points that are well characterized at the cellular level, including the epithelial-to-mesenchymal transition (EMT) (reviewed in ref. Citation35), which is likely to bear a close similarity to the process of mesenchymal-to-epithelial transition (MET) that takes place during normal kidney development and is regulated by Wnt signaling, namely Wnt-4.Citation28,Citation36 There is some circumstantial evidence that the Wnt pathway is indeed connected with renal fibrosis, based on the finding of nuclear accumulation of β-catenin during induction of the EMT in association with induced fibrosis response.Citation37
Additional observations suggest roles for Wnt signaling in renal fibrosis. Besides β-catenin, expression of the Wnt inhibitors Dkk1 and sFRP is increased in an animal model of renal fibrosis in which the kidney has a unilateral obstruction.Citation38 More specifically, sFRP4 accumulates in the perivasculature of the kidney but in spite of this the total amount of protein in the affected kidney decreases. This may be due to the reduced potential of the damaged kidney cells for utilizing the circulating sFRP4.Citation38 Interestingly, when recombinant sFRP4 was injected into the kidney it transiently downregulated the β-catenin and fibronectin levels and also reduced the number of myofibroblasts associated with the development of renal fibrosis, the latter suggesting that secreted Wnt inhibitors may suppress the development of an induced fibrosis response. Other studies involving chemically induced renal fibrosis have suggested further roles for Wnt-4 in the fibrotic disease process.Citation39–Citation41 The potential beneficial roles of Wnt-4 in experimental fibrosis may be connected with the functions of matrilysin.Citation41 Collectively, inhibition of the action of the Wnt pathway may contribute to renal fibrosis, perhaps by controlling the EMT.Citation38 Whether these associations are significant would need to be verified in studies with conditional knock-out mouse models.
Renal Wnt Signaling in Acute Renal Failure and Diabetes
Ischaemia can lead to severe acute renal failure, and it is important to develop new ways to manage this condition, including exploitation of the induced cell proliferation connected with the regenerative response to ischaemia. Wnt-4 expression has been shown in a rat model to be increased after ischaemia, to co-localize with aquaporin-1 expression in the proximal tubules and to be downregulated after perfusion. The action of Wnt-4 may be to promote cell proliferation, perhaps via the control of cyclin D1 in the tubular cells, in order to promote recovery from ischaemia.Citation42
In addition to the roles of the Wnt-4 signal, β-catenin and TCF/LEF-1 seem to be associated with recovery from sub-lethal renal ischaemic injury, since both β-catenin and TCF proteins transiently accumulate in the nucleus in response to ischaemia and the action of catenins (α and β) and E-cadherin may concurrently be translocated from the basolateral cellular location to be connected more closely with the cytoskeleton and may involve Wnt signaling.Citation43
A few studies have started to raise the possibility that changes in Wnt signaling may also be connected with diabetic nephropathy, a condition that severely compromises kidney function. Cultured mesangial cells exposed to high glucose become apoptotic, thus proliferating less in association with lower Wnt-1, -3a, -4 and -5a expression. This glucose-induced apoptosis can be reduced by overexpression of Wnt-4 or -5a in association with increased β-catenin stability.Citation44 Collectively, the available evidence raises the possibility that Wnt signaling may be associated with both acute experimental ischaemia and diabetic nephropathy, but more precisely defined functional studies with conditional knock-out or gain-of-function mouse models will certainly be needed in order to reveal these possibilities in more detail.
The research carried out so far is starting to suggest that changes in Wnt signaling are connected with the development of several severe kidney diseases. Following the discovery that Wnt signals play a critical role in controlling the tubular ureteric bud and nephron differentiation processes, the pathway has been a candidate for association with the prognosis for kidney diseases. Our current sophisticated functional genomic mouse technologies mean that these associations can be now studied in detail to reveal the causal mechanisms behind the disease process. The discovery that the Wnt signaling pathway is connected with the development of kidney diseases should provide ways of generating new therapies for these kidney diseases in the future.
