Abstract
Maternal dexamethasone administration promotes fetal maturation such that thermoregulation is improved following premature delivery and is thus comparable with a full term birth. In the present study we determined the impact of dexamethasone on both the mothers’ metabolic status together with adipose tissue function in the newborn. Glucocorticoid action, adipokine gene expression and mitochondrial protein abundance were measured in perirenal adipose tissue of neonatal sheep that were born into either a warm (30oC) or cool (15oC) ambient temperature at 140 days of gestation (dGA; term ~147 dGA), either two days after maternal dexamethasone administration, or at 146 dGA for controls. Dexamethasone administration resulted in a reduction in maternal food intake in conjunction with raised plasma cortisol and free triiodothyronine. In offspring of dexamethasone administered mothers, plasma cortisol was lower and non-esterified fatty acids (NEFA) higher than controls. Glucocorticoid receptor (GR), 11β-hydroxysteroid dehydrogenase (11β-HSD1), interleukin-6 and uncoupling protein (UCP)1 and 2 mRNA together with voltage dependent anion channel, cytochrome c protein and UCP1 abundance were all increased by dexamethasone administration and being born into a cool ambient temperature. Gene expression of tumor necrosis factor α, adiponectin and peroxisome proliferator-activated receptor transcription factor γ were unaffected by dexamethasone. The abundance of mRNA for the GR, 11β-HSD1, UCP1 and 2 mRNA together with each protein were positively correlated to plasma NEFA and negatively correlated to plasma cortisol. In conclusion, despite reduced maternal food intake dexamethasone promotes maturation of glucocorticoid action and mitochondrial protein abundance in the newborn, an adaptation dependent on delivery temperature.
Introduction
The increase in fetal plasma cortisol is one endocrine factor that is critical in enabling the newborn to adapt to the extrauterine environment.Citation1,Citation2 In particular this enables the newborn to initiate non-shivering thermogenesis an adaptation that is mediated in part by promoting the rapid appearance of the brown adipose tissue specific uncoupling protein (UCP)1 which is uniquely able to generate very large amounts of heat.Citation3,Citation4 In precocious thermoregulators, including humans and sheep, adipose tissue comprises both brown and white adipocytes of which the latter posses UCP2 but not UCP1.Citation5 Changes in adipose tissue at the time of birth include an increase in glucocorticoid action mediated by a rise in receptor mRNA abundance in conjunction with an increase in local cortisol action due to increased 11β-hydroxysteroid dehydrogenase type 1 (11β HSD1) which is the dominant 11βHSD in this tissue.Citation5,Citation6 Moreover, changes in adipocyte function around the time of birth, particularly in the mitochondria are not confined to UCPs but include an increase in abundance of the voltage-dependent anion channel (VDAC) which is located with the outer mitochondrial membrane together with cytochrome c within the mitochondrial space.Citation7 Taken together these adaptations within adipose tissue of the newborn are critical in ensuring the newborn is effectively able to meet the metabolic demands at birthCitation8 and can be further dependent on maternal thyroid status.Citation9
Other important secretory products from white adipose tissue include the adipokines such as leptin, interleukin (IL)-6, tumor necrosis factor (TNF)α and adiponectin.Citation10 At the same time the peroxisome proliferator-activated receptor (PPAR) transcription factor γ is highly abundant in brown adipose tissue of the new bornCitation11 but the main factors regulating its expression are currently unknown. It is established that although leptin mRNA is abundant in adipose tissue of the new born sheep it is not affected by fetal cortisol status,Citation12 maternal dexamethasone or ambient temperature.Citation13 The extent to which other major adipokines, particularly those linked to inflammation, or PPAR γ may be up regulated by dexamethasone and/or ambient temperature in the newborn is currently not known and was therefore investigated.
