Abstract
Secreted signaling molecules of the Wnt family have been found to play a central role in controlling embryonic development of a wide range of taxa from Hydra to humans. The most extensively studied Wnt signaling pathway is the canonical Wnt pathway, which controls gene expression by stabilizing β-catenin, and regulates a multitude of developmental processes. More recently, noncanonical Wnt pathways, which are β-catenin-independent, have been found to be important developmental regulators. Understanding the mechanisms of Wnt signaling is essential for the development of novel preventive and therapeutic approaches of human diseases. Limb development is a paradigm to study the principles of Wnt signaling in various developmental contexts. In the developing vertebrate limb, Wnt signaling has been shown to have important functions during limb bud initiation, limb outgrowth, early limb patterning, and later limb morphogenesis events. This review provides a brief overview on the diversity of Wnt-dependent signaling events during embryonic development of the vertebrate limb.
Introduction
At the limb initiation stage, lateral plate mesoderm at the fore and hind limb level is induced to grow and form limb buds. Ongoing proliferation of lateral plate mesenchymal cells in the presumptive limb fields concomitant with decreased proliferation in the flank leads to initial formation of the buds.Citation1 Shortly thereafter, cells from the lateral edges of the adjacent somites begin migrating into the limb to form limb muscles.Citation2–Citation4 Like limb muscles, nerves, pigment cells, and vasculature also originate outside the limb, while other limb tissues, including the mesenchyme, connective tissue, cartilage, bone and tendons, derive from the lateral plate mesoderm. In the distal ectoderm of the early limb bud, the formation of a local ectodermal thickening, the apical ectoderm ridge (AER), is induced by the underlying limb mesoderm. In all tetrapods, the AER forms at the distal tip of the ectodermal pocket, running along the antero-posterior axis of the limb bud at the boundary of dorsal and ventral ectoderm, and is necessary for proximo-distal limb outgrowth.Citation5,Citation6 Three well-characterized signaling centres coordinate the overall limb patterning and growth along the three spatial axes: The AER directs the proximal-distal (P/D) limb outgrowth; the zone of polarizing activity (ZPA) in the posterior limb mesenchyme patterns the limb along the anterior-posterior (A/P) axis; and the non-AER limb ectoderm sets up the dorsal-ventral (D/V) polarity.Citation7,Citation8 Signals originating from these signaling centers are integrated to set up spatial coordinates to pre-pattern the limb mesenchyme, and finally the various cell types to be specified. During later limb morphogenesis, skeletal elements, muscles, tendons, blood vessels, etc., form and mature to make the limb a functional organ.Citation9,Citation10
Wnt Signaling Pathways
Wnts are secreted proteins that control a multitude of diverse developmental processes, but also tumorigenesis.Citation11–Citation15 They signal through the canonical, β-catenin dependent pathway, or at least two different noncanonical pathways (; refs. Citation16–Citation18). Central to this pathway is the regulation of β-catenin activity, which depends on its protein abundance and nuclear localization. In the absence of Wnt, glycogen synthase kinase 3 (GSK-3) phosphorylates β-catenin, which allows the ubiquitin ligase complex to bind and tag β-catenin for proteasome- mediated degradation. In the presence of Wnts, Wnt ligand binds to its coreceptors Frizzled and LRP5/6, so that the cytoplasmatic target of Frizzled, Dishevelled, is activated and suppresses GSK-3 activity. As a result, β-catenin is not phosphorylated and thus not degraded, resulting in accumulation of β-catenin in the cytoplasm which is translocated to the nucleus. There it binds to transcription factors of the LEF/TCF family and converts them from repressors to activators, which triggers downstream gene transcription.Citation14,Citation19 In this pathway, Arrow (LRP5/6) recruits Axin to the membrane and this interaction leads to Axin degradation upon canonical Wnt signaling. As a consequence, β-catenin is no longer bound by Axin, resulting in reduced β-catenin degradation and cytoplasmic sequestration. Thus, canonical Wnt signaling controls gene expression by allowing β-catenin to accumulate when Wnts are present and by reducing nuclear β-catenin levels when Wnts are absent.
The second pathway is the planar cell polarity pathway, in which Frizzled activates JNK and directs asymmetric cytoskeletal organization and coordinated polarization of cells within the plane of epithelial sheets.Citation20,Citation21 Both of these pathways utilise Dishevelled (Dsh): the β-catenin pathway is dependent on all three functional domains present in Dsh (DIX, PDZ and DEP) whilst the JNK pathway only requires the DEP domain. Dsh is connected via Daam1 to downstream effectors such as the small GTPase Rho and Rho-associated kinase (ROCK). The product of the Wnt target gene naked (Nkd) was recently identified as an antagonist for Wnt signaling that binds to Dsh and blocks β-catenin but stimulates the JNK pathway. This pathway is e.g. utilised in Drosophila planar cell polarity regulation and by Wnt11 in convergent extension movements during gastrulation. Thirdly, the Wnt/Ca2+ pathway lead to release of intracellular calcium, possibly via G-proteins.Citation17,Citation22 This pathway involves activation of phospholipase C (PLC) and protein kinase C (PKC). Elevated Ca2+ can activate the phosphatase calcineurin, which leads to dephosphorylation of the transcription factor NF-AT and its accumulation in the nucleus. In Xenopus embryos, NF-AT activity suppresses canonical Wnt signals during axis formation.Citation23,Citation24
Wnt Signaling and Limb Bud Initiation
At the onset of limb development, the limb buds initially form as a result of an interplay between fibroblast growth factor (FGF) and Wnt signaling, both acting downstream of the T-box transcription factors Tbx5 and Tbx4 in the developing forelimb and hindlimb, respectively.Citation25–Citation27 In the chick limb bud, Wnt2b in the presumptive forelimb region and Wnt8c in the presumptive hindlimb region, both signal through β-catenin/TCF to maintain the expression of FGF 10 in the lateral plate mesoderm (LPM) at the presumptive limb level ().Citation28 Implantation of cells that express β-catenin, which acts as a constitutively active molecule, into the flank region can ectopically induce FGF10 expression and limb bud outgrowth. In contrast, misexpression of Axin, which is an antagonist of the β-catenin pathway, downregulates FGF10 expression and affects limb outgrowth.Citation28
In the avian embryo, Kawakami and colleagues found that at the level of the presumptive forelimb, Wnt2b is expressed in both the IM and the LPM. Local application of cells expressing Wnt2b in the flank region induced FGF10 expression and subsequently resulted in ectopic limb outgrowth. Further, ectopic application of FGF8 was found to induce Wnt2b expression prior to the induction of FGF10, indicating that Wnt2b is downstream of FGF8 but upstream of FGF10. Similarly in the hindlimb, Wnt-8c has been shown to mediate the FGF8/FGF10 regulatory loop. Wnt-8c is only expressed in the caudal LPM of the prospective hindlimb and precedes the expression of FGF10 in this region. Moreover, Wnt8c, like Wnt2b, can induce the expression of FGF10, making Wnt8c a candidate mediator of limb bud initiation in the hindlimb region. Thus, the β-catenin pathway drives the initiation of limb bud formation in both fore and hind limb levels, albeit distinct Wnt molecules activate this pathway in the two limb fields. However, in the mouse embryo the expression patterns of Wnt2b and Wnt8c were not detected.Citation25 Therefore, it is not clear whether in mammals a Wnt ligand expressed in the early LPM or IM before limb bud formation is required for limb initiation. Nevertheless, canonical Wnt signaling was shown to sustain high levels of FGF10 expression during limb initiation also in the mouse, even though Tbx5 alone is able to activate FGF10 expression.Citation25 Another member of the Wnt family, Wnt10a, has been found to be expressed in the chick limb ectoderm and to induce FGF8 via the canonical Wnt pathway.Citation29
Wnt Signaling During Limb Bud Outgrowth
The AER is induced at the junction between the dorsal and ventral ectoderm at an early stage of limb bud formation, and produces growth factors such as FGF8 and FGF4 to promote proximo-distal (P/D) limb outgrowth. Wnt3a has been proposed to act downstream of FGF10 and to induce AER formation and FGF8 expression through the canonical Wnt pathway.Citation30 Inactivation of mesenchymal β-catenin leads to degeneration of the AER and limb truncation.Citation31 Thus, in the chick, the β-catenin/LEF pathway is thought to play a major role in both in the initiation of limb bud outgrowth and in the establishment of AER. Surprisingly, Wnt3a is not expressed in murine limb ectoderm, and limb development is normal in Wnt3a mutant mice.Citation32,Citation33 However, mice lacking TCF-1 and LEF-1 develop significantly smaller limb buds and lack a functional AER. This indicates that limb outgrowth is initiated but stalls due to the absence of a functional AER.Citation34 Wnt3, which is signaling via the β-catenin pathway, has been found to be involved in the formation and maintenance of the AER in mouse, similar to Wnt3a in the chick.Citation35
It is not only the presence of a functional AER, but also continued proliferation of the limb bud mesenchyme that is necessary for limb outgrowth. In the limb, loss of Wnt5a activity results in reduced proliferation of the progressive zone underneath the AER, a progressive shortening of the appendicular skeleton in a proximal-to-distal fashion and a total loss of the most distal structures such as the digits.Citation36 Wnt5a is expressed in the distal mesenchyme underneath the AER in both chick and mouse.Citation37,Citation38 It is not known through which pathway Wnt5a acts in this context. In other systems, Wnt5a was shown to act via either the canonicalCitation39 or non-canonical pathwayCitation22,Citation40 depending on which type of Frizzled receptor is present.