Abbreviations
| ADPKD | = | autosomal dominant polycystic kidney disease |
| ARPKD | = | autosomal recessive polycystic kidney disease |
| ALK3 | = | activin-like kinase 3 |
| Apc | = | adenomatous polyposis coli |
| Bmp | = | bone morphogenic protein |
| CTNNB1 | = | gene encoding β-catenin |
| DDS | = | denysdrash syndrome |
| Dkk | = | dickkopf |
| FZD | = | frizzled |
| GAD1 | = | glutamic acid decarboxylase |
| GSK-3β | = | glycogen synthase kinase |
| HEK | = | human embryonic kidney cells |
| HGKD | = | hypoplastic glomerulocystic kidney disease |
| HIG-2 | = | hypoxia-inducible protein-2 |
| HNF-1β | = | hepatocyte nuclear factor |
| jck | = | juvenile cystic kidneys |
| MADH | = | methylamine dehydrogenase |
| MDCK | = | madin-darby canine kidney epithelial cells |
| MMP | = | matrilysin, matrix metalloproteinase |
| sFRP | = | secreted frizzled-related protein |
| TCF | = | T-cell factor |
| UUO | = | unilateral ureteric obstruction |
| WAGR syndrome | = | wilms' tumor, aniridia, genitourinary abnormalities, mental retardation |
| WTX | = | wilms' tumor gene in the X chromosome |
Figures and Tables
Figure 1 Schematic representation of the expression pattern of the Wnt family members in the embryonic kidney. The Wnt-6, -7b, -9b and -11 genes are expressed in the epithelial ureteric bud that will form the future collecting duct of the kidney. Of these, Wnt-9b serves as a signal mediating reciprocal inductive signalling from the ureteric bud to the kidney mesenchyme to trigger nephrogenesis, while Wnt-11 controls ureteric bud branching morphogenesis to create the collecting duct tree. Wnt-4 is the only Wnt family member that has so far been found to be expressed in the pretubular mesenchymal cells. Wnt-4 is essential for controlling nephrogenesis. Wnt-2b gene is transiently expressed in the perinephric mesenchyme and may control ureteric branching from the mesenchymal periphery.Citation5
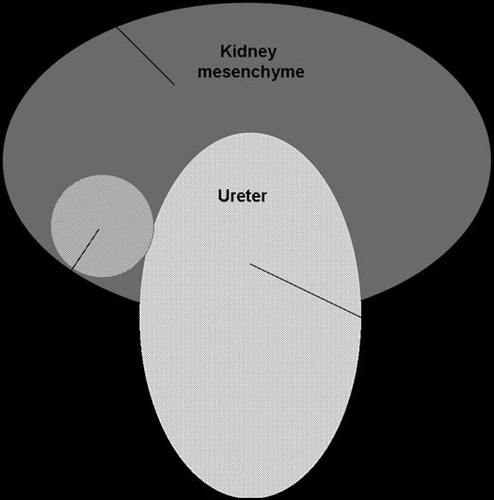
Table 1 Association of the Wnt pathway components with kidney diseases
References
- Saxén L. Organogenesis of the kidney 1987; Cambridge Cambridge University Press
- Logan CY, Nusse R. The wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004; 20:781 - 810
- Sharpe C, Lawrence N, Martinez Arias A. Wnt signaling: A theme with nuclear variations. Bioessays 2001; 23:311 - 318
- Merkel CE, Karner CM, Carrol TJ. Molecular regulation of kidney development: is the answer blowing in the Wnt?. Pediatr Nephrol 2007; 22:1825 - 1838
- Lin Y, Liu A, Zhang S, Ruusunen T, Kreidberg JA, Peltoketo H, Drummond I, Vainio S. Induction of ureter branching as a response to wnt-2b signaling during early kidney organogenesis. Dev Dyn 2001; 222:26 - 39
- Kispert A, Vainio S, McMahon AP. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development 1998; 125:4225 - 4234
- Itaranta P, Lin Y, Perasaari J, Roel G, Destree O, Vainio S. Wnt-6 is expressed in the ureter bud and induces kidney tubule development in vitro. Genesis 2002; 32:259 - 268
- Kispert A, Vainio S, Shen L, Rowitch DH, McMahon AP. Proteoglycans are required for maintenance of wnt-11 expression in the ureter tips. Development 1996; 122:3627 - 3637
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 2005; 9:283 - 292
- Sadler GJ, Anderson MR, Moss MS, Wilson PG. Metastases from renal cell carcinoma presenting as gastrointestinal bleeding: Two case reports and a review of the literature. BMC Gastroenterol 2007; 7:4
