Abstract
Angiogenesis represents the outgrowth of new blood vessels from existing ones, a physiologic process that is vital to supply nourishment to newly forming tissues during development and tissue remodeling and repair (wound healing). Regulation of angiogenesis in the healthy body occurs through a fine balance of angiogenesis-stimulating factors and angiogenesis inhibitors. When this balance is disturbed, excessive or deficient angiogenesis can result and contribute to development of a wide variety of pathological conditions. The therapeutic stimulation or suppression of angiogenesis could be the key to abrogating these diseases. In recent years, tissue engineering has emerged as a promising technology for regenerating tissues or organs that are diseased beyond repair. Among the critical challenges that deter the practical realization of the vision of regenerating functional tissues for clinical implantation, is how tissues of finite size can be regenerated and maintained viable in the long-term. Since the diffusion of nutrients and essential gases to cells, and removal of metabolic wastes is typically limited to a depth of 150-250 μ m from a capillary (3 - 10 cells thick), tissue constructs must mandatorily permit in-growth of a blood capillary network to nourish and sustain the viability of cells within. The purpose of this article is to provide an overview of the role and significance of hyaluronan (HA), a glycosaminoglycan (GAG) component of connective tissues, in physiologic and pathological angiogenesis, its applicability as a therapeutic to stimulate or suppress angiogenesis in situ within necrotic tissues in vivo, and the factors determining its potential utility as a pro-angiogenic stimulus that will enable tissue engineering of neo-vascularized and functional tissue constructs for clinical use.
Introduction
Angiogenesis represents the outgrowth of new blood vessels from existing ones, an important physiologic process that occurs in the body both under healthy and pathological conditions. Angiogenesis is vital to supply nourishment to newly forming tissues during development, a phenomenon termed specifically as vasculogenesis,Citation1,Citation2 and during tissue regeneration in vivo or in vitro, often achieved using principles of tissue engineering.Citation3 The rapid development of blood capillaries that temporarily infilter injured tissues is also critical to their remodeling and repair, a phenomenon termed wound healing.Citation4 Regulation of angiogenesis in the healthy body occurs through a balance of angiogenesis-stimulating factors and angiogenesis inhibitors. Under pathological conditions, the body can lose the ability to balance these two contradictory signaling mechanisms to result in either excessive or alternately insufficient outgrowth of new capillaries, from existing vessels, a phenomenon that is unique in being transient, since the capillaries normally disappear as the pathological condition regresses.Citation5,Citation6 For example, in many diseases (e.g., cancer, diabetic blindness, age-related macular degeneration, rheumatoid arthritis, psoriasis and more than 70 other conditions), where angiogenesis is involved, new blood vessels are generated in excess and lead to the hyper-growth of diseased tissue masses, called tumors. In addition, when these newly-formed blood vessels form networks that lead into major circulatory conduits, they promote the metastasis of cancer by transporting tumor cells to other locations within the body. In yet other diseases (e.g., coronary artery disease, stroke and delayed wound healing), insufficient formation of new blood vessels can impede proper tissue nourishment, and cause their hypoxic death.Citation7 Accordingly, clinical therapies aimed at regulating angiogenesis by restoring the fine homeostatic balance between angiogenic promoters and inhibitors and thereby arresting the etiology and pathology of a number of pathological conditions are of clinical interest. With the advent of “regenerative medicine” and its in vitro counterpart, “tissue engineering,” the potential to manufacture complex tissues and organs on demand is fast becoming a reality.
Among the critical challenges that deter the practical realization of the vision of regenerating functional tissues for clinical implantation, is how tissues of finite size can be regenerated and maintained viable in the long-term. Since the diffusion of nutrients and essential gases to cells is typically limited to a depth of 150–250 µm from a capillary (3–10 cells thick), tissue constructs must mandatorily permit in-growth of a blood capillary network to nourish and sustain the viability of cells within.Citation8,Citation9 The purpose of this article is to provide an overview of the role and significance of hyaluronan (HA), a glycosaminoglycan (GAG) component of connective tissues, in physiologic and pathological angiogenesis, and its potential utility to engineering neo-vascularized and functional tissue constructs.