Wnt Signaling in Limb Patterning
AER formation is intimately linked to dorso-ventral (D/V) patterning of the limb ectoderm. Because canonical Wnt signaling is required upstream of Engrailed 1(En1) in the ventral ectoderm for determining ventral limb identity,Citation34,Citation35 the AER formation defects in the limb-specific mutants of Wnt3 or β-catenin might be a result of a failure to pattern the ventral ectoderm properly. This hypothesis is consistent with the observation that formation of the mature AER involves the convergence of broadly distributed ventral precursor cells towards the D/V boundary of the limb field; expression of AER marker genes such as FGF8 is initially observed in a broad domain of thickened ventral ectoderm and progressively becomes restricted to the mature AER at the distal margin in the mouse.Citation41–Citation44 Another ectoderm-derived Wnt ligand that is thought to play a role in AER formation is Wnt7a. Unlike Wnt3, Wnt7a is expressed specifically in the dorsal ectoderm, where it activates Lmx1b in the dorsal limb mesenchyme, which encodes a LIM-homeodomain transcription factor that determines the dorsal mesodermal cell fate in the limb.Citation37,Citation38,Citation45–Citation47 Misexpression of Lmx1 dorsalises the ventral distal limb bud showing that it is a key downstream mediator of Wnt7a signaling.Citation47,Citation48 Strikingly, loss of Wnt7a function in the mouse resulted in embryos with ventralized paws having sole pads on both surfaces, showing that Wnt7a is necessary for specifying dorsal cell fates in the distal limb.Citation46 To restrict the activity of Wnt7a to dorsal ectoderm requires the activity of Engrailed-1 in the ventral ectoderm.Citation42,Citation49 Ectopic expression of En-1 throughout the limb ectoderm in chick, including the dorsal ectoderm, results in suppression of Wnt7a in the dorsal ectoderm,Citation49 whereas in En-1 deficient mice, Wnt7a expands into the ventral ectoderm of the limb bud, leading to double-dorsal paws.Citation42
Next to its decisive role in P/D limb outgrowth and patterning, the AER is also important for antero-posterior (A/P) limb patterning, as it indirectly maintains Shh expression in the Zone of Polarizing Activity (ZPA) through a positive feedback loop between Shh in the posterior limb mesenchyme and FGF4 expressed in the AER.Citation50–Citation53 Canonical Wnt signaling is required for AER maintenance. First, the AER forms but is not maintained, when β-catenin is ablated slightly later in limb development.Citation35 Second, AER activity and structure change in response to alterations of canonical Wnt signaling. Dkk1 encodes a secreted antagonist of the canonical Wnt pathway and is, in the developing limb bud, expressed in the AER.Citation54–Citation56 In the Dkk1-/- mouse embryo, FGF8 expression in the AER is stronger, and is expanded both dorsally and ventrally in the limb bud as a result of upregulated canonical Wnt signaling. An expanded AER is also observed in En-1-deficient mice, indicating an interaction of Dkk, En-1 and Wnt7a during mouse limb development.Citation57 This is in line with data on Lrp6, which is a Wnt coreceptor required for the canonical Wnt signaling pathway.Citation58–Citation60 In Lrp6-/- embryo, FGF8 expression is significantly reduced, indicating that the AER degenerates prematurely as a result of weakened canonical Wnt signaling.Citation59,Citation61 These results demonstrate that after AER formation, proper modulation of canonical Wnt signaling is necessary to maintain normal function and structure of the AER. Interestingly, Thalidomide-induced limb deformities in human patients have now been proposed to be at least in part due to defects in the interaction of Dkk1, Wnt and BMP signaling.Citation62
Apart from its role in DV patterning and AER formation, Wnt7a is also involved in AP patterning, as Wnt7a deficient mice suffer from loss of posterior skeletal elements. This suggests that Wnt7a signaling may be necessary for normal function of the ZPA. Consistent with this, the expression of Shh, which mediates ZPA activity, is progressively lost in Wnt7a deficient limbs.Citation46 After removal of dorsal limb ectoderm, both Shh expression and posterior skeletal development can be rescued by applying Wnt7a in combination with FGF4, arguing that maintenance of Shh expression is under the combinatorial influence of Wnt and FGF signaling. However, the activity of Wnt7a in regulating Shh expression is independent of its dorsalizing activity, as Shh expression is normal in Lmx1-/- limbs.Citation45 Since the expression of Axin2 and Nkd, both being transcriptional targets and antagonists of canonical Wnt signaling, is located dorsally in the limb mesoderm,Citation63–Citation65 it will be interesting to investigate whether Axin2 and Nkd expression depends on Wnt7a or β-catenin signaling and whether any of them is involved in D/V patterning, Shh expression, or the establishment of the difference between dorsal and ventral ectoderm in response to Wnt3 signaling.
Wnt Signaling in Limb Morphogenesis
Next to their functions in overall limb patterning, Wnts control the morphogenesis of specific tissues in the limb such as musculature, synovial joints, cartilage, and bone.
Muscle differentiation.
Precursors of limb musculature are formed by deepithelization of the lateral dermomyotome and subsequent migration of somitic mesenchymal myogenic precursor into the limb bud.Citation3,Citation4 Wnts play a number of roles during muscle development including the initiation of myogenesis. The myogenic precursor cells are specified by Wnts secreted from the neural tube and the overlying surface ectoderm through induction of Pax3,Citation66 likely via the canonical pathway. Once at their homing sites within the limb bud, the precursor cells switch on the expression of the myogenic determination factors MyoD and Myf5. There is compelling evidence indicating the requirement of canonical Wnt signaling in the induction of myogenic differentiation. Blocking canonical Wnt signaling leads to reduction in myocyte numbers in the chick limb bud.Citation67 Further, the expression of the Wnt antagonist Sfrp2 in uncommitted myogenic precursors is downregulated as the myogenic cells differentiate.Citation68–Citation70 Recently it was shown that Wnt6 from the limb ectoderm promotes limb myogenesis via Pax3 and Myf5, whereas the other putative signaling pathway involving MyoD expression is negatively regulated by Wnt6 signaling,Citation71 and that Wnt6 can compensate for the ectoderm during the formation of limb muscle cells.Citation72 In addition, Wnt signaling modulates other aspects of myogenic development such as migration and cell morphology. A recent study has identified a Wnt-dependent TCF4 binding site in the enhancer region of Msx1, which represses differentiation in migratory myoblasts.Citation73 In the limb bud, N-cadherin, which is essential for myogenic migration and during chondrogenesis, is maintained by Wnt7a.Citation74 Myoblasts terminally differentiate into slow or fast muscle fibres that have different metabolic activities and contain different isoforms of myofibrillar contractile proteins, such as myosin heavy chain (MHC). The specific combination of slow and fast muscle fibres in each muscle determines its function. Recent cell labelling experiments in chick limb buds indicate that the specification of slow and fast myotubes involves both extrinsic and intrinsic mechanisms.Citation75 Wnt family members such as Wnt5a and Wnt11 may be involved in instructing the fate determination of slow and fast muscle fibres, likely through noncanonical pathways.Citation67 The ability of Wnts to induce stem cells to differentiate into a specific muscle cell type has tremendous impact on tissue engineering and repairing. Adult muscle stem cells are induced to differentiate into myoblasts by canonical Wnt signaling, showing that the generation and regeneration of skeletal muscles are governed by a common mechanism.Citation76 A better understanding of how Wnt signaling regulates muscle development will be very beneficial for therapy of neuromuscular diseases in the future.