- Togashi A, Katagiri T, Ashida S, Fujioka T, Maruyama O, Wakumoto Y, Sakamoto Y, Fujime M, Kawachi Y, Shuin T, Nakamura Y. Hypoxia-inducible protein 2 (HIG2), a novel diagnostic marker for renal cell carcinoma and potential target for molecular therapy. Cancer Res 2005; 65:4817 - 4826
- Janssens N, Andries L, Janicot M, Perera T, Bakker A. Alteration of frizzled expression in renal cell carcinoma. Tumor Biol 2004; 25:161 - 171
- Dahl E, Wiesmann F, Woenckhaus M, Stoehr R, Wild PJ, Veeck J, Knuchel R, Klopocki E, Sauter G, Simon R, Wieland WF, Walter B, Denzinger S, Hartmann A, Hammerschmied CG. Frequent loss of SFRP1 expression in multiple human solid tumors: Association with aberrant promoter methylation in renal cell carcinoma. Oncogene 2007; 26:5680 - 5691
- Guo A, Salomoni P, Luo J, Shih A, Zhong S, Gu W, Pandolfi PP. The function of PML in p53-dependent apoptosis. Nat Cell Biol 2000; 2:730 - 736
- Gumz ML, Zou H, Kreinest PA, Childs AC, Belmonte LS, LeGrand SN, Wu KJ, Luxon BA, Sinha M, Parker AS, Sun LZ, Ahlquist DA, Wood CG, Copland JA. Secreted frizzled-related protein 1 loss contributes to tumor phenotype of clear cell renal cell carcinoma. Clin Cancer Res 2007; 13:4740 - 4749
- Sansom OJ, Griffiths DF, Reed KR, Winton DJ, Clarke AR. Apc deficiency predisposes to renal carcinoma in the mouse. Oncogene 2005; 24:8205 - 8210
- Shiina H, Igawa M, Breault J, Ribeiro-Filho L, Pookot D, Urakami S, Terashima M, Deguchi M, Yamanaka M, Shirai M, Kaneuchi M, Kane CJ, Dahiya R. The human T-cell factor-4 gene splicing isoforms, Wnt signal pathway, and apoptosis in renal cell carcinoma. Clin Cancer Res 2003; 9:2121 - 2132
- Lowe LH, Isuani BH, Heller RM, Stein SM, Johnson JE, Navarro OM, Hernanz-Schulman M. Pediatric renal masses: Wilms tumor and beyond. Radiographics 2000; 20:1585 - 1603
- Koesters R, Ridder R, Kopp-Schneider A, Betts D, Adams V, Niggli F, Briner J, von Knebel Doeberitz M. Mutational activation of the beta-catenin proto-oncogene is a common event in the development of wilms' tumors. Cancer Res 1999; 59:3880 - 3882
- Li CM, Kim CE, Margolin AA, Guo M, Zhu J, Mason JM, Hensle TW, Murty VV, Grundy PE, Fearon ER, D'Agati V, Licht JD, Tycko B. CTNNB1 mutations and overexpression of Wnt/beta-catenin target genes in WT1-mutant wilms' tumors. Am J Pathol 2004; 165:1943 - 1953
- Fukuzawa R, Heathcott RW, Sano M, Morison IM, Yun K, Reeve AE. Myogenesis in Wilms' tumors is associated with mutations of the WT1 gene and activation of bcl-2 and the Wnt signaling pathway. Pediatr Dev Pathol 2004; 7:125 - 137
- Maiti S, Alam R, Amos CI, Huff V. Frequent association of beta-catenin and WT1 mutations in wilms tumors. Cancer Res 2000; 60:6288 - 6292
- Rivera MN, Kim WJ, Wells J, Driscoll DR, Brannigan BW, Han M, Kim JC, Feinberg AP, Gerald WL, Vargas SO, Chin L, Iafrate AJ, Bell DW, Haber DA. An X chromosome gene, WTX, is commonly inactivated in wilms tumor. Science 2007; 315:642 - 645
- Major MB, Camp ND, Berndt JD, Yi X, Goldenberg SJ, Hubbert C, Biechele TL, Gingras AC, Zheng N, Maccoss MJ, Angers S, Moon RT. Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science 2007; 316:1043 - 1046
- Sim EU, Smith A, Szilagi E, Rae F, Ioannou P, Lindsay MH, Little MH. Wnt-4 regulation by the wilms' tumor suppressor gene, WT1. Oncogene 2002; 21:2948 - 2960
- Haber DA, Sohn RL, Buckler AJ, Pelletier J, Call KM, Housman DE. Alternative splicing and genomic structure of the wilms tumor gene WT1. Proc Natl Acad Sci USA 1991; 88:9618 - 9622