Angiogenic Cascade
The initiation of angiogenesis begins with the local release of pro- and anti-angiogenic growth factors by endothelial cells (ECs). Such release occurs in response to disease- or injury-induced inflammation or by the depleted rate of breakdown and hence accumulation of Hypoxia-Inducible Factor-1a (HIF-1a) in hypoxia-prone diseased/injured tissues.Citation10 In this phase, termed vessel de-stabilization, these released biomolecules diffuse into the surrounding tissue and bind to specific cell-surface receptors located on quiescent, macrovascular ECs of nearby post-capillary- and small terminal venules.Citation11,Citation12 Thereupon, the ECs become activated and prompt intracellular pathways that signal them to upregulate production and release of enzymes called proteases. These enzymes specifically break down structural matrix molecules (e.g., collagen, elastin). The proteases also cleave small holes in the basement membrane, a layer of extracellular matrix underlying the ECs, that anchors the cells to underlying connective tissue, and create a route for the ECs to migrate from the parent vessel to toward the source of chemotactic angiogenesis-stimulating factors (e.g., a diseased tissue) and thus proliferate. The ECs migrate through the vascular bed using cell surface adhesive receptors (integrins) that interact and bind to extracellular matrix structures, as they traction forward, and with the help of matrix metalloproteinase enzymes (MMPs). The MMPs dissolve the tissue in front of the migrating cells in order to accommodate the sprouting vessel. Such migration of ECs occurs at a rate of a few millimeters per day. The tissue is remodeled once the ECs extend toward the diseased tissue and align to form a capillary lumen. These free-ending conduits coalesce end to end to form blood vessel loops capable of circulating blood. As these capillaries form, the ECs stop proliferating, synthesize new basement membrane and establish inter-cell and EC-basement membrane junctions to stabilize the formed conduits. Specialized structural support in the form of smooth muscle cells (SMCs) and pericytes that surround the endothelial tubes further stabilize the newly-formed blood capillaries. Blood flow then begins through the loop to either facilitate tissue remodeling and wound healing, or in some cases encourage tissue growth by providing essential gases and nutrients, such as within tumors.
Different from the above-mentioned “sprouting” of blood capillaries from pre-existing ones, neo-vascularization during early embryonic tissue development initiates by recruitment of extra-embryonic primordial cells, termed angioblasts, to form “blood islands.” At the periphery of these islands, the angioblasts flatten and differentiate into ECs, while those at the center form blood cell precursors. These “blood islands” progressively coalesce to form the beginnings of what eventually evolve into mature neo-capillaries.Citation13 A number of recent studiesCitation14–Citation17 have shown that even in adults, endothelial progenitor cells (EPCs), generated by the bone marrow, can be introduced into circulation, mobilized by tissue ischemia and honed into the targeted sites of neo-vascularization. Thus postnatal tissue vascularization can occur both by angiogenesis and vasculogenesis.Citation18
Angiogenesis and Pathologic Tissue Remodeling
Despite the commonality of EC activation to initiating both physiologic and pathologic angiogenesis, the former differs from the latter in two respects. First, EC proliferation is very tightly controlled during physiologic angiogenesis (e.g., in wound healing), while significantly less fettered under pathologic conditions.Citation19 Second, blood capillaries formed as a normal physiologic event, can continue to exist as such, or mature into arterioles/venules, if and as long as a demand for nutrition and growth exists; the capillaries however disappear once the tissue reparative/regenerative event is complete, due to apoptotic (programmed) death of ECs by transient upregulation of angiogenic inhibitors and other mediators.Citation20 This kind of transience in microvessel recruitment is absent under pathological conditions so that chronic accumulation of microvessels can occur to contribute to the etiology and pathology of so called “angiogenic diseases.”
Just as the progression of angiogenesis and vasculogenesis is intimately regulated at each step via the mediation of angiogenic simulators, secreted by activated ECs, or other accessory cell types (e.g., inflammatory macrophages, pericytes),Citation21 the regression of these phenomena and of the formed microvessels is prompted by the appropriately-timed upregulation in synthesis of angiogenesis inhibitors, which can variably prompt cells to apoptose,Citation5,Citation6 deter ECM production,Citation22 induce ECM degradationCitation23,Citation24 and thus alter EC phenotype to render them less migratory and more quiescent.Citation25 Conversely, the pathologic exhuberance of microvascular networks may be due to downregulation of the negative regulators of angiogenesis.Citation26,Citation27 lists several known angiogenic stimulators and inhibitors.Citation28
Broadly, pathologies involving angiogenic aberrations represent great heterogeneity in tissue type impacted and their etiology and progression, though they may be broadly classified into two categories. First, there are those diseases that involve endothelial hyper-proliferation and chronic inflammation. These include hemangiomasCitation29 (localized tumors), psoriasisCitation30 (excessive skin production and inflammation), Kappsi's SarcomaCitation31 (dermal/oral/gastrointestinal nodules), diabetic retinopathyCitation32 (retinal damage due to excessive, leaky capillaries), endometriosisCitation33 (extra-uterine tissue growth) and rheumatoid arthritisCitation34 (chronic auto-immune disorder of joints). Excessive angiogenesis can also contribute to atherosclerosis, wherein dense microvascular networks develop within the wall of major vessels to cause vascular leakiness and hemorrhage, then thrombus-occlusion.Citation35 Such an outcome can also induce hypoxia in distal tissues served by the afflicted vessel, and thus HIF-1a release by cells, to propagate pro-angiogenic phenomena.Citation10 More commonly, an imbalance of angiogenic promoters and inhibitors within tumors can spontaneously switch some tumor cells into an angiogenic phenotype thus initiating rapid and intense neo-vascularization that can sustain tumor cell viability and propagate tumor growth.Citation36 In some cases, the neo-microvascular network so established can facilitate the escape and transport of tumor cells from the primary tumor site to distal organs, resulting in tumor metastasis (spreading).