It is well established that maternal dexamethasone administration promotes the maturation of fetal brown adipose tissue and enables the newborn to better adapt to the thermal challenge after birth.Citation14 Consequently even though these offspring are born ∼1 week prematurely they do not become hypothermic and are as effective at adapting to the thermal challenge of the extrauterine environment as sheep born at term. The extent to which changes in other characteristics of adipose tissue may contribute to this adaptation are not known, neither is the extent to which these may be directly influenced by delivery temperature. It has not yet been determined whether such adaptations are due to changes in glucocorticoids alone or are mediated in part by the accompanying rise in lipolysis and increase in plasma non-esterified fatty acids (NEFA).Citation14 In the present study, the effect of delivery temperature, i.e. being born into a warm or cool ambient temperature was examined with respect to the abundance of mitochondrial proteins and glucocorticoid action within brown adipose tissue. At the same time, the extent to which these neonatal changes may be amplified by maternal adaptations following dexamethasone administration was determined. This analysis was undertaken on perirenal adipose tissue which constitutes ∼80% of fat mass in the newborn sheep.Citation15 Importantly, adaptations of this type may not only impact of adipose tissue at birth but could potentially extend into later life given the long lasting changes previously described within ovine adipose tissue following maternal nutritional manipulation at defined stages of gestation.Citation5
Changes in maternal glucocorticoid and thyroid status will also affect metabolism, resulting in an increase in hepatic gluconeogenesis in conjunction with stimulation of lipolysis and a concomitant rise in plasma NEFA.Citation16,Citation17 At the same time, maternal food intake may decrease; an adaptation that has been repeatably demonstrated in the pregnant ratCitation18–Citation20 and although it has no effect on outcome at birth it is clearly of relevance when considering the longer term outcomes.Citation18,Citation19 Reduced maternal food consumption is also likely to impact on maternal thyroid statusCitation21 that can be important in determining postnatal outcomes.Citation22 In the present study therefore the extent to which maternal dexamethasone administration may effect both the maternal metabolic and hormonal environment, in particular her thyroid status, as well as her food intake was also examined.
Results
Effect of maternal dexamethasone administration on maternal food intake, plasma hormones and metabolites and hepatic 5′MDI activity.
Maternal food intake of roughage, but not concentrate, was significantly reduced by dexamethasone that resulted in twice as much hay being left uneaten [daily amount of hay not eaten on the three days before dexamethasone 378 ± 94; daily amount of hay not eaten following dexamethasone administration 719 ± 76 g (n = 4) (p < 0.01)] thereby contributing to a 15% reduction in estimated metabolisable energy intake following dexamethasone [Pre-dexamethasone: 15.4 ± 0.5; Post dexamethasone: 13.2 ± 0.6 MJ/day (p < 0.05)]. In controls, food intake remained unchanged over the same period when it was 15.7 ± 0.4 MJ/day.
Maternal dexamethasone had no effect on plasma glucose or NEFA, but resulted in increased plasma concentrations of free T3 and cortisol, whereas T4 was reduced (). The activity of type I 5′MDI was also higher in livers of dexamethasone administered mothers compared with controls [Dexamethasone − 1907 ± 180; Controls − 1486 ± 203 pmol I-released/mg protein/h (p < 0.05)].
Effect of maternal dexamethasone administration and delivery temperature on UCP1, UCP2, GR and 11βHSD1 mRNA, and VDAC and cytochrome c mitochondrial protein abundance in perirenal adipose tissue in the neonatal lamb.
Maternal dexamethasone resulted in increased UCP2 mRNA together with VDAC cytochrome c protein abundance in fetal adipose tissue compared with controls, whilst mRNA abundance for UCP1, GR and 11βHSD1 were unaffected ( and ). There were no significant differences in plasma concentrations of cortisol (Control − 55 ± 9; dexamethasone administered − 85 ± 16 nmol/l) or free T3 (Control − 1.47 ± 0.39; dexamethasone administered − 0.76 ± 16 pmol/l) in the fetuses whereas plasma NEFA were increased by dexamethasone [Control − 0.45 ± 0.05; dexamethasone administered − 0.87 ± 0.08 nmol/l (p < 0.01)].
In the neonate, irrespective of delivery temperature, maternal dexamethasone resulted in increased mRNA abundance for all genes measured, in conjunction with increased VDAC cytochrome c protein abundance. At the same time CD offspring had higher UCP1, 2, GR and 11βHSD1 mRNA together with VDAC and cytochrome c protein abundance compared with their WD siblings. At 6 h after birth plasma concentrations of cortisol were reduced in offspring born to dexamethasone administered mothers whereas plasma NEFA were raised, adaptations that were greatest in the CD group [Cortisol − CD: controls − 103 ± 14; dexamethasone administered − 39 ± 11 nmol/l (p < 0.01); NEFA − CD: controls − 0.53 ± 0.04; dexamethasone administered − 1.68 ± 0.11 nmol/l (p < 0.001)].
A number of significant relationships were observed between the molecular, endocrine and metabolic parameters measured in all groups of offspring. Plasma cortisol concentration was negatively correlated with mRNA abundance for UCP2, UCP1, GR and 11βHSD1 together with VDAC and cytochrome c protein abundance (). In contrast, plasma NEFA was positively correlated with each of these measurements (). Partial correlation analysis revealed that plasma NEFA concentration was a significant independent determinant of UCP2 gene (correlation coefficient = 0.6173; p = 0.006) expression in contrast to plasma cortisol concentration.