Skeletal development.
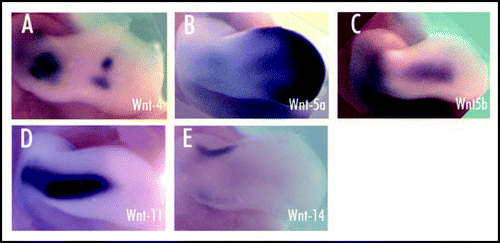
Wnt signaling controls several key developmental processes during skeletal morphogenesis in the limb. It has been shown that canonical Wnt signaling acts to suppress chondrocyte differentiation from condensed mesenchymal progenitors.Citation74,Citation77,Citation78 Several candidate Wnt genes (4, 5a, 5b, 11, 14) are expressed in the mesenchyme surrounding the developing cartilage elements () along with their receptors Fz4 and Fz6, and the Wnt antagonists Dkk2 and Dkk3.Citation77,Citation79–Citation85 Wnt1, 7a and 14 have been shown to inhibit chondrogenesis in micromass culture. Thus, the presence of Wnt antagonists, particularly Sfrp3 which is expressed in the central mesenchymal condensation, may allow chondrogenesis to proceed.Citation77,Citation78,Citation86 However, in vitro studies indicate that misexpression of the Wnt antagonist Frzb-1 inhibits chondrogenesis, whereas misexpression of constitutively active LEF-1 or Wnt8 promotes chondrocyte differentiation.Citation87 The inhibition by Wnt7a has been correlated with the maintenance of N-cadherin expression and hence cell adhesion which halts chondrogenesis just after the initiation stage.Citation74 This process seems to be mediated by Frz7 and the MAPK-pathway.Citation88,Citation89 Misexpression of Wnt5a or dominant negative forms of its receptors Fz1 and Fz7 results in the formation of shorter skeletal elements due to a delay in chondrocyte differentiation. Conversely, Wnt5a was shown to promote chondrocyte differentiation in the distal limb bud by inhibiting canonical Wnt activity.Citation90
Other Wnts such as Wnt14 have been implicated in initiating synovial joint-forming interzone cells prior to cartilage segmentation.Citation77,Citation81 Ectopic expression of Wnt14 in the chick limb bud induces morphological and molecular changes in chondrocytes characteristic of early stages of joint formation, such as the expression of Gdf5, and Wnt14 can induce dedifferentiation of chondrocytes in micromass cultures, a process that occurs during normal joint development.Citation77 In addition, several other Wnts such as Wnt4 and Wnt5a are also expressed in the future joint region.Citation77,Citation91
It is interesting to note that Wnt5a and Wnt4 have opposing effects on chondrocyte maturation. Misexpression of Wnt5a causes a delayed appearance of hypertrophic chondrocyte, Wnt4 misexpression results in an acceleration of chondrocyte maturation.Citation81 Wnt9a deficient mice show a relatively mild phenotype in joint formation, such as synovial chondroid metaplasia in some joints, while mice mutant for Wnt4 have no anomaly in joints. However, mice which are double mutant for Wnt4 and Wnt9a show severe phenotypes, suggesting that Wnt4 and Wnt9a play redundant roles in the maintenance of joint integrity.Citation92 Overexpression of Wnt9a or β-catenin in early differentiating chondrocytes causes ectopic joint formation and conditional inactivation of β-catenin by crossbreeding with Col2a1-Cre mice results in the disappearance of Gdf5 expression and a reduction of joint formation.Citation93 Conditional deletion of β-catenin in mouse limbs also results in fused joints.Citation94 These findings suggest that Wnt/ β-catenin signaling is necessary and sufficient for joint development initiation. Defective Wnt signaling has been described to be manifest in many skeletal diseases. For instance, the Wnt pathway has been implicated in degenerative joint diseases such as rheumatoid arthritis.Citation95 Mutations in the WISP3 gene, which encodes for Wnt1-induced signaling protein subfamily of the connective tissue growth factor (CTGF) family, are associated with the autosomal recessive skeletal disorder progressive pseudorhematoid dysplasia (PPD).Citation96 Mutation of LRPs, which are expressed in osteoclasts, results in osteoporosis-pseudoglioma syndrome.Citation97 This syndrome is characterized by a low bone mass and increased vulnerability to fractures and deformation. Wnt signaling has also been implicated in osteoarthritis where Sfrp4 expression has been shown to correlate with apoptotic chondrocytes within degenerating articular cartilage.Citation98
Figures and Tables
Fig 1 Three Wnt signaling pathways (modified from Huelsken and Behrens, 2002). (A) The Wnt/β-catenin pathway. Wnt signal is transduced through the frizzled receptor (Fz) via dishevelled (Dsh), which represses the axin/glycogen synthase kinase-3β (GSK3β) complex, which induces the degradation of β-catenin. Accumulated Cytoplasmic β-catenin is translocated to the nucleus where it binds with Tcf/Lef, thus activating the transcription of its target gene. (B) The planar cell polarity (PCP) pathway activated in response to Wnt signaling signals via the small GTPases Rho and Cdc42 to c-Jun N-terminal kinase (JNK), acting primarily on the cytoskeleton (C) An alternative pathway stimulates the release of intracellular Ca2+, activating protein kinase C (PKC) and Ca2+/calmodulin-dependent kinase II (CamKII).
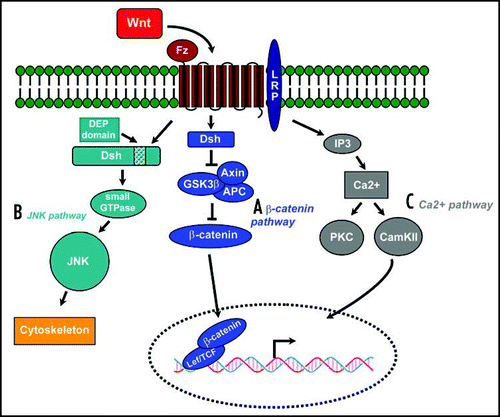
Fig 2 Model of early limb bud initiation in chick (modified from Kawakami et al., 2001). (A) Prior to limb initiation (stage 12), FGF8 (in grey) is expressed in the intermediate mesoderm (IM) adjacent to the presumptive forelimb area. FGF10 (in blue) is expressed in the lateral plate mesoderm (LPM), and in the segmental plate (SP). In the caudal portion of the LPM, FGF10 is coexpressed with Wnt8c (in red). Somites are indicated with their corresponding segment numbers. (B) During limb induction (HH stage 14), both FGF8 (in grey) and Wnt2b (in red) are expressed in the IM. Wnt2b is also expressed in the LPM of the presumptive forelimb area and signals through β-catenin to regulate FGF10 specifically in the prospective forelimb. FGF10 expression remains in the caudal LPM in a diffuse pattern. (C) At HH stage 16, expression of Wnt2b and FGF10 is confined to the forelimb bud field in the LPM. Additionally, expression of FGF10 is now confined to the presumptive hindlimb bud field. It may be Wnt8c in the caudal LPM contributes to this restriction of FGF10. In the LPM of the presumptive limb areas, FGF10 signals to the surface ectoderm (SE) to induce Wnt3a (green) resulting in activation of FGF8. For reason of simplicity, only the expression patterns in the relevant regions are shown, while the expression in somites and other areas is neglected.
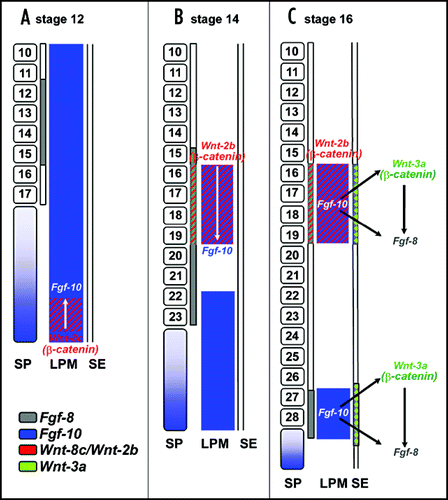
Fig 3 Expression of Wnt genes (4, 5a, 5b, 11, 14) in the mesenchyme surrounding the developing cartilage elements of HH-stage 26 chick forelimb. Limbs shown in dorsal view (Loganathan et al., 2005). (A) Expression of Wnt 4 in the central elbow region and in the joint interzones of the wrist. (B) Wnt 5a expression is seen at the distal tip of the limb bud and in the entire AER, with lower expression levels proximally. (C) Expression of Wnt 5b in the dorsal and ventral mesenchyme of the limb. (D) Wnt 11 transcripts in the dorsal and ventral mesenchyme of the limb. (E) Wnt 14 expression as a transverse stripe in the mesenchyme of the presumptive joint region.
Acknowledgments
Work in our laboratory is sponsored by the Deutsche Forschungsgemeinschaft DFG (SFB592, GRK1104 to M.S.) and the European NoE MYORES (M.S.).
References
- Searls RL, Janners MY. The initiation of limb bud outgrowth in the embryonic chick. Dev Biol 1971; 24:198 - 213
- Chevallier A, Kieny M, Mauger A. Limb-somite relationship: origin of the limb musculature. J Embryol Exp Morphol 1977; 41:245 - 258
- Christ B, Jacob HJ, Jacob M. [Origin of wing musculature. Experimental studies on quail and chick embryos]. Experientia 1974; 30:1446 - 1449
- Christ B, Jacob HJ, Jacob M. Experimental analysis of the origin of the wing musculature in avian embryos. Anat Embryol (Berl) 1977; 150:171 - 186
- Rowe DA, Fallon JF. The proximodistal determination of skeletal parts in the developing chick leg. J Embryol Exp Morphol 1982; 68:1 - 7
- Summerbell D. Interaction between the proximo-distal and antero-posterior co-ordinates of positional value during the specification of positional information in the early development of the chick limb-bud. J Embryol Exp Morphol 1974; 32:227 - 237
- Niswander L. Interplay between the molecular signals that control vertebrate limb development. Int J Dev Biol 2002; 46:877 - 881
- Tickle C. Patterning systems—from one end of the limb to the other. Dev Cell 2003; 4:449 - 458
- Christ B, Brand-Saberi B. Limb muscle development. Int J Dev Biol 2002; 46:905 - 914
- Mariani FV, Martin GR. Deciphering skeletal patterning: clues from the limb. Nature 2003; 423:319 - 325
- Huelsken J, Behrens J. The Wnt signalling pathway. J Cell Sci 2002; 115:3977 - 3978
- Huelsken J, Birchmeier W. New aspects of Wnt signaling pathways in higher vertebrates. Curr Opin Genet Dev 2001; 11:547 - 553
- Nusse R. Wnt signaling in disease and in development. Cell Res 2005; 15:28 - 32
- Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science 2000; 287:1606 - 1609
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol 1998; 14:59 - 88
- Barrow JR. Wnt/PCP signaling: a veritable polar star in establishing patterns of polarity in embryonic tissues. Semin Cell Dev Biol 2006; 17:185 - 193
- Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet 2000; 16:279 - 283
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004; 20:781 - 810
- Eastman Q, Grosschedl R. Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Curr Opin Cell Biol 1999; 11:233 - 240
- Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 1998; 94:109 - 118
- Li L, Yuan H, Xie W, Mao J, Caruso AM, McMahon A, Sussman DJ, Wu D. Dishevelled proteins lead to two signaling pathways. Regulation of LEF-1 and c-Jun N-terminal kinase in mammalian cells. J Biol Chem 1999; 274:129 - 134
- Sheldahl LC, Park M, Malbon CC, Moon RT. Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr Biol 1999; 9:695 - 698
- Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 1997; 275:1930 - 1934
- Saneyoshi T, Kume S, Amasaki Y, Mikoshiba K. The Wnt/calcium pathway activates NF-AT and promotes ventral cell fate in Xenopus embryos. Nature 2002; 417:295 - 299
- Agarwal P, Wylie JN, Galceran J, Arkhitko O, Li C, Deng C, Grosschedl R, Bruneau BG. Tbx5 is essential for forelimb bud initiation following patterning of the limb field in the mouse embryo. Development 2003; 130:623 - 633
- Naiche LA, Papaioannou VE. Loss of Tbx4 blocks hindlimb development and affects vascularization and fusion of the allantois. Development 2003; 130:2681 - 2693
- Takeuchi JK, Koshiba-Takeuchi K, Suzuki T, Kamimura M, Ogura K, Ogura T. Tbx5 and Tbx4 trigger limb initiation through activation of the Wnt/Fgf signaling cascade. Development 2003; 130:2729 - 2739
- Kawakami Y, Capdevila J, Buscher D, Itoh T, Rodriguez Esteban C, Izpisua Belmonte JC. WNT signals control FGF-dependent limb initiation and AER induction in the chick embryo. Cell 2001; 104:891 - 900
- Narita T, Sasaoka S, Udagawa K, Ohyama T, Wada N, Nishimatsu S, Takada S, Nohno T. Wnt10a is involved in AER formation during chick limb development. Dev Dyn 2005; 233:282 - 287
- Kengaku M, Capdevila J, Rodriguez-Esteban C, De La Pena J, Johnson RL, Belmonte JC, Tabin CJ. Distinct WNT pathways regulating AER formation and dorsoventral polarity in the chick limb bud. Science 1998; 280:1274 - 1277
- Hill TP, Taketo MM, Birchmeier W, Hartmann C. Multiple roles of mesenchymal beta-catenin during murine limb patterning. Development 2006; 133:1219 - 1229
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev 1994; 8:174 - 189
- Yoshikawa Y, Fujimori T, McMahon AP, Takada S. Evidence that absence of Wnt-3a signaling promotes neuralization instead of paraxial mesoderm development in the mouse. Dev Biol 1997; 183:234 - 242
- Galceran J, Farinas I, Depew MJ, Clevers H, Grosschedl R. Wnt3a-/--like phenotype and limb deficiency in Lef1(-/-)Tcf1(-/-) mice. Genes Dev 1999; 13:709 - 717
- Barrow JR, Thomas KR, Boussadia-Zahui O, Moore R, Kemler R, Capecchi MR, McMahon AP. Ectodermal Wnt3/beta-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev 2003; 17:394 - 409
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 1999; 126:1211 - 1223
- Dealy CN, Roth A, Ferrari D, Brown AM, Kosher RA. Wnt-5a and Wnt-7a are expressed in the developing chick limb bud in a manner suggesting roles in pattern formation along the proximodistal and dorsoventral axes. Mech Dev 1993; 43:175 - 186