Deficient angiogenesis can also contribute to poor cell viability and health, and lead to pathogenesis of conditions such as myocardial and cerebral ischemias (restricted blood supply)Citation37 and deficient wound/ulcer healing.Citation38 Recent inclusions to this list are tissue constructs regenerated using principles of tissue engineering.
Relevance of Angiogenesis to Tissue Engineering
In the past decade, tissue engineering has emerged as a promising technology for potential regeneration of tissues and organs on demand. Broadly, the term “tissue engineering” is defined as per Langer and VacantiCitation39 as “an interdisciplinary field that applies the principles of engineering and life sciences towards development of biologic substitutes that restore, maintain, or improve tissue function of a while organ.”
Although the initial envisioned benefit of tissue engineering was its therapeutic applicability, diagnostic uses (e.g., model systems to test drug uptake, cytotoxicity, metabolism, etc.) have since been identified. These enable simple and non-invasive characterization of interactions between the three inherent components of tissue-engineered constructs, namely cells, biomaterial scaffolds and regulatory biomolecules. Depending on the regenerative potential of the tissues targeted for replacement, tissue constructs may either be grown in vitro and implanted in a fully functional state, or alternately may be implanted when ultrastructurally underdeveloped, and allowed to undergo maturation in vivo to acquire full functionality as the incorporated cellular scaffold biodegrades. Despite rapid advances in the field in terms of identifying and optimizing cell sources, cellular cues and culture protocols to faithfully regenerate those tissues that contain labile or stable cell types, two critical challenges limit our present ability to regenerate tissue constructs for clinical use. The first is our inability to scale up culture processes to regenerate finite-sized tissue constructs that would be dimensionally and mechanically appropriate for clinical use, and the second is the problem of maintaining such constructs viable in the long-term. Since cells in vivo typically derive nourishment from nutrients and essential gases diffusing from capillaries no more than 3–10 cell layers away (150–250 µm),Citation8,Citation9 mimicking such a microenvironment within tissue-engineered constructs is a must; in the absence of this, the viability and performance of cells within the bulk of the construct will be compromised due to insufficient nutrition and lack of removal of metabolic wastes. In this context, the ability to stimulate angiogenesis within tissue-engineered constructs is recognized as a major need in the field. In addition, there is a vital need to be able to regulate angiogenesis so as to adequately fulfill the metabolic needs of the tissue, and yet deter possible adverse consequences of excessive capillary ingrowth.
Cell Sources for Angiogenic Tissue-Engineering
Two major cell types have been incorporated within or recruited to tissue-engineered scaffolds or constructs towards inducing neoangiogenesis within. These are terminally-differentiated endothelial cells,Citation40,Citation41 and bone marrow-derived endothelial progenitor cells (EPCs).Citation14–Citation17 For creating vascularized constructs in vitro, autologous cells are isolated from tissue biopsies from the patient, the EPCs typically from peripheralCitation42 and as explored more recently, from unrelated umbilical cord blood,Citation43 number-expanded in culture and then seeded within biomaterial scaffolds. Microvascular ingrowth from host vasculature can then be prompted by endogenous release of angiogenic growth factors (HIF-1, VEGF)Citation44 by these cells, upon induction by hypoxic conditions within the constructs when they are first implanted in vivo. Likewise, EPCs circulating in the blood stream can be recruited by local hypoxia and shear stress at the site of implant to hone in, proliferate, tubulize and thus prompt neovascularization within.
Cues for Induced Neo-Vascularization of Engineered Tissues
Since such spontaneous neo-vascularization as described above is rarely adequate, providing additional cellular cues to prompt the same have been investigated. These have included controlled, localized delivery of pro-angiogenic/EC-sepcific mitogenic growth factors such as fibroblast growth factors (FGF),Citation45 vascular endothelial growth factor (VEGF),Citation45 platelet-derived growth factor (PDGF),Citation46 hepatocyte growth factor (HGF)Citation47 from scaffolds into which they are directly tethered or mixed, or from microspheres into which they are incorporated.Citation3,Citation48 Since angiogenesis can be difficult to regulate temporally and spatially, either when prompted within the engineered tissue constructs or within ischemic tissues, more recent approaches have sought to modulate the process via inducing simultaneous expression of genes of multiple angiogenic factors through delivery of engineered transcription factors.Citation49
Three-dimensional cellular substrates in the form of scaffolds can also critically enhance the outcome of angiogenic stimulation of cells, specifically the ability of cells to proliferateCitation50 and vessels to in-grow, branch, and network in a given space. Scaffolds conducive to significant angiogenesis in vivo have been recognized to consistently exhibit high porosity (>85–90%),Citation51 and an interconnected network of pores of size greater than 250 µm.Citation52 A wide variety of synthetic polymer scaffolds exhibiting these architectural facets and in many cases, controlled delivery of angiogenic factors, have been shown to facilitate limited neovascularization. Select examples include poly-methacrylates,Citation53 polylactides (PLA),Citation54 synthetic hydroxyapatite,Citation55 polyanhydrides (PA),Citation56 polycaprolactone (PCLA),Citation54 fumarate-based polyestersCitation57 and self-assembling synthetic peptides.Citation58 More recently, due to concerns that synthetic polymers tend to induce non-physiologic cellular responses, particularly due to lack of biological signaling, there has been a shift towards biodegradable scaffolds based on natural materials, either standalone, or including those based on chemically-derivatized, or underivatized components of extracellular matrices (ECM). Salient examples include collagenCitation59 and fibrin hydrogels,Citation60 including those incorporating bFGF release,Citation48,Citation61 collagen-elastin blends,Citation62 gelatin hydrogels,Citation63 chitosan,Citation64 alginateCitation65 and glycosaminoglycans, particularly hyaluronan (HA) and its derivatives.Citation66 In the case of most of these materials, however, chemical derivatization and/or crosslinking necessary to impart appropriate mechanics, degradation profiles, and the requisite architecture, often involves the biologically active functional groups on these molecules, to alter their native biologic properties.
Of the various biologic molecules of interest, hyaluronan (HA) has been increasingly studied in the context of a pro-angiogenic tool for tissue engineering, due to its role in initiating and regulating angiogenesis under physiologic and pathologic scenarios. From a biomaterials perspective, its amenability to chemical derivatization/crosslinking with little change to its inherent biocompatibility, non-immunogenicity and non-cytotoxicity is also highly attractive.
Hyaluronan (HA): A Versatile Biologic Molecule
Hyaluronan, also referred to as hyaluronic acid (HA) or hyaluronate, is a naturally occurring non-sulfated glycosaminoglycan (GAG) component in the extracellular matrix of connective, epithelial and neural tissues. Among the specific tissue types that contain significant HA content are synovial fluid, vitreous humor, cartilage, blood vessels, skin and the umbilical cord. In its various forms, HA plays a seminal role in embryonic/neonatal tissue development,Citation67 and provides structural and cell-signaling cues, to maintain tissue homeostasis, and direct the initiation and progression of various pathological conditions, including the aberrations of angiogenesis.
GAGs are long, unbranched polymers of repeating disaccharide units. GAG types differ from one another in the identity of their disaccharide unit, geometry of glycosidic linkage, extent of sulfation and the nature of the core proteins that may be covalently bound to the polysaccharide chains. HA is a unique member of the GAG family in being neither sulfated nor bound to a core protein. It is a long-chain polymer of up to 25,000 repeats of D-glucuronic acid and D-N-acetylglucosamine monomers, linked by alternating β-1,4 and β-1,3 glycosidic bonds (). In vivo, HA is predominantly distributed in the ECM as a long-chain polymer, typically ranging between 1–4 MDa in size, though the chains can exhibit molecular weights as high as 10 MDa.Citation68 Shorter HA chains ranging from less than 105 Da to 106 Da are also synthesized de novo, under physiologic conditions.Citation69
In vivo, HA is synthesized by a class of enzymes, termed Hyaluronan Synthases (HAS). The enzymes are membrane bound and cyclically add the pertinent monomer units to the end of the forming polysaccharide chain, as it is extended to the extracellular space, via the plasma membrane. Three HAS isoforms: HAS-1, -2 and -3, corresponding to genes located at different chromosome locations, are involved in HA biosynthesis, through their catalytic activities and the sizes of synthesized HA chains vary; HAS-3 is the most active while HAS-1 the least active. HAS-1 and -2 typically synthesize HA of larger sizes 2 × 105–2 × 106 Da while HAