Abstract
The increasing incidence of obesity in the developed and developing world in the last decade has led to a need to define our understanding of the physiological mechanisms which can predispose individuals to weight gain in infancy, childhood and adulthood. There is now a considerable body of evidence which has shown that the pathway to obesity may begin very early in life, and that exposure to an inappropriate level of nutrition during prenatal and/or early postnatal development can predispose individuals to obesity in later life The brain is at the heart of the regulation of appetite and food preferences, and it is increasingly being recognised that the development of central appetitive structures is acutely sensitive to the nutritional environment both before and immediately after birth. This review will summarise the body of work which has highlighted the critical role of the brain in the early origins of obesity and presents some perspectives as to the potential application of these research findings in the clinical setting.
Introduction
It is widely accepted that the incidence of obesity in the developed and developing world has increased over the last decade, giving rise to the current global epidemic of obesity, and it is possible to cite any number of journal articles or public health statistics to support this.Citation1,Citation2 The dramatic rise in the prevalence of obesity has brought with it a substantial increase in the incidence of associated comorbidities, such as insulin resistance, cardiovascular disease, type 2 diabetes and the metabolic syndrome.Citation3 The social and economic burden of the current obesity epidemic is vast and increasing,Citation1,Citation2 and despite a wide-spread recognition of the problem, it is proving exceptionally difficult to bring under control. In this context, it is important that a growing body of evidence has demonstrated clearly that the origins of obesity may lie very early in life. It has been demonstrated that exposure to either an inappropriately high or inappropriately low level of nutrition during prenatal and early postnatal development can have significant implications for the capacity of an individual to regulate their body weight after birth, and can therefore predispose these individuals to obesity in later life.Citation4–Citation6 The brain is at the heart of the regulation of appetite and food preferences, and it is increasingly being recognized that the development of appetitive structures is acutely sensitive to the nutritional environment both before and immediately after birth. This review will summarize the body of work, by our group and others, which has highlighted the critical role of the brain in the programming of obesity and presents some perspectives as to the potential application of these research findings in the clinical setting.
The Regulation of Appetite in the Adult
A detailed summary of this network is beyond the scope of the present review, and so will only be dealt with briefly here. There is now, however, a series of contemporary and comprehensive reviews describing the complex neural network which is responsible for the regulation of appetite and metabolism in the adult.Citation7–Citation9
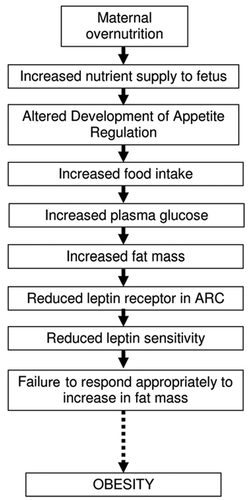
The control of appetite and energy balance is mediated by a collection of hypothalamic neuropeptides which are expressed principally in the arcuate nucleus (ARC) of the hypothalamus ().Citation7,Citation8,Citation10,Citation11 This hypothalamic neural network integrates signals relating to energy supply, energy utilization and total energy reserves, in order to appropriately regulate food intake and energy expenditure to maintain energy balance.Citation8 The neurons which express these neuropeptides have extensive projections to other hypothalamic nuclei including the dorsomedial hypothalamus (DMH), lateral hypothalamus (LHA) and paraventricular nucleus (PVN), where further orexigenic signals are recruited.Citation7 The hormonal and metabolic signals relating to energy intake, energy utilization and total energy reserves are received by the first order neurons in the ARC, and from here are relayed to second-order neurons in other hypothalamic nuclei. The terminals from all orexigenic and anorexigenic signals converge finally on the PVN, and it has been suggested that this hypothalamic region is responsible for integrating these signals and for initiating the appropriate metabolic adjustments to maintain energy balance.Citation12
Among the hypothalamic appetite regulators, the best described are the orexigenic neuropepetides, neuropeptide Y (NPY) and agouti related peptide (AGRP) and the anorexigenic neuropeptides, pro-opiomelanocortin (POMC)-derived α-melanocyte-stimulating hormone (α-MSH) and cocaine and amphetamine regulated transcript (CART), all of which are expressed in the ARC. NPY is the most potent stimulator of appetite thus far identified, and central administration of NPY into the PVN results in a significant increase in appetite drive and food intake.Citation13–Citation16 Both the expression of NPY mRNA in the ARC and the release of NPY into the PVN are markedly increased in response to fasting or food restriction both in vivo and in vitro.Citation17 Conversely, NPY expression is downregulated in response to signals of increased energy stores, including leptin, insulin and glucose.Citation8,Citation18,Citation19
The melanocortin signalling system provides the opposing anorexigenic drive, based on POMC-derived α-MSH, which acts via the melanocortin receptors (MC3R and MC4R) to suppress food intake.Citation20–Citation23 The expression of POMC mRNA in the ARC is upregulated during periods of high energy intake and is reduced by fasting or food restriction.Citation24,Citation25 Agouti-related protein (AGRP) acts as an endogenous antagonist of MCRs, and increases in AGRP expression therefore inhibit melanocortin signalling and increase appetite.Citation26 Thus, the physiological effects of melanocortin signalling are dependent on the balance between POMC (α-MSH) and AGRP expression within the ARC.
CART is another important component of the central appetite-regulating network, acting as a potent inhibitor of feeding. Central administration of CART peptide dramatically reduces feeding in rodents, whilst blocking the actions of CART with CART antibodies results in significant increases in food intake.Citation27 In the rodent, CART is coexpressed with POMC in the ARC which suggests that CART may interact with the melanocortin signalling system. Leptin receptors are present on CART positive neurons in the hypothalamus, and leptin increases c-Fos activity in CART peptide-positive neurons.Citation28,Citation29 Furthermore, CART mRNA expression in the ARC is reduced during fasting in rodents.Citation30
The Regulation of Appetite In Utero
There are obvious distinctions between the fetus and the adult in the requirement for a system for the regulation of energy balance. The fetus receives its nutrition entirely by transplacental transfer from the maternal circulation and therefore has a limited capacity to respond to alterations in nutrient supply by altering nutrient intake.Citation31 At birth, however, the newborn has an immediate requirement for a functional system for controlling energy intake and energy expenditure. Furthermore, the neonate must be able to maintain a state of positive energy balance in order to promote growth and adipose tissue deposition during early neonatal life. Whilst many researchers have classically regarded the fetus as being a passive participant in the process of nutrient exchange in utero, it is becoming increasingly clear that the appetite-regulating neural network is present before birth in humans and higher-order mammals, including non-human primates and sheep.Citation32–Citation35 Importantly, there is a growing body of evidence demonstrating that the nutritional environment to which the individual is exposed during prenatal and perinatal development has long term consequences for the function of the appetite regulating neural network; and this clearly impacts on the way in which an individual regulates its feeding behavior, energy balance and ultimately body weight throughout later life.