- Parr BA, Shea MJ, Vassileva G, McMahon AP. Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development 1993; 119:247 - 261
- He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, Varmus H. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science 1997; 275:1652 - 1654
- Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 1997; 390:410 - 413
- Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development 1995; 121:439 - 451
- Loomis CA, Harris E, Michaud J, Wurst W, Hanks M, Joyner AL. The mouse Engrailed-1 gene and ventral limb patterning. Nature 1996; 382:360 - 363
- Mahmood R, Bresnick J, Hornbruch A, Mahony C, Morton N, Colquhoun K, Martin P, Lumsden A, Dickson C, Mason I. A role for FGF-8 in the initiation and maintenance of vertebrate limb bud outgrowth. Curr Biol 1995; 5:797 - 806
- Michaud JL, Lapointe F, Le Douarin NM. The dorsoventral polarity of the presumptive limb is determined by signals produced by the somites and by the lateral somatopleure. Development 1997; 124:1453 - 1463
- Chen H, Lun Y, Ovchinnikov D, Kokubo H, Oberg KC, Pepicelli CV, Gan L, Lee B, Johnson RL. Limb and kidney defects in Lmx1b mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet 1998; 19:51 - 55
- Parr BA, McMahon AP. Dorsalizing signal Wnt-7a required for normal polarity of D-V and A-P axes of mouse limb. Nature 1995; 374:350 - 353
- Riddle RD, Ensini M, Nelson C, Tsuchida T, Jessell TM, Tabin C. Induction of the LIM homeobox gene Lmx1 by WNT7a establishes dorsoventral pattern in the vertebrate limb. Cell 1995; 83:631 - 640
- Vogel A, Rodriguez C, Warnken W, Izpisua Belmonte JC. Dorsal cell fate specified by chick Lmx1 during vertebrate limb development. Nature 1995; 378:716 - 720
- Logan C, Hornbruch A, Campbell I, Lumsden A. The role of Engrailed in establishing the dorsoventral axis of the chick limb. Development 1997; 124:2317 - 2324
- Laufer E, Nelson CE, Johnson RL, Morgan BA, Tabin C. Sonic hedgehog and Fgf-4 act through a signaling cascade and feedback loop to integrate growth and patterning of the developing limb bud. Cell 1994; 79:993 - 1003
- Niswander L, Jeffrey S, Martin GR, Tickle C. A positive feedback loop coordinates growth and patterning in the vertebrate limb. Nature 1994; 371:609 - 612
- Yang Y, Niswander L. Interaction between the signaling molecules WNT7a and SHH during vertebrate limb development: dorsal signals regulate anteroposterior patterning. Cell 1995; 80:939 - 947
- Zuniga A, Haramis AP, McMahon AP, Zeller R. Signal relay by BMP antagonism controls the SHH/FGF4 feedback loop in vertebrate limb buds. Nature 1999; 401:598 - 602
- Mao J, Wang J, Liu B, Pan W, Farr GH 3rd, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell 2001; 7:801 - 809
- Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, Niehrs C, Belmonte JC, Westphal H. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell 2001; 1:423 - 434
- Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol 2001; 11:951 - 961
- Adamska M, MacDonald BT, Sarmast ZH, Oliver ER, Meisler MH. En1 and Wnt7a interact with Dkk1 during limb development in the mouse. Dev Biol 2004; 272:134 - 144
- Adamska M, Billi AC, Cheek S, Meisler MH. Genetic interaction between Wnt7a and Lrp6 during patterning of dorsal and posterior structures of the mouse limb. Dev Dyn 2005; 233:368 - 372
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 2000; 407:535 - 538
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature 2000; 407:530 - 535
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A 2003; 100:3299 - 3304
- Knobloch J, Shaughnessy JD Jr, Ruther U. Thalidomide induces limb deformities by perturbing the Bmp/Dkk1/Wnt signaling pathway. Faseb J 2007; 21:1410 - 1421
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol 2002; 22:1184 - 1193
- Soshnikova N, Zechner D, Huelsken J, Mishina Y, Behringer RR, Taketo MM, Crenshaw EB 3rd, Birchmeier W. Genetic interaction between Wnt/beta-catenin and BMP receptor signaling during formation of the AER and the dorsal-ventral axis in the limb. Genes Dev 2003; 17:1963 - 1968
- Yan D, Wallingford JB, Sun TQ, Nelson AM, Sakanaka C, Reinhard C, Harland RM, Fantl WJ, Williams LT. Cell autonomous regulation of multiple Dishevelled-dependent pathways by mammalian Nkd. Proc Natl Acad Sci U S A 2001; 98:3802 - 3807
- Munsterberg AE, Kitajewski J, Bumcrot DA, McMahon AP, Lassar AB. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes Dev 1995; 9:2911 - 2922
- Anakwe K, Robson L, Hadley J, Buxton P, Church V, Allen S, Hartmann C, Harfe B, Nohno T, Brown AM, Evans DJ, Francis-West P. Wnt signalling regulates myogenic differentiation in the developing avian wing. Development 2003; 130:3503 - 3514
- Cossu G, De Angelis L, Borello U, Berarducci B, Buffa V, Sonnino C, Coletta M, Vivarelli E, Bouche M, Lattanzi L, Tosoni D, Di Donna S, Berghella L, Salvatori G, Murphy P, Cusella-De Angelis MG, Molinaro M. Determination, diversification and multipotency of mammalian myogenic cells. Int J Dev Biol 2000; 44:699 - 706
- Ladher RK, Church VL, Allen S, Robson L, Abdelfattah A, Brown NA, Hattersley G, Rosen V, Luyten FP, Dale L, Francis-West PH. Cloning and expression of the Wnt antagonists Sfrp-2 and Frzb during chick development. Dev Biol 2000; 218:183 - 198