There was also a negative correlation between plasma cortisol and NEFA (R2 = 0.71, p < 0.0001). At the same time UCP1 mRNA and its translated protein were positively correlated as was mRNA abundance for GR and 11βHSD1 (), whilst UCP2 mRNA plus VDAC and cytochrome c abundance were all positively correlated with 11βHSD1 mRNA. Partial correlation analysis revealed that plasma NEFA concentration was a significant independent determinant of UCP1 (correlation coefficient = 0.6003; p = 0.008) and 11βHSD1 (correlation coefficient = 0.4675; p = 0.050) mRNA, and VDAC (correlation coefficient = 0.6579; p = 0.003) and cytochrome c (correlation coefficient = 0.7896; p < 0.0001) protein expression in perirenal adipose tissue, in contrast to plasma cortisol concentration. Neither plasma NEFA nor cortisol concentrations were significant independent determinants of GR gene expression.
Effect of maternal dexamethasone administration and delivery temperature on IL-6, adiponectin, TNFα and PPARγ mRNA in perirenal adipose tissue in the neonate.
Maternal dexamethasone resulted in increased IL-6 mRNA abundance but only in those neonates delivered into a cool ambient temperature (). There was no effect of either maternal dexamethasone or delivery temperature on adiponectin, TNFα or PPARγ mRNA abundance (data not shown).
Discussion
We have shown for the first time in a large animal species that maternal administration of dexamethasone reduces her food intake, although this effect is confined to roughage rather than concentrate intake, but has no deleterious effects on her metabolic and endocrine profile. Interestingly, maternal plasma cortisol was raised which is in accord with the immediate effects of a more pronounced degree of nutrient restriction (i.e. 50%) in late gestation.Citation23 Taken together these findings indicate that feeding behavioral responses may potentially be more important in comparison to centrally mediated mechanisms regulate the stress axis in pregnancy.Citation24 At the same time dexamethasone promotes adipose tissue maturation with the result that the newborn was better adapted to meet the cold challenge of the extrauterine environment.Citation14 Consequently even though offspring born to dexamethasone administered mothers were born ∼1 week prematurely by caesarean section they were comparable to near term controls. The magnitude of neonatal response to maternal dexamethasone was dependent not only on the change in neonatal plasma cortisol that accompanied maternal dexamethasone but the concomitant rise in plasma NEFA concentrations. It should, however, be noted that all animals in the present study exhibited high plasma cortisol concentrations that are essential for effective adaptation to the extrauterine environment.Citation25
We have also shown specific effects on adipose tissue with an increase in each mitochondrial protein studied either at the mRNA or protein level although this only became apparent after birth. In contrast of the adipokines investigated only IL-6 mRNA abundance was affected by maternal dexamethasone and is in accord with previous findings on leptin.Citation13 These results therefore support the hypothesis that only those processes within adipose tissue that are necessary to promote the mobilization of NEFA and concomitant utilization as an energy source are initiated at birth. This adaptation is clearly in contrast to that seen in the fetus when plasma NEFA remain at basal values when cortisol and T3 primarily regulate UCP abundance and glucocorticoid action.Citation6 Furthermore, in the newborn sheep there appear to be minimal changes in the potential ability of adipose tissue to secrete inflammatory hormones. Our observation that IL-6 was specifically up regulated in those offspring born to mothers given dexamethasone and then exposed to a cool ambient temperature support a role for IL-6 in non-shivering thermogenesis as seen in rodents.Citation26
Delivery into a cool ambient had the greatest stimulatory effect on adipose tissue with respect to all the measurements we have made. This effect is likely to reflect the additional sympathetic stimulation that accompanies cold exposureCitation27 that further acts to ensure the newborn is able to meet the increased metabolic demands necessary to fully restore its body temperature.Citation14,Citation28 An important component of this process is the rise in plasma NEFA,Citation29 the concentration of which was strongly correlated with all mRNA and protein measurements made on adipose tissue. These findings indicate the close relationship between the rate of metabolic substrate supply to brown adipose tissue and the increase in UCP1 both at the level of mRNA and protein. The increase in UCP1 was accompanied by a parallel rise in both VDAC and cytochrome c abundance which is not unexpected as both increase at birth and are know to be sensitive to cortisol status during pre- and postnatal development.Citation7,Citation30 The further enhancement in the abundance of each of these proteins in conjunction with the parallel rise in glucocorticoid action as indicated by the increase in mRNA for both the GR and 11βHSD1 confirm the tightly controlled co-ordination of these processes.Citation31 This is not unexpected because of the critical need for the newborn to effectively initiate nonshivering thermogenesis in order to prevent hypothermia which can be life threatening.Citation32 Indeed, given the close relationship between mRNA abundance and protein within the brown adipocyte it is very likely that the changes in glucocorticoid expression at the mRNA level will be translated into functional effects. This could be expected, as we have observed positive relationships between GR mRNA and mitochondrial protein abundance. Another notable finding in our study was that delivery into a warm ambient temperature actually resulted in a reduction of all parameters measured to levels lower than those observed in the fetus, irrespective of maternal dexamethasone administration. The most likely explanation for this is that warm delivery results in a lower metabolic stimulusCitation14,Citation28 thereby contributing to a significant reduction in rate of appearance of neonatal stimulatory hormones including thyroid hormones,Citation33 as well as prolactinCitation34 which all impact on adipose tissue function in the neonate and can be affected by cortisol.Citation4 Neither delivery temperature nor dexamethasone, however, have any effect on local production of T3 via the enzyme 5′MDI in liver or adipose tissue,Citation14 thus contrasting with the effect seen in the maternal liver.