As stated earlier, the neuronal circuitry linking the different regions of the hypothalamus, and which is critical to the integrated regulation of feeding behavior, is present before birth in the sheep and non-human primate, as well as in the human.Citation32–Citation34,Citation36 In the rodent, however, this neuronal circuitry is not fully established until 16 days after birth.Citation33,Citation37,Citation38 It is therefore clear that the critical windows of development during which exposure to altered levels of nutrients will have long-term effects on the development of this axis are different in rodents than for large animal models and humans, and that this becomes important when considering the potential clinical implications of current research findings.
Is the Central Neural Network for Appetite Regulation Functional Before Birth?
Historically, the fetus has been regarded as a passive recipient of transplacental nutrition, with limited capacity to respond to peripheral signals of current nutritional status. It is now, however, becoming increasingly clear that the system which regulates appetite in postnatal life is already responsive to signals of nutritional status, i.e., glucose and insulin, before birth. Over ten years ago, Singh and colleagues showed that exposure to hyperglycemia and hyperinsulinemia before birth in the rat decreased NPY expression in the fetal hypothalamus in late gestation.Citation39 It has also been demonstrated that depriving rat pups of access to maternal milk for 24 to 36 hours results in a significant increase in the expression of NPY mRNA in the ARC from as early as postnatal day 2, suggesting that the appetite-regulating system is responsive to nutrient deprivation early in postnatal life, before the neuronal circuitry is fully established.Citation33 Since then, similar studies have been conducted in large animal models, such as the sheep, in which these systems develop before birth, as in the human.
In order to establish whether and to what extent the neuronal circuitry could respond to increased glucose supply before birth in sheep, we determined the effect of fetal hyperglycemia and hyperinsulinemia in late gestation on the expression of the appetite-regulating neuropeptides. Intrafetal glucose infusion (at 130–140 days gestation; term = ∼150 days) was associated with a 2.5-fold increase in plasma glucose and ∼2-fold increase in plasma insulin concentrations in the fetal circulation, whilst plasma leptin concentrations were not affected.Citation40 Importantly, this prenatal hyperglycemia resulted in a significant increase in the expression of POMC mRNA within the ARC (). The expression of the anorexigenic neuropeptide, CART, and the orexigenic peptides, NPY and AGRP, was not altered in glucose-infused fetuses.Citation40 These findings provided evidence that POMC-containing neurons within the fetal hypothalamus, but not those containing NPY, AGRP or CART, could respond directly to altered concentrations of glucose and/or insulin in the fetal circulation in late gestation. Further studies of mid-gestation ovine fetuses (81 days) have also found a positive relationship between POMC mRNA in the ARC and fetal glycemia at this earlier stage (C.L. Adam, et al., unpublished data).
In a separate study we demonstrated that maternal under- nutrition during late pregnancy in the ewe, resulting in fetal hypoinsulinemia and hypoglycaemia, was associated with increased NPY expression in the fetal ARC.Citation34 We have therefore suggested that this critical orexigenic component of the appetite-regulating neural network may be more responsive to decreases, rather than increases, in nutrient supply, with a primary role to limit thermogenesis and promote feeding in the immediate postnatal period. Greenwood and colleagues have reported that low birth weight lambs have an increased voluntary feed intake relative to current body weight in the immediate postnatal period,Citation41 and Owens and colleagues have more recently reported an earlier and more sustained intensity of suckling in the first 90 minutes post-fasting in lambs whose intrauterine growth was experimentally restricted.Citation42 It has therefore been suggested that prentatal upregulation of NPY mRNA expression in prenatally undernourished newborns may be a mechanism which promotes feeding immediately following birth and thereby contributes to the accelerated postnatal growth rate often present in growth-restricted newborns.Citation43 However, whilst an interesting and potentially important premise, the extent to which programming of appetite may contribute to catch-up growth remains poorly defined, and a direct link between prenatal upregulation of NPY and increased food intake in the early postnatal period has yet to be established.
The adipose tissue hormone, leptin, is arguably one of the most important regulators of the central neural network in adult life,Citation44 and there is now considerable evidence that leptin may also play an important role in the regulation of energy balance before birth. Leptin is synthesized by the fetal perirenal adipose tissue from as early as 90 d gestation in the fetal sheep (term ∼150 d gestation),Citation45 and fetal plasma leptin concentrations are directly related to adiposity in fetuses of ewes fed at or above maintenance energy requirements in late pregnancy,Citation46 suggesting the leptin may have a role as a peripheral signal of adipose stores before birth. In sheep and humans, unlike the rodent, fat stores are deposited before birth and it has been established that fetal adipose tissue is the major source of fetal leptin in these species.Citation45,Citation47 Intrafetal leptin infusion for 4 days from 137–141 d gestation resulted in a significant reduction of relative fat mass in ovine fetuses and an increase in the abundance of uncoupling-protein 1 (UCP-1) protein in perirenal adipose tissue.Citation48 These results suggested that leptin could act before birth to stimulate lipolysis in adipose depots and increase endogenous thermogenesis, and led us to investigate the capacity for leptin to regulate the activity of the central neural network in fetal life.
Leptin infusion in the fetal sheep from 131–134 d gestation resulted in a six-fold increase in plasma leptin concentrations, but no change in the expression of either the anorexigenic (POMC and CART) or orexigenic (AGRP and NPY) neuropeptides in the fetal hypothalamus at the end of the infusion period.Citation49 In this same study, however, there was some evidence for an inverse relationship between leptin concentrations on the day of tissue collection and the expression of NPY mRNA in the fetal ARC. Whilst further studies are clearly warranted, we have suggested that this may be a consequence of the emergence of leptin responsiveness within the population of NPY-expressing neurons at 134 d gestation in the sheep.Citation49 Interestingly, Proulx and colleagues had previously shown that leptin treatment in the neonatal rodent, a timing equivalent to late gestation in the sheep and human, could alter the expression of appetite-regulating neuropeptides NPY and POMC in the rostral ARC, but was without any affect on food intake, which suggested that leptin sensitivity may emerge before the completion of neuronal connections between discreet hypothalamic regions.Citation50
The limited responsiveness of the appetite-regulating neuropeptides to leptin early in development is consistent with the results of rodent studies,Citation51 and suggests that the sensitivity of appetite-regulating neuropeptides to leptin emerges relatively late in the development of this network. Our work in the fetal and neonatal sheep has led us to suggest that the reason for the limited capacity to respond to leptin in utero may be a consequence of the relatively low level of gene expression for the signalling form of the leptin receptor (OBRb) in ARC neurons. In the adult sheep, leptin receptors are expressed abundantly in the ARC, and at a lower level in the ventromedial hypothalamus (VMH),Citation52 whereas during fetal and early postnatal life, this distribution is reversed.Citation35,Citation40 Furthermore, the abundance of OBRb expression in the ARC relative to the VMN increases across gestation and into early postnatal life; OBRb is undetectable in the ARC at 134 d gestation (Muhlhausler et al., unpublished observations), but increases relative to expression in the VMH at 141 d gestation,Citation40 and further increases by 30 d of postnatal age ().Citation53
Experimental Evidence for the Programming of Appetite and Obesity
Historical studies by Oscai and colleagues were perhaps the first to demonstrate in an experimental paradigm the phenomenon that appetite in adult life could be related to early life experience in the rodent, a period equivalent to late gestation in the sheep and human. These studies demonstrated that the amount consumed by rats during the suckling period correlated directly with their intake during ad libitum feeding in adult life.Citation54 These observations were confirmed by Plagemann and colleagues, who showed that exposure to overnutrition (i.e., hyperglycemia and hyperinsulinemia) either before birth or in the early postnatal period resulted in persistent hyperphagia and the development of obesity by 3 weeks of age.Citation55 Importantly, the continued work in Plagemann's laboratory over the past decade has now demonstrated convincingly that exposure to prenatal or postnatal overnutrition in the rodent results in substantial changes in the development of the hypothalamic architecture.Citation55,Citation56
In rats, exposure to a diabetic environment before birth or to increased early postnatal nutrition as a result of small litter rearing results in a significant increase the number of neurons within the hypothalamus expressing the orexigenic neuropeptides, NPY and galanin, by 3 weeks of ageCitation57,Citation58 and, importantly this increase persists into adulthood (17 months of age).Citation59 In offspring born to control mothers that are raised exclusively on milk from diabetic dams, NPY and AGRP levels in the ARC are increased and POMC and α-MSH levels are decreased at weaning, changes which are consistent with an increased orexigenic drive.Citation60 In all of these studies, the changes are detectable at the end of the critical hypothalamic differentiation period (postnatal day 21 in the rodent) and precede the onset of obesity, which suggests that they may contribute directly to the development of the obese phenotype. The morphological changes in hypothalamic architecture are also associated with changes in the responsiveness of hypothalamic neurons to nutrient-related signals; for example, the capacity of leptin to inhibit the activity of ARC neurons and the excitatory role of CART, melanocortins and NPY on PVN neurons are permanently diminished in postnatally over-nourished offspring.Citation61,Citation62
More recently, we have demonstrated that prenatal overnutrition results in alterations to the regulation of appetite in early postnatal life in the sheep, an animal model which mimicks human development more closely than the rodent. In this study, we showed that lambs whose mothers were fed on a high plane of nutrition in late pregnancy exhibited relative hyperphagia (increased food intake as a proportion of their current body weight) during the first 3 weeks of postnatal life, coupled with an increase in plasma glucose concentrations.Citation53 These findings suggest that exposure to an increased nutrient supply before birth may result in reduced sensitivity to satiety signals, or an exaggerated response to signals of negative energy balance, in the early postnatal period. Lambs of overnourished ewes also had significantly higher relative subcutaneous adiposity compared to controls. Importantly, in lambs of well-fed ewes, increased levels of adiposity were associated with reduced expression of the leptin receptor (OBRb) in the hypothalamic ARC, which is consistent with the development of central leptin resistance. Whilst it appeared that the appetite-stimulating neuropeptides NPY and AGRP, as well as the appetite-inhibiting precursor molecule POMC, were all regulated appropriately by signals of current nutritional status, lambs of over-nourished ewes failed to appropriately upregulate the expression of CART in the ARC in response to increases in body fat mass.Citation53 These findings suggest that a failure to appropriately upregulate appetite- inhibitory pathways may contribute to the development of an obese phenotype in this experimental model ().
A decreased nutrient supply during prenatal and early postnatal development has also been show to program appetite and body composition. Pups born to severely nutrient restricted rat dams are growth restricted at birth, are hyperphagic in postnatal life and develop obesity, insulin resistance and hyperleptinemia in adulthood.Citation63,Citation64 This hyperphagia is amplified when the offspring are fed a hypercaloric diet in the post-weaning period,Citation63 which suggests that the set point of the regulation of appetite in these offspring is permanently reset at an elevated level. However, Plagemann et al. also found that specifically restricting maternal protein during pregnancy and lactation resulted in hypophagia and reduced body weight in the offspring; this appeared to be the consequence of hypoplasia of neurons expressing NPY and galanin in the ARC, PVN and LHA at weaning.Citation65,Citation66
Whilst the underlying mechanisms for programming the appetite-regulating neural network are still not fully understood, Plagemann's studies implicate insulin as a key regulator of these adaptations. Both maternal diabetes and early postnatal overnutrition result in perinatal hyperinsulinemia, and peripheral or intra-hypothalamic insulin treatment during the perinatal period also results in hyperphagia, increased weight gain, obesity, hyperinsulinemia and insulin resistance in adult life.Citation67,Citation68 In rodent models of maternal diabetes, treatments which normalize maternal glucose concentrations also prevent the development of obesity and metabolic disturbances in the offspring.Citation69 Fetal and perinatal insulin and glucose concentrations are clearly important, however, recent studies have now indicated a critical role for leptin in the development of appetitive structures.
Leptin and the Development of the Appetite Regulating Neural Network
It is well established that leptin plays a central role in regulating the expression of the appetite-regulating neuropeptides, and therefore regulating food intake, in the adult. The long form of the leptin receptor (OBRb) is expressed on NPY/AGRP and POMC/CART neurons within the hypothalamic ARC, and it has been demonstrated that centralCitation70 or peripheralCitation71 administration of leptin decreases NPY/AGRP gene expression and increases POMC/CART mRNA expression within ARC neurons.Citation72,Citation73 It has recently become clear that leptin may play a critical role in regulating the development of the appetitive structures much earlier in life, both before and after birth.
A series of studies have demonstrated that leptin is both permissive for the development of appetite-regulating neural circuitry and, importantly, that exposure to altered levels of leptin during critical windows in its development may have permanent effects on the structure and function of this neural network. Mice that are leptindeficient (ob/ob mice) fail to develop adequate projections from the ARC to other hypothalamic regions, and as a consequence their ability to regulate appetite in response to external cues is severely comprised. In a critical study, Bouret and colleagues showed that the development of these neural projections could be restored by the administration of exogenous leptin during the neonatal period.Citation74 In this same study, it was also shown that the administration of exogenous leptin to adult ob/ob mice was without effect on the density of neuronal projections.Citation74 This therefore suggested that there were critical windows in the development of appetitive structures during which the presence of leptin was a prerequisite for the formation of the hypothalamic architecture. Bouret and colleagues also showed that leptin could act in vitro to promote the development of neuronal outgrowths from arcuate neurons in culture, confirming the direct role of leptin in the development of the neuronal circuitry.Citation75
The involvement of leptin in the normal development of hypothalamic appetitive structures may be central to the programming of obesity in models of fetal growth restriction. Vickers and colleagues have reported that subcutaneous delivery of leptin to growth-restricted offspring of nutrient restricted rat dams in the early postnatal period normalizes the postnatal hyperphagia, and prevents the later development of obesity and hyperleptinemia in the offspring.Citation76 It has therefore been suggested that leptin levels in the circulation during fetal and early neonatal life may have implications for the development of energy balance regulatory systems in postnatal life. The underlying mechanism, and the critical window during which leptin treatment can be effective is yet to be clearly defined, and similar studies by Yura and colleagues have failed to demonstrate comparable effects.Citation77
Treatment of rat dams with leptin during pregnancy and lactation has been shown to provide the offspring with resistance to the weight gain on a high-fat diet after weaning when compared to the offspring of saline-treated controls. The increased ability to resist weight gain on a high fat diet has been attributed to the combined effects of increased energy expenditure, increased insulin-sensitivity and reduced ad libitum food intake in the offspring of leptin-treated dams.Citation78,Citation79 In another study, rats were administered subcutaneously with either leptin or vehicle from birth until weaning, and thereafter both control and leptin-treated offspring were maintained on either a control or a high-fat diet until 6 months of age. The leptin-treated offspring exhibited an enhanced resilience to weight gain on the high-fat diet when compared to controls.Citation80 Importantly, this enhanced resilience was attributed to an altered response of the central neural network to chronic high-fat feeding. Thus, high-fat feeding was associated with reduced expression of OBRb in the hypothalami of control offspring, but not in those of offspring that had been treated with leptin in the neonatal period. Furthermore, neonatal leptin treatment resulted in increased expressed of POMC mRNA during high-fat feeding which was not present in the control group.Citation80 In contrast, the regulation of the potent appetite stimulator, NPY, was unaffected by neonatal leptin administration. It would therefore appear that exposure to increased leptin concentrations during critical developmental windows can permanently modify the neural network and can potentially be beneficial for resistance against the impact of high-fat feeding in postnatal life and in counteracting the impact of fetal programming.
These findings clearly have potential clinical applications, and the prospect of leptin-supplemented infant formulas as a potential strategy for reducing the burden of childhood obesity has already been raised.Citation79 As with all relatively new areas of research, however, it is important to understand the biological implications of such a treatment, and to exercise caution in extrapolating results from altricial species to the human neonate. It is also the case that there are several studies which have failed to demonstrate any effect of neonatal leptin treatment in rodents on weight gain, appetite or body composition in the offspring.Citation77,Citation81 Therefore, whilst leptin appears to have an important role in the development of the hypothalamic circuitry, there is still a need for further study of the potential role of leptin in the programming of obesity.
Food Preferences: Is a Taste for Junk-Food Passed onto the Next Generation?
One of the most important concepts to arise in the field of programming of appetite and food intake is the notion that food preferences, and not just control of appetite per se, may be established before birth.Citation82 It is becoming increasingly clear that the impact of specific foods on the regulation of satiety is dependent not only on the quantity of food ingested, but also the quality.Citation83 Thus, highly palatable foods (high in saturated fat and/or simple sugars) promote overfeeding and rapid weight gain to a greater extent than other foods. There is emerging evidence that a preference for high fat, high sugar foods may be programmed in utero.
Langley-Evan's group provided the first evidence that exposure to perturbations of the nutritional environment in utero could alter food preferences in the offspring. In these studies, offspring of mothers exposed to a low protein diet during pregnancy and lactation were provided with ad libitum access to a selection of high-protein, high-fat and control chow after weaning, allowing measurement of the relative intake of the respective diets. Exposure to a low-protein diet in utero resulted in an increased preference for high-fat foods in the female offspring.Citation84 This provides a potential mechanism for the programming of obesity in these offspring which has been widely demonstrated by this and other groups.Citation82,Citation85 Whilst a link between maternal low-protein feeding and a preference for high-fat foods has been demonstrated, it is perhaps pertinent that the low-protein diet is also a high-carbohydrate diet, since the level of fat is maintained at a constant level. The observed association may therefore be attributable to the influence of high carbohydrate rather than low protein.
A more recent studyCitation86 has further highlighted the potential importance of the intrauterine environment for the determination of food preferences in the offspring. In this study, pregnant dams were offered either a control rat chow or a highly palatable ‘cafeteria-style’ diet, consisting of a veritable smorgasbord of donuts, cakes, pies and potato chips. Dams were maintained on this cafeteria-style (“junk food”) diet either during pregnancy only or during both pregnancy and lactation. After weaning, offspring from all dams were given ad libitum access to either a junk food diet or a control diet, and the feed intake and weight gain assessed. The offspring of dams fed a junk food diet during pregnancy and lactation consumed significantly more of the junk food than did control offspring, but both groups of offspring ate similar amounts of the chow diet. It would appear, therefore, that maternal junk-food consumption during pregnancy programs a taste for junk food in the offspring.Citation86 Importantly, the offspring of mothers fed a junk food diet during pregnancy, but switched to a control diet during lactation, showed no preference for junk foods compared to the control group. Therefore, as with the programming of the hypothalamic appetite regulating system, there appear to be critical windows for the programming of food preferences, and such programmed changes may potentially be reversible if strategies for intervention are applied during the period in which this system is still undergoing maturation. The challenge lies in understanding the critical windows for the development of these systems in the human, and in identifying potential intervention strategies that are safe, effective and can be implemented on a population level.
Conclusion and Perspective
The rising incidence of obesity is currently a major public health issue in both the developed and the developing world.Citation1 It is becoming increasing clear that, in addition to lifestyle and environmental factors, the susceptibility to obesity may have origins in early life, and that altered development of key regulatory systems within the brain is likely to play a critical role in this process.Citation53,Citation87 It is clear that the fetus has the capacity to respond and adapt to altered levels of nutrient supply, and that exposure to either inappropriately high or inappropriately low levels of nutrition before birth can permanently alter the set-point of appetite regulation in rodents, humans and large animal models. We and others have shown that the consequences of being exposed to an increased plane of nutrition before birth manifest early in postnatal development, and that these changes are consistent with further propagation of the obese phenotype in these individuals.Citation53,Citation87 There remains a certain level of debate as to the most important signal for the development of appetitive structures and for influencing the functional development of these systems; glucose, insulin and leptin have been implicated as key players, and the weight of the evidence suggests that leptin may be more important for structural development of the network,Citation37 whilst insulin and glucose may play a greater role in the control of functional development and programming.Citation56 It is also clear that there is an interaction between the prenatal and postnatal nutritional environments, and that while the development of the neuronal circuitry is complete at birth in humans and higher order mammals, the density of neuronal connections increases across the early postnatal period in these species.Citation33 This therefore raises the possibility that the developmental consequences of the prenatal nutritional environment could potentially be corrected, or at least modified, by interventions introduced in early neonatal life. Leptin has been implicated as a key factor regulating the development of the appetitive structures in rodents,Citation37,Citation74 and these studies have suggested that leptin supplementation during critical windows of development may have the potential to correct the adverse consequences of leptin deficiency.
Whilst both prenatal under-and over-nutrition have been implicated in the programming of appetite, it is arguably the case that prenatal overnutrition is by far the more common exposure in modern Western society.Citation1 More women that ever before are entering pregnancy with a high BMI,Citation88 which has clear consequences for the regulation of appetite and energy balance in the offspring before and after birth. Maternal obesity and diets dominated by high fat and high sugar foods are significant risk factors for the development of obesity and potentially for a preference for junk foods in the offspring, providing a driving force for the well-described intergenerational cycle of obesity. Our understanding of the physiological mechanisms that underlie the development of obesity has developed considerably over the past decade. The challenge now is to identify safe and effective strategies, which can be utilized during critical windows in pre and postnatal development in order to reduce the long-term adverse impact of prenatal overnutrition on metabolic health in the offspring at a population level. Now that we have an improved understanding of the importance of maternal nutrition for the long-term metabolic health of the offspring, we have a responsibility to engage with clinicians and public health professionals in order to increase awareness of this research and to work with these groups to develop detailed and meaningful nutritional guidelines for women entering pregnancy.
Abbreviations
| ARC | = | arcuate nucleus |
| PVN | = | paraventricular nucleus |
| VMN | = | ventromedial hypothalamus |
| NPY | = | neuropeptide Y |
| POMC | = | pro-opiomelanocortin |
| AGRP | = | agouti related peptide |
| CART | = | cocaine and amphetamine regulated transcript |
| OBRb | = | long (signalling) isoform of the leptin receptor |
Figures and Tables
Figure 1 A schematic overview of the appetite regulatory pathways in the adult hypothalamus. (From ref. Citation9 with permission.)
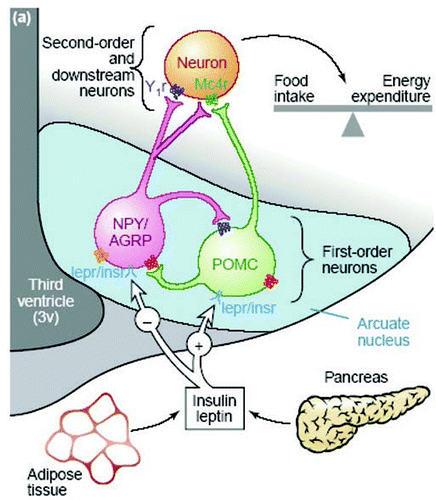
Figure 2 Autoradiographs depicting the distribution of POMC mRNA expression in the fetal ARC in saline infused (A) and glucose infused (B) fetuses at 140 ± 1 d gestation (term ∼150 d gestation). (C) The effect of glucose infusion on the expression of POMC mRNA in the fetal arcuate nucleus. * denotes p < 0.05 compared to the saline infused fetuses. ARC: arcuate nucleus, 3V: third ventricle.
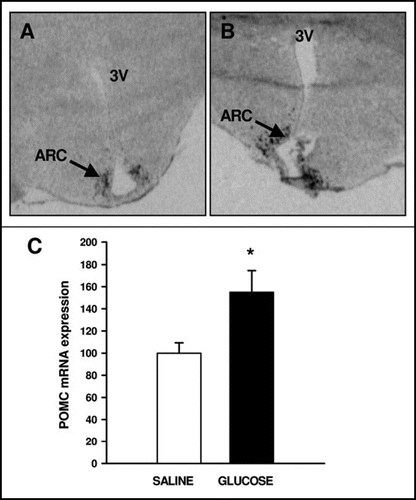
Figure 3 Autroradiographs depicting typical patterns of OBRb expression in the fetal sheep at (A) 134 d gestation (B) 141 d gestation and (C) 30 d of postnatal age. (D) Graph depicting the ratio of expression of OBRb in the arcuate nucleus (ARC) and ventromedial hypothalamus (VMH) at 134 d gestation, 141 d gestation and 30 d of postnatal age. Different letters denote significant differences between age groups p < 0.05. ARC: arcuate nucleus, VMN: ventromedial hypothalamus, 3V: third ventricle.
Acknowledgements
B.S.M. is the recipient of an Peter Doherty Postdoctoral Fellowship from the National Health and Medical Research Council of Australia.
References
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006; 295:1549 - 1555
- Haslam DW, James WP. Obesity. Lancet 2005; 366:1197 - 1209
- Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 2006; 83:461 - 465
- Prentice AM. Obesity and its potential mechanistic basis. British Medical Bulletin 2001; 60:51 - 67
- McMillen IC, Adam CL, Muhlhausler BS. Early origins of obesity: programming the appetite regulatory system. J Physiol (Lond) 2005; 565:9 - 17
- McMillen IC, Robinson JS. Developmental Origins of the Metabolic Syndrome: Prediction, Plasticity and Programming. Physiol Rev 2005; 85:571 - 633
- Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocrine Reviews 1999; 20:68 - 100
- Williams G, Bing C, Cai XJ, Harrold JA, King PJ, Liu XH. The hypothalamus and the control of energy homeostasis: different circuits, different purposes. Physiol Behav 2001; 74:683 - 701
- Barsh GS, Schwartz MW. Genetic approaches to studying energy balance: perception and integration. Nat Rev Genet 2002; 3:589 - 600
- Schwartz M, Seeley R, Campfield A, Burn P, Baskin D. Identification of targets of leptin action in rat hypothalamus. J Clin Invest 1996; 98:1101 - 1106
- Niswender KD, Baskin DG, Schwartz MW. Insulin and its evolving partnership with leptin in the hypothalamic control of energy homeostasis. Trends Endocrinol Metab 2004; 15:362 - 369
- Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron 2002; 36:199 - 211
- Raposinho PD, Pedrazzini T, White RB, Palmiter RD, Aubert ML. Chronic neuropeptide Y infusion into the lateral ventricle induces sustained feeding and obesity in mice lacking either Npy1r or Npy5r expression. Endocrinology 2004; 145:304 - 310
- Stanley BG, Leibowitz SF. Neuropeptide Y injected in the paraventricular hypothalamus: A powerful stimulant of feeding behavior. Proc Natl Acad Sci USA 1985; 82:3940 - 3943
- Woods S, Seeley R, Porte D, Schwartz M. Signals that regulate food intake and energy homeostasis. Science 1998; 280:1378 - 1383
- Gehlert DR. Role of hypothalamic neuropeptide Y in feeding and obesity. Neuropeptides 1999; 33:329 - 338
- Schwartz MW, Erickson JC, Baskin DG, Palmiter RD. Effect of Fasting and Leptin Deficiency on Hypothalamic Neuropeptide Y Gene Transcription in Vivo Revealed by Expression of a lacZ Reporter Gene. Endocrinology 1998; 139:2629 - 2635
- Dallman MF, Akana SF, Strack AM, Hanson ES, Sebastian RJ. The neural network that regulates energy balance is responsive to glucocorticoids and insulin and also regulates HPA axis responsivity at a site proximal to CRF neurons. Annals of the New York Acadamy of Sciences 1995; 771:730 - 742
- Elias CF, Asckenasi C, Lee C, Kelly J, Ahima RS, Bjørbæk C, Flier JK, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron 1999; 23:775 - 786
- Hwa JJ, Ghibaudi L, Gao J, Parker EM. Central melanocortin system modulates energy intake and expenditure of obese and lean Zucker rats. American Journal of Physiology (Regulatory and Integrative Comparative Physiology) 2001; 281:444 - 451
- Tritos NA, Maratos-Flier E. Two important systems in energy homeostasis: melanocortins and melanin-concentrating hormone. Neuropeptides 1999; 33:339 - 349
- Vergoni AV, Bertolini A. Role of melanocortins in the central control of feeding. European J Pharmacol 2000; 405:25 - 32
- Yeo GSH, Farooqi IS, Challis BG, Jackson RS, O'Rahilly S. The role of melanocortin signalling in the control of body weight: evidence from human and murine genetic models. Q J Med 2000; 93:7 - 14
- Pritchard LE, Turnbull AV, White A. Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signalling and obesity. J Endocrinol 2002; 172:411 - 421
- Mizuno TM, Makimura H, Silverstein J, Roberts JL, Lopingco T, Mobbs CV. Fasting regulates hypothalamic neuropeptide Y, agouti-related peptide, and proopiomelanocortin in diabetic mice independent of changes in leptin or insulin. Endocrinology 1999; 140:4551 - 4557
- Rossi M, Kim MS, Morgan DGA, Small CJ, Edwards CMB, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, Smith DM, Yagaloff K, Ghatei MA, Bloom SR. A C-terminal fragment of agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology 1998; 139:4428 - 4431
- Hunter RG, Philpot K, Vicentic A, Dominguez G, Hubert GW, Kuhar MJ. CART in feeding and obesity. Trends Endocrinol Metab 2004; 15:454 - 459
- Oliveira J, Vani X, Fazio MA, Miranda MTM, da Silva JM, Bittencourt JC, Elias CF, Miranda A. Leptin fragments induce c-Fos immunoreactivity in rat hypothalamus. Regul Peptid 2005; 127:123 - 132
- Vrang N, Kristensen P, Tang-Christensen M, Larsen P. Effects of leptin on arcuate pro-opiomelanocortin and cocaine-amphetamine-regulated transcript expression are independent of circulating levels of corticosterone. J Neuroendocrinol 2002; 14:880 - 886
- Li HY, Hwang HW, Hu YH. Functional characterizations of cocaine- and amphetamine-regulated transcript mRNA expression in rat hypothalamus. Neurosci Lett 2002; 323:203 - 206
- Hay WW. Placental transport of nutrients to the fetus. Hormone Res 1994; 42:215 - 222
- Grayson BE, Allen SE, Billes SK, Williams SM, Smith MS, Grove KL. Prenatal development of hypothalamic neuropeptide systems in the nonhuman primate. Neuroscience 2006; 143:975 - 986
- Grove KL, Smith MS. Ontogeny of the hypothalamic neuropeptide Y system. Physiology & Behavior 2003; 79:47 - 63
- Warnes KE, Morris MJ, Symonds ME, Phillips ID, Clarke IJ, Owens JA, McMillen IC. Effects of increasing gestation, cortisol and maternal undernutrition on hypothalamic neuropeptide Y expression in the sheep fetus. J Neuroendocrinol 1998; 10:51 - 57
- Muhlhausler BS, McMillen IC, Rouzaud G, Findlay PA, Marrocco EM, Rhind SM, Adam CL. Appetite regulatory neuropeptides are expressed in the sheep hypothalamus before birth. J Neuroendocrinol 2004; 16:502 - 507
- Koutcherov Y, Mai JK, Paxinos G. Hypothalamus of the human fetus. J Chem Neuroanat 2003; 26:253 - 270
- Bouret SG, Draper SJ, Simerly RB. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci 2004; 24:2797 - 2805
- Grove KL, Brogan RS, Smith MS. Novel expression of neuropeptide Y (NPY) mRNA in hypothalamic regions during development: region-specific effects of maternal deprivation on NPY and agouti-related protein mRNA. Endocrinology 2001; 142:4771 - 4776
- Singh BS, Westfall TC, Devaskar SU. Maternal diabetes-induced hyperglycemia and acute intracerebral hyperinsulinism suppress fetal brain neuropeptide Y concenrations. Endocrinology 1997; 138:963 - 969
- Muhlhausler BS, Adam CL, Marrocco EM, Findlay PA, Roberts CT, McFarlane JR, Kauter KG, McMillen IC. Impact of glucose infusion on the structural and functional characteristics of adipose tissue and on hypothalamic gene expression for appetite regulatory neuropeptides in the sheep fetus during late gestation. J Physiol 2005; 565:185 - 195
- Greenwood PL, Hunt AS, Hermanson JW. A.W.B. Effects of birth weight and postnatal nutrition on neonatal sheep: I. Body growth and composition, and some aspects of energetic efficiency. J Anim Sci 1998; 76:2354 - 2367
- De Blasio MJ, Gatford KL, Robinson JS, Owens JA. Placental restriction of fetal growth reduces size at birth and alters postnatal growth, feeding activity, and adiposity in the young lamb. Am J Physiol Regul Integr Comp Physiol 2007; 292:875 - 886
- Hokken-Koelega AC, De Ridder MA, Lemmen RJ, Den Hartog H, De Muinck Keizer-Schrama SM, Drop SL. Children born small for gestational age: Do they catch up?. Pediatr Res 1995; 38:267 - 271
- Jequier E. Leptin signaling adiposity, and energy balance. Ann N Y Acad Sci 2002; 967:379 - 388
- Yuen BSJ, McMillen IC, Symonds ME, Owens PC. Abundance of leptin mRNA in fetal adipose tissue is related to fetal body weight. J Endocrinol 1999; 163:11 - 14
- Muhlhausler BS, Roberts CT, McFarlane JR, Kauter KG, McMillen IC. Fetal leptin is a signal of fat mass independent of maternal nutrition in ewes fed at or above maintenance energy requirements. Biol Reprod 2002; 67:493 - 499
- Ehrhardt RA, Bell AW, Boisclair YR. Spatial and developmental regulation of leptin in fetal sheep. Am J Physiol Regul Integr Comp Physiol 2002; 282:1628 - 1635
- Yuen BSJ, Owens PC, Muhlhausler BS, Roberts CK, Symonds ME, Keisler DH, McFarlane JR, Kauter K, Evens Y, McMillen IC. Leptin alters the structural and functional characteristics of adipose tissue before birth. FASEB J 2003; 17:1102 - 1104
- Muhlhausler BS, Dorosz JD, Adam CL, Findlay PA, McMillen IC. Hypothalamic NPY Expression is inversely correlated with plasma leptin concentrations in the late gestation fetal sheep. Reprod Sci 2007; 14:207
- Proulx K, Richard D, Walker CD. Leptin regulates appetite-related neuropeptides in the hypothalamus of developing rats without affecting food intake. Endocrinology 2002; 143:4683 - 4692
- Ahima RS, Hileman SM. Postnatal regulation of hypothalamic neuropeptide expression by leptin: implications for energy balance and body weight regulation. Regul Peptid 2000; 92:1 - 7
- Williams LM, Adam CL, Mercer JG, Moar KM, Slater D, Hunter L, Findlay PA, Hoggard N. Leptin receptor and neuropeptide Y gene expression in the sheepbrain. J Neuroendocrinol 1999; 11:165 - 169
- Muhlhausler BS, Adam CL, Findlay PA, Duffield JA, McMillen IC. Increased maternal nutrition alters development of the appetite-regulating network in the brain. FASEB J 2006; 20:1257 - 1259
- Oscai LB, McGarr JA. Evidence that the amount of food consumed in early life fixes appetite in the rat. Am J Physiol Regul Integr Comp Physiol 1978; 235:141 - 144
- Plagemann A, Harder T, Janert U, Rake A, Rittel F, Rohde W, Dorner G. Malformations of hypothalamic nuclei in hyperinsulinemic offspring of rats with gestational diabetes. Dev Neurosci 1999; 21:58 - 67
- Plagemann A, Harder T, Rake A, Janert U, Melchior K, Rohde W, Dorner G. Morphological alterations of hypothalamic nuclei due to intrahypothalamic hyperinsulinism in newborn rats. Int J Dev Neurosci 1999; 17:37 - 44
- Plagemann A, Harder T, Rake A, Melchior K, Rittel F, Rohde W, Dorner G. Hypothalamic insulin and neuropeptide Y in the offspring of gestational diabetic mother rats. Neuroreport 1998; 9:4069 - 4073
- Plagemann A, Harder T, Rake A, Voits M, Fink H, Rohde W, Dorner G. Perinatal elevation of hypothalamic insulin, acquired malformation of hypothalamic galaninergic neurons, and syndrome X-like alterations in adulthood of neonatally overfed rats. Brain Res 1999; 836:146 - 155
- Plagemann A, Harder T, Melchior K, Rake A, Rohde W, Dorner G. Elevation of hypothalamic neuropeptide Y-neurons in adult offspring of diabetic mother rats. Neuroreport 1999; 10:3211 - 3316
- Fahrenkrog S, Harder T, Stolaczyk E, Melchior K, Franke K, Dudenhausen JW, Plagemann A. Cross-fostering to diabetic rat dams affects early development of mediobasal hypothalamic nuclei regulating food intake, body weight, and metabolism. J Nutr 2004; 134:648 - 654
- Davidowa H, Li Y, Plagemann A. Altered responses to orexigenic (AGRP, MCH) and anorexigenic (MSH, CART) neuropeptides of paraventricular hypothalamic neurons in early postnatally overfed rats. Eur J Neurosci 2003; 18:613 - 621
- Davidowa H, Plagemann A. Decreased inhibition by leptin of hypothalamic arcuate neurons in neonatally overfed young rats. Neuroreport 2000; 11:2795 - 2798
- Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal Origins of hyperphagia, obesity and hypertension and postnatal amplification by hypercaloric nutrition. American Journal of Physiology (Endocrinology and Metabloism) 2000; 279:83 - 87
- Breier BH, Vickers MH, Ikenasion BA, Chan KY, Wong WPS. Fetal programming of appetite and obesity. Mol Cell Endocrinol 2001; 185:73 - 79
- Plagemann A, Harder T, Rake A, Melchior K, Rohde W, Dorner G. Hypothalamic nuclei are malformed in weanling offspring of low protein malnourished rat dams. J Nutr 2000; 130:2582 - 2589
- Plagemann A, Waas T, Harder T, Rittel F, Ziska T, Rohde W. Hypothalamic neuropeptide Y levels in weaning offspring of low-protein malnourished mother rats. Neuropeptides 2000; 34:1 - 6
- Harder T, Plagemann A, Rohde W, Dorner G. Syndrome X-like alterations in adult female rats due to neonatal insulin treatment. Metabolism 1998; 47:855 - 862
- Harder T, Rake A, Rohde W, Doerner G, Plagemann A. Overweight and increased diabetes susceptibility in neonatally insulin-treated adult rats. Endocr Regul 1999; 33:25 - 31
- Harder T, Aerts L, Franke K, Van Bree R, Van Assche FA, Plagemann A. Pancreatic islet transplantation in diabetic pregnant rats prevents acquired malformation of the ventromedial hypothalamic nucleus in their offspring. Neurosci Lett 2001; 299:85 - 88
- Wang Q, Bing C, Al-Barazanji K, Mossakowaska DE, Wang X, McBay DL, Neville WA, Taddayon M, Pickavance L, Dryden S, Thomas MEA, McHale MT, Gloyer IS, Wilson S, Buckingham R, Arch JRS, Trayhurn P, Williams G. Interactions between leptin and hypothalamic neuropeptide Y neurons in the control of food intake and energy homeostasis in the rat. Diabetes 1997; 46:335 - 341
- Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, Hale J, Hoffmann J, Hsiung HM, Kriauciunas A, MacKeller W, Rosteck PR, Schoner B, Smith D, Tinsley FC, Zhang X, Heiman M. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature 1995; 377:530 - 532
- Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology 1997; 138:4489 - 4492
- Vergoni AV, Schioth HB, Bertolini A. Melanocortins and feeding behavior. Biomed Pharmacother 2000; 54:129 - 134
- Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 2004; 304:108 - 110
- Bouret SG, Simerly RB. Minireview: Leptin and development of hypothalamic feeding circuits. Endocrinology 2004; 145:2621 - 2626
- Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Neonatal leptin treatment reverses developmental programming. Endocrinology 2005; 146:4211 - 4216
- Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, Kawamura M, Takemura M, Kakui K, Ogawa Y, Fujii S. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab 2005; 1:371 - 378
- Stocker C, O'Dowd J, Morton NM, Wargent E, Sennitt MV, Hislop D, Glund S, Seckl JR, Arch JRS, Cawthorne MA. Modulation of susceptibility to weight gain and insulin resistance in low birthweight rats by treatment of their mothers with leptin during pregnancy and lactation. Int J Obes Relat Metab Disord 2003; 28:129 - 136
- Stocker CJ, Wargent E, O'Dowd J, Cornick C, Speakman JR, Arch JRS, Cawthorne MA. Prevention of diet-induced obesity and impaired glucose tolerance in rats following administration of leptin to their mothers. Am J Physiol Regul Integr Comp Physiol 2007; 292:1810 - 1818
- Pico C, Oliver P, Sanchez J, Miralles O, Caimari A, Priego T, Palou A. The intake of physiological doses of leptin during lactation in rats prevents obesity in later life. Int J Obes 2007; 31:1199 - 1209
- Schmidt I, Fritz A, Scholch C, Schneider D, Simon E, Plagemann A. The effect of leptin treatment on the development of obesity in overfed suckling Wistar rats. Int J Obes Relat Metab Disord 2001; 25:1168 - 1174
- Langley-Evans SC, Bellinger L, McMullen S. Animal models of programming: early life influences on appetite and feeding behavior. Matern Child Nutr 2005; 1:142 - 148
- Fulton S, Richard D, Woodside B, Shizgal P. Food restriction and leptin impact brain reward circuitry in lean and obese Zucker rats. Behav Brain Res 2004; 155:319 - 329
- Bellinger L, Lilley C, Langley-Evans SC. Prenatal exposure to a maternal low-protein diet programmes a preference for high-fat foods in the young rat. Br J Nutr 2004; 92:513 - 520
- Ozanne SE. Metabolic programming in animals. Br Med Bull 2001; 60:143 - 152
- Bayol SA, Farrington SJ, Stickland NC. A maternal ‘junk food’ diet in pregnancy and lactation promotes an exacerbated taste for ‘junk food’ and a greater propensity for obesity in rat offspring. Br J Nutr 2007; 98:843 - 851
- Plagemann A, Harder T, Rake A, Waas T, Melchior K, Ziska T, Rohde W, Dorner G. Observations on the orexigenic hypothalamic neuropeptide Y-system in neonatally overfed weanling rats. J Neuroendocrinol 1999; 11:541 - 546
- LaCoursiere DY, Bloebaum L, Duncan JD, Varner MW. Population-based trends and correlates of maternal overweight and obesity, Utah 1991–2001. Am J Obstet Gynecol 2005; 192:832 - 839