- Holmes G, Boterashvili S, English M, Wainwright B, Licht J, Little M. Two N-terminal self-association domains are required for the dominant negative transcriptional activity of WT1 Denys-Drash mutant proteins. Biochem Biophys Res Commun 1997; 233:723 - 728
- Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by wnt-4. Nature 1994; 372:679 - 683
- Costantini F. Renal branching morphogenesis: Concepts, questions, and recent advances. Differentiation 2006; 74:402 - 421
- Le Floch N, Rivat C, De Wever O, Bruyneel E, Mareel M, Dale T, Gespach C. The pro-invasive activity of wnt-2 is mediated through a noncanonical wnt pathway coupled to GSK-3beta and c-Jun/AP-1 signaling. FASEB J 2005; 19:144 - 146
- Haumaitre C, Fabre M, Cormier S, Baumann C, Delezoide AL, Cereghini S. Severe pancreas hypoplasia and multicystic renal dysplasia in two human fetuses carrying novel HNF1beta/MODY5 mutations. Hum Mol Genet 2006; 15:2363 - 2375
- Qian CN, Knol J, Igarashi P, Lin F, Zylstra U, Teh BT, Williams BO. Cystic renal neoplasia following conditional inactivation of apc in mouse renal tubular epithelium. J Biol Chem 2005; 280:3938 - 3945
- Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, Kahn A, Vandewalle A, Perret C. Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene 2001; 20:5972 - 5981
- Hu MC, Piscione TD, Rosenblum ND. Elevated SMAD1/beta-catenin molecular complexes and renal medullary cystic dysplasia in ALK3 transgenic mice. Development 2003; 130:2753 - 2766
- Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: Pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 2004; 15:1 - 12
- Taki M, Kamata N, Yokoyama K, Fujimoto R, Tsutsumi S, Nagayama M. Downregulation of wnt-4 and upregulation of wnt-5a expression by epithelial-mesenchymal transition in human squamous carcinoma cells. Cancer Sci 2003; 94:593 - 597
- Kim K, Lu Z, Hay ED. Direct evidence for a role of beta-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int 2002; 26:463 - 476
- Surendran K, Schiavi S, Hruska KA. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol 2005; 16:2373 - 2384
- Surendran K, McCaul SP, Simon TC. A role for wnt-4 in renal fibrosis. Am J Physiol Renal Physiol 2002; 282:431 - 441
- Atala A, Freeman MR, Mandell J, Beier DR. Juvenile cystic kidneys (jck): A new mouse mutation which causes polycystic kidneys. Kidney Int 1993; 43:1081 - 1085
- Surendran K, Simon TC, Liapis H, McGuire JK. Matrilysin (MMP-7) expression in renal tubular damage: Association with Wnt4. Kidney Int 2004; 65:2212 - 2222
- Terada Y, Tanaka H, Okado T, Shimamura H, Inoshita S, Kuwahara M, Sasaki S. Expression and function of the developmental gene wnt-4 during experimental acute renal failure in rats. J Am Soc Nephrol 2003; 14:1223 - 1233
- Price VR, Reed CA, Lieberthal W, Schwartz JH. ATP depletion of tubular cells causes dissociation of the zonula adherens and nuclear translocation of beta-catenin and LEF-1. J Am Soc Nephrol 2002; 13:1152 - 1161
- Lin CL, Wang JY, Huang YT, Kuo YH, Surendran K, Wang FS. Wnt/beta-catenin signaling modulates survival of high glucose-stressed mesangial cells. J Am Soc Nephrol 2006; 17:2812 - 2820
- Gumz ML, Zou H, Kreinest PA, Childs AC, Belmonte LS, LeGrand SN, Wu KJ, Luxon BA, Sinha M, Parker AS, Sun LZ, Ahlquist DA, Wood CG, Copland JA. Secreted frizzled-related protein 1 loss contributes to tumor phenotype of clear cell renal cell carcinoma. Clin Cancer Res 2007; 13:4740 - 4749