- Petropoulos H, Skerjanc IS. Beta-catenin is essential and sufficient for skeletal myogenesis in P19 cells. J Biol Chem 2002; 277:15393 - 15399
- Geetha-Loganathan P, Nimmagadda S, Prols F, Patel K, Scaal M, Huang R, Christ B. Ectodermal Wnt-6 promotes Myf5-dependent avian limb myogenesis. Dev Biol 2005; 288:221 - 233
- Geetha-Loganathan P, Nimmagadda S, Huang R, Scaal M, Christ B. Role of Wnt-6 in limb myogenesis. Anat Embryol (Berl) 2006; 211:183 - 188
- Miller KA, Barrow J, Collinson JM, Davidson S, Lear M, Hill RE, Mackenzie A. A highly conserved Wnt-dependent TCF4 binding site within the proximal enhancer of the anti-myogenic Msx1 gene supports expression within Pax3-expressing limb bud muscle precursor cells. Dev Biol 2007; 311:665 - 678
- Tufan AC, Tuan RS. Wnt regulation of limb mesenchymal chondrogenesis is accompanied by altered N-cadherin-related functions. Faseb J 2001; 15:1436 - 1438
- Kardon G, Campbell JK, Tabin CJ. Local extrinsic signals determine muscle and endothelial cell fate and patterning in the vertebrate limb. Dev Cell 2002; 3:533 - 545
- Polesskaya A, Seale P, Rudnicki MA. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell 2003; 113:841 - 852
- Hartmann C, Tabin CJ. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell 2001; 104:341 - 351
- Rudnicki JA, Brown AM. Inhibition of chondrogenesis by Wnt gene expression in vivo and in vitro. Dev Biol 1997; 185:104 - 118
- Christiansen JH, Dennis CL, Wicking CA, Monkley SJ, Wilkinson DG, Wainwright BJ. Murine Wnt-11 and Wnt-12 have temporally and spatially restricted expression patterns during embryonic development. Mech Dev 1995; 51:341 - 350
- Christiansen JH, Monkley SJ, Wainwright BJ. Murine WNT11 is a secreted glycoprotein that morphologically transforms mammary epithelial cells. Oncogene 1996; 12:2705 - 2711
- Hartmann C, Tabin CJ. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development 2000; 127:3141 - 3159
- Kawakami Y, Wada N, Nishimatsu SI, Ishikawa T, Noji S, Nohno T. Involvement of Wnt-5a in chondrogenic pattern formation in the chick limb bud. Dev Growth Differ 1999; 41:29 - 40
- Loganathan PG, Nimmagadda S, Huang R, Scaal M, Christ B. Comparative analysis of the expression patterns of Wnts during chick limb development. Histochem Cell Biol 2005; 123:195 - 201
- Monaghan AP, Kioschis P, Wu W, Zuniga A, Bock D, Poustka A, Delius H, Niehrs C. Dickkopf genes are co-ordinately expressed in mesodermal lineages. Mech Dev 1999; 87:45 - 56
- Tanda N, Kawakami Y, Saito T, Noji S, Nohno T. Cloning and characterization of Wnt-4 and Wnt-11 cDNAs from chick embryo. DNA Seq 1995; 5:277 - 281
- Stott NS, Jiang TX, Chuong CM. Successive formative stages of precartilaginous mesenchymal condensations in vitro: modulation of cell adhesion by Wnt-7A and BMP-2. J Cell Physiol 1999; 180:314 - 324
- Enomoto-Iwamoto M, Kitagaki J, Koyama E, Tamamura Y, Wu C, Kanatani N, Koike T, Okada H, Komori T, Yoneda T, Church V, Francis-West PH, Kurisu K, Nohno T, Pacifici M, Iwamoto M. The Wnt antagonist Frzb-1 regulates chondrocyte maturation and long bone development during limb skeletogenesis. Dev Biol 2002; 251:142 - 156
- Tufan AC, Daumer KM, DeLise AM, Tuan RS. AP-1 transcription factor complex is a target of signals from both WnT-7a and N-cadherin-dependent cell-cell adhesion complex during the regulation of limb mesenchymal chondrogenesis. Exp Cell Res 2002; 273:197 - 203
- Tufan AC, Daumer KM, Tuan RS. Frizzled-7 and limb mesenchymal chondrogenesis: effect of misexpression and involvement of N-cadherin. Dev Dyn 2002; 223:241 - 253
- Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol 2003; 162:899 - 908
- Church V, Nohno T, Linker C, Marcelle C, Francis-West P. Wnt regulation of chondrocyte differentiation. J Cell Sci 2002; 115:4809 - 4818
- Spater D, Hill TP, O'Sullivan R J, Gruber M, Conner DA, Hartmann C. Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development 2006; 133:3039 - 3049
- Guo X, Day TF, Jiang X, Garrett-Beal L, Topol L, Yang Y. Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev 2004; 18:2404 - 2417
- Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell 2005; 8:727 - 738
- Sen M, Reifert J, Lauterbach K, Wolf V, Rubin JS, Corr M, Carson DA. Regulation of fibronectin and metalloproteinase expression by Wnt signaling in rheumatoid arthritis synoviocytes. Arthritis Rheum 2002; 46:2867 - 2877
- Hurvitz JR, Suwairi WM, Van Hul W, El-Shanti H, Superti-Furga A, Roudier J, Holderbaum D, Pauli RM, Herd JK, Van Hul EV, Rezai-Delui H, Legius E, Le Merrer M, Al-Alami J, Bahabri SA, Warman ML. Mutations in the CCN gene family member WISP3 cause progressive pseudorheumatoid dysplasia. Nat Genet 1999; 23:94 - 98
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 2001; 107:513 - 523
- James IE, Kumar S, Barnes MR, Gress CJ, Hand AT, Dodds RA, Connor JR, Bradley BR, Campbell DA, Grabill SE, Williams K, Blake SM, Gowen M, Lark MW. FrzB-2: a human secreted frizzled-related protein with a potential role in chondrocyte apoptosis. Osteoarthritis Cartilage 2000; 8:452 - 463