As for UCP1, the abundance of UCP2 mRNA was greatest in adipose tissue sampled from those offspring born into a cool ambient temperature and whose mothers had been given dexamethasone. This adaptation was accompanied by a parallel increase in GR and 11βHSD1 mRNA, for which there was a strong positive relationship with UCP2 mRNA abundance. These findings indicate the sensitivity of both UCP1 and 2 to glucocorticoid status around the time of birth.Citation12,Citation35 The extent to which a change in local cortisol synthesis via the enzyme 11βHSD1, which is predicted to be enhanced,Citation36 rather than direct stimulation of the GR receptor that is one potential stimulus for the rise in UCP remains to be clarified. With regard to the more immediate effects of maternal dexamethasone administration on fetal adipose tissue maturation, it was only gene expression for UCP2 that was raised prior to the onset of breathing. Unfortunately it is not currently technically feasible to establish whether this change would be immediately translated into protein because all available antibodies cross react with UCPCitation37 and none are presently specific for ovine UCP2.Citation31 In ovine tissues which do not posses UCP1, namely the lung, it has been possible to make measurements of both mRNA and protein and a close correlation between each has been shown,Citation35 contradicting findings in adult rats.Citation37 This has led to the suggestion that the translation of UCP2 mRNA to protein may differ between species,Citation35 particularly in those which are born mature at birth and need to rapidly increase their metabolic rate and thus oxygen requirements. In this regard UCP2 may play a pivotal role, in the lung at least,Citation35 so it would be of great interest to extend the present study to further tissues in order to see whether parallel effects are seen.
It is known that in contrast to UCP1, which is lost from ovine adipose tissue soon after birthCitation7 and is barely detectable by one month of age as white adipose tissue dominates, that the abundance of UCP2 increases further after birth.Citation5 This rise parallels the increase in adipose tissue mass and may be indicative of the differential location of UCP2 being primarily present within white rather than brown adipocytes. We also observed a close relationship between changes in UCP2 mRNA and cytochrome c abundance which extends previous findings from fetal studies in which cortisol status has been significantly altered.Citation30 A positive relationship of this type could be indicative of the combined roles of UCP2 and cytochrome c in apoptosisCitation38 which may be the mechanism by which the transition from brown to white adipocytes is rapidly brought about after birth. Indeed the rate of this transition after birth can be determined by the temperature in which the offspring are reared.Citation27 The extent to which developmental changes in UCP2 during the early period of white adipose tissue growth contribute to the role of UCP2 in reactive oxygen species production and related processes as indicated from studies in which UCP2 has been deleted and in vitro systemsCitation39 remains to be established.
In conclusion, we have shown for the first time the close relationship between changes in key mitochondrial proteins and glucocorticoid action within adipose tissue of the newborn. These adaptations at birth are strongly influenced by delivery temperature and are enhanced by maternal dexamethasone administration thereby improving the ability of the newborn to effectively adapt to the cold exposure of the extrauterine environment.
Materials and Methods
Animals and experimental design.
Eight multiparous Bluefaced-Leicester cross Swaledale sheep of recorded mating date and confirmed as bearing triplets were entered into the study. Each animal was individually housed from four weeks before predicted delivery date and fed a daily diet of 0.4–0.6 kg of concentrate and 1.2 kg of hay, sufficient to fully meet the total energy requirements for a late gestation sheep.Citation40 Food intake was recorded daily throughout. A summary of the experimental protocol as previously establishedCitation13,Citation28,Citation34 is illustrated in . In addition, all sheep had jugular vein catheters inserted under local anaesthesia to enable maternal blood sampling to be carried out on 137 days and 139 days gestation from each sheep. At 138 days gestation, four sheep were randomly selected and given an intramuscular injection of dexamethasone (16 mg i.e. 0.09 mg/kg/day (Dexadreson; National Veterinary Supplies, Stoke, UKCitation14) whereas controls were given a placebo injection of vehicle. Caesarean section delivery was then performed on 140 days gestation (as described by ref. Citation28) while the four control mothers under went caesarean section birth on 146 days gestation. Offspring born to controls were delivered at this later time point, rather than 140 days gestation, when they remain hypothermic and rarely survive beyond 2 hours after birth if born by caesarean section.Citation32 All sheep produced live triplets.
One newborn from each mother was immediately placed in a warm ambient temperature of 30°C (i.e. warm delivered; WD) and its sibling into a cool ambient temperature of 15°C (i.e. cool delivered; CD). Each newborn also had a jugular vein catheter. inserted under local anaesthesia to enable hourly blood sampling.Citation14 The remaining fetus and the mother were both euthanased by intravenous administration of barbiturate (100 mg/kg pentobarbital sodium; Euthatal, National Veterinary Supplies). From the fetus, perirenal adipose tissue was immediately removed, placed in liquid nitrogen, and stored at −80°C until analyzed, whilst from the mother a representative portion of the liver (i.e. 20 g from the same position of the right lobe from each animal) was taken. Similarly, perirenal adipose tissue was sampled from WD and CD offspring at 6 hours of age after intravenous administration of barbiturate. All offspring remained unfed. There was no difference in total conceptus weight between groups and although lambs born to controls weighed (3.77 ± 0.22 kg) more than those born to dexamethasone exposed mothers (3.21 + 0.15 kg) these weights are in accord with those found in triplet bearing sheep of the breed used in the present study.Citation41
Laboratory analyses.
Protein detection. Mitochondria were prepared from 1 g of perirenal adipose tissue and the abundance of UCP1, VDAC and cytochrome c determined (as previously described in ref. Citation7).
Hepatic activity of type I iodothyronine 5′-monodeiodinase (5′MDI) was also determined in protein homogenates prepared from the maternal liver by measuring the rate of release of 125I- from 125I reverse triiodothyronine (T3) (as described by ref. Citation33).
Messenger RNA detection.
Total RNA was isolated from perirenal adipose tissue using Tri-Reagent (Sigma, Poole, UK) and the expression of UCP1, UCP2, GR (type 2) and 11βHSD1 mRNA determined by reverse transcriptase-polymerase chain reaction (RT-PCR), as previously described in detail by Gnanalingham et al.Citation5,Citation6
In the case of PPAR γ, IL-6, TNF and adiponectin mRNA were determined by quantitative real-time PCR performed on Quantica real-time thermocycler (Techne Incorporated, Barloworld Scientific Ltd, Stone, UK) using a 2x SYBR Green I master mix (Abgene AB-1159) in a 20 µl reaction volume, containing 1 µl reverse transcriptase reaction. A sequenced and isolated PCR amplicon was used to produce a standard curve, to ensure equal PCR amplification efficiency. 18S ribosomal RNA was used as a housekeeping gene. Previously unpublished PCR primers sequences are shown in . Gene expressions were measured by the 2-ΔCT method.Citation42 Each assay was performed in duplicate on all samples from each group of sheep. Levels of gene expression were expressed as relative values using fetal control values as a reference (1.0).
Plasma hormone and metabolite analyses.
Plasma concentrations of NEFA and glucose were measured enzymatically.Citation33 The plasma concentrations of cortisol and free T3 were measured by a magnetic solid phase immunoenzymatic assay kit (serozyme kit number 14391504 and 12861504 respectively)Citation43 and total thyroxine (T4) and T3 by radioimmunoassay.Citation33
Statistical analyses.
The effects of maternal dexamethasone administration and ambient temperature within groups were determined by ANOVA. Significant correlations between molecular indices and physiological parameters were undertaken by a two-tailed Spearman's Rank Order Test (SPSS v11.0, SPSS Inc.) in controls and dexamethasone administered neonates at six hours of age i.e. closest to the time of tissue sampling. In addition partial correlation analysis was undertaken when there was more than one variable correlated with the measured molecular parameter, to determine which variable had the greatest effect. Values are presented as means ± SEM and p < 0.05 was taken to indicate a significant difference.
Figures and Tables
Figure 1 Summary of experimental protocol used to determine the effect of delivery temperature and maternal dexamethasone administration on the abundance of mRNA and mitochondrial proteins in perirenal adipose tissue of the neonatal lamb. dGA; days of gestation, term ∼147 dGA.
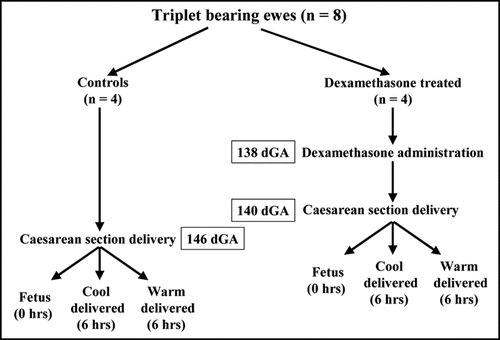
Figure 2 Abundance of (A) uncoupling protein (UCP)-1 mRNA, (B) UCP2 mRNA, (C) glucocorticoid receptor (GR) mRNA and (D) 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) mRNA in perirenal adipose tissue sampled from fetuses delivered by caesarean section at 140 days of gestation (dGA; term ∼147 dGA) following maternal dexamethasone (Dex) administration or at 146 dGA (control group) into warm and cool ambient temperatures. Example images of gene mRNA expression are given. Values are means with their standard errors (n = 4 per individual group). *p < 0.05, **p < 0.01, mean value significantly different from respective control group.
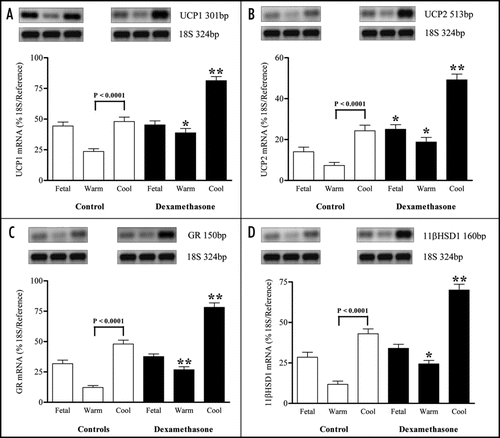
Figure 3 Abundance of (A) voltage-dependent anion channel (VDAC) and (B) cytochrome c proteins in perirenal adipose tissue sampled from fetuses delivered by caesarean section at 140 days of gestation (dGA; term ∼147 dGA) following maternal dexamethasone (Dex) administration or at 146 dGA (control group) into warm and cool ambient temperatures. Examples of protein expression are given. Values are means with their standard errors (n = 4 per individual group). ** P < 0.01, mean value significantly different from respective control group.
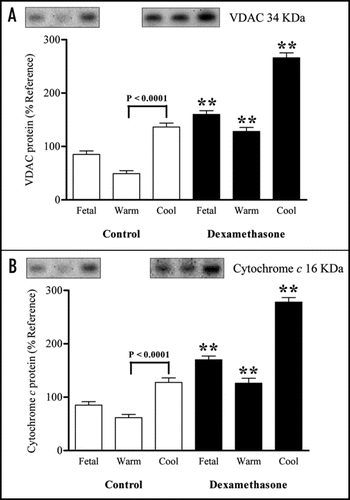
Figure 4 Abundance of interleukin 6 mRNA in perirenal adipose tissue sampled from fetuses delivered by caesarean section at 140 days of gestation (dGA; term ∼147 dGA) following maternal dexamethasone (Dex) administration or at 146 dGA (control group) into warm and cool ambient temperatures. Values are means with their standard errors (n = 4 per individual group).
Table 1 Effect of maternal dexamethasone administration on maternal plasma hormones and metabolites
Table 2 Significant negative relationship between plasma cortisol concentration and molecular measurements made in perirenal adipose tissue sampled from fetuses and neonates delivered by caesarean section into warm and cool ambient temperatures at either 140 days gestation following maternal dexamethasone administration or at 146 days (Controls) (n = 24)
Table 3 Significant positive relationship between plasma non-esterified fatty acid concentration relationships between molecular measurements made in perirenal adipose tissue sampled from fetuses and neonates delivered by caesarean section into warm and cool ambient temperatures at either 140 days gestation following maternal dexamethasone administration or at 146 days (Controls) (n = 24)
Table 4 Significant positive relationships between molecular measurements made in perirenal adipose tissue sampled from fetuses and neonates delivered by caesarean section into warm and cool ambient temperatures at either 140 days gestation following maternal dexamethasone administration or at 146 days (Controls) (n = 24)
Acknowledgments
The Special Trustees for Nottingham University Hospitals and the European Union Sixth Framework Programme for Research and Technical Development of the European Community—The Early Nutrition Programming Project (FOOD-CT-2005-007036) supported this work.
References
- Liggins GC. The role of cortisol in preparing the fetus for birth. Rep Fertil Dev 1994; 6:141 - 150
- Fowden AL, Li J, Forhead AJ. Glucocorticoids and the preparation for life after birth: are there long-term consequences of the life insurance?. Proc Nutr Soc 1998; 57:113 - 122
- Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Canon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochemica et Biophysica Acta 2001; 1504:82 - 106
- Symonds ME, Mostyn A, Pearce S, Budge H, Stephenson T. Endocrine and nutritional regulation of fetal adipose tissue development. J Endocrinol 2003; 179:293 - 299
- Gnanalingham MG, Mostyn A, Symonds ME, Stephenson T. Ontogeny and nutritional programming of adiposity: potential role of glucocorticoid sensitivity and uncoupling protein-2. Am J Physiol 2005; 289:R1407 - R1415
- Gnanalingham MG, Mostyn A, Forehead AJ, Fowden AL, Symonds ME, Stephenson T. Increased uncoupling protein-2 mRNA abundance and glucocorticoid sensitivity in adipose tissue in the sheep fetus during late gestation is dependent on plasma cortisol and triiodothyronine. J Physiol, Lon 2005; 567:283 - 292
- Mostyn A, Wilson V, Dandrea J, Yakubu DP, Budge H, Alves-Guerra MC, et al. Ontogeny and nutritional manipulation of mitochondrial protein abundance in adipose tissue and the lungs of postnatal sheep. Br J Nutr 2003; 90:323 - 328
- Mostyn A, Pearce S, Stephenson T, Symonds ME. Hormonal and nutritional regulation of adipose tissue mitochondrial development and function in the newborn. Experimental and Clinical Endocrinology and Diabetes 2004; 112:2 - 9
- Symonds ME, Bird JA, Clarke L, Gate JJ, Lomax MA. Nutrition, temperature and homeostasis during perinatal development. Exp Physiol 1995; 80:907 - 940
- Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: An update. Clin Endocrinol 2006; 64:355 - 365
- Bispham J, Gardner DS, Gnanalingham MG, Stephenson T, Symonds ME, Budge H. Maternal nutritional programming of fetal adipose tissue development: differential effects on messenger ribonucleic acid abundance for uncoupling proteins and peroxisome proliferator-activated and prolactin receptors. Endocrinology 2005; 146:3943 - 3949
- Mostyn A, Pearce S, Budge H, Elmes M, Forehead AJ, Fowden AL, et al. Influence of cortisol on adipose tissue development in the fetal sheep during late gestation. J Endocrinol 2003; 176:23 - 30
- Bispham J, Budge H, Mostyn A, Dandrea J, Clarke L, Keisler D, et al. Ambient temperature, maternal dexamethasone, and postnatal ontogeny of leptin in the neonatal lamb. Pediatr Res 2002; 52:85 - 90
- Clarke L, Heasman L, Symonds ME. Influence of maternal dexamethasone administration on thermoregulation in lambs delivered by caesarean section. J Endocrinol 1998; 156:307 - 314
- Alexander G, Bell AW. Quantity and calculated oxygen consumption during summit metabolism of brown adipose tissue in newborn lambs. J Dev Physiol 1975; 26:214 - 220
- Lecavalier L, Bolli G, Gerich J. Glucagon-cortisol interactions on glucose turnover and lactate gluconeogenesis in normal humans. Am J Physiol 1990; 258:E569 - E575
- Symonds ME, Bryant MJ, Lomax MA. Lipid metabolism in shorn and unshorn pregnant sheep. Brit J Nutr 1989; 62:35 - 49
- Holness MJ, Sugden MC. Dexamethasone during late gestation exacerbates peripheral insulin resistance and selectively targets glucose-sensitive functions in beta cell and liver. Endocrinology 2001; 142:3742 - 3748
- Woods LL, Weeks DA. Prenatal programming of adult blood pressure: role of maternal corticosteroids. Am J Physiol 2005; 289:R955 - R962
- Woods LL. Maternal glucocorticoids and prenatal programming of hypertension. American J Physiol 2006; 291:R1069 - R1075
- Bispham J, Gopalakrishnan GS, Dandrea J, Wilson V, Budge H, Keisler DH, et al. Maternal endocrine adaptation throughout pregnancy to nutritional manipulation: consequences for maternal plasma leptin and cortisol and the programming of fetal adipose tissue development. Endocrinology 2003; 144:3575 - 3585
- Symonds ME, Clarke L. Influence of thyroid hormones and temperature on adipose tissue development and lung maturation. Proc Nutr Soc 1996; 55:567 - 575
- Edwards LJ, McMillen IC. Maternal undernutrition increases arterial blood pressure in the sheep fetus during late gestation. J Physiol, Lon 2001; 533:561 - 570
- Brunton PJ, Bales J, Russell JA. Neuroendocrine stress but not feeding responses to centrally administered neuropeptide Y are suppressed in pregnant rats. Endocrinology 2006; 147:3737 - 3745
- Alexander G, Bell AW. The role of the adrenal in the metabolic response of young lambs to the cold. J Dev Physiol 1982; 4:53 - 73
- Li G, Klein RL, Matheny M, King MA, Meyer EM, Scarpace PJ. Induction of uncoupling protein 1 by central interleukin-6 gene delivery is dependent on sympathetic innervation of brown adipose tissue and underlies one mechanism of body weight reduction in rats. Neuroscience 2002; 115:879 - 889
- Symonds ME, Bird JA, Sullivan C, Wilson V, Clarke L, Stephenson T. Effect of delivery temperature on endocrine stimulation of thermoregulation in lambs born by cesarean section. J Appl Physiol 2000; 88:47 - 53
- Clarke L, Heasman L, Firth K, Symonds ME. Influence of route of delivery and ambient temperature on thermoregulation in newborn lambs. Am J Physiol 1997; 272:R1931 - R1939
- Alexander G, Williams D. Shivering and nonshivering thermogenesis during summit metabolism in young lambs. J Physiol, Lon 1968; 198:251 - 276
- Gnanalingham MG, Giussani DA, Sivathondan P, Forehead AJ, Stephenson T, Symonds ME, et al. Chronic umbilical cord compression results in premature maturation of lung and brown adipose tissue in the late gestation ovine fetus. Am J Physiol 2005; 289:E456 - E465
- Gnanalingham MG, Mostyn A, Gardner DS, Stephenson T, Symonds ME. Developmental regulation of adipose tissue: Nutritional manipulation of local glucocorticoid action and uncoupling protein 2. Adipocytes 2005; 1:221 - 228
- Clarke L, Bird JA, Lomax MA, Symonds ME. Effect of β3-adrenergic agonist (Zeneca D7114) on thermoregulation in near-term lambs delivered by cesarean section. Pediatr Res 1996; 40:330 - 336
- Clarke L, Darby CJ, Lomax MA, Symonds ME. Effect of ambient temperature during 1st day of life on thermoregulation in lambs delivered by cesarean section. J Appl Physiol 1994; 76:1481 - 1488
- Bispham JR, Heasman L, Clarke L, Ingleton PM, Stephenson T, Symonds ME. Effect of maternal dexamethasone treatment and ambient temperature on prolactin receptor abundance in brown adipose and hepatic tissue in the fetus and newborn lamb. J Neuroendocrinol 1999; 11:849 - 856
- Gnanalingham MG, Mostyn A, Dandrea J, Yakubu DP, Symonds ME, Stephenson T. Ontogeny and nutritional programming of uncoupling protein-2 and glucocorticoid receptor mRNA in the ovine lung. J Physiol, Lon 2005; 565:159 - 169
- Whorwood CB, Firth KM, Budge H, Symonds ME. Maternal undernutrition during early- to mid-gestation programmes tissue-specific alterations in the expression of the glucocorticoid receptor, 11β-hydroxysteroid dehydrogenase isoforms and type 1 angiotensin II receptor in neonatal sheep. Endocrinology 2001; 142:2854 - 2864
- Pecqueur C, Alves-Guerra M-C, Gelly C, Lévi-Meyrueis C, Couplan E, Collins S, et al. Uncoupling protein-2: in vivo distribution, induction upon oxidative stress and evidence for translational regulation. J Biol Chem 2001; 276:8705 - 8712
- Voehringer DW, Hirschberg DL, Xiao J, Lu Q, Roederer M, Lock CB, et al. Gene microarray identification of redox and mitochondrial elements that control resistance or sensitivity to apoptosis. PNAS 2000; 97:2680 - 2685
- Chevillotte E, Giralt M, Miroux B, Ricquier D, Villarroya F. Uncoupling Protein-2 controls adiponectin gene expression in adipose tissue through the modulation of reactive oxygen species production. Diabetes 2007; 56:1042 - 1050
- Agricultural Research Council. Requirements for energy. The Nutritional Requirements of Ruminant Livestock 1980; Slough, UK Commonwealth Agricultural Bureau 115 - 119
- Gardner DS, Buttery PJ, Daniel Z, Symonds ME. Factors affecting birth weight in sheep: Maternal environment. Reproduction 2007; 133:297 - 307
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2T Method. Methods 2001; 25:402 - 408
- Bird JA, Spencer JAD, Mould T, Symonds ME. Endocrine and metabolic adaptation following caesarean section or vaginal delivery. Arch Dis Child Fetal Neonatal Ed 1996; 74:F132 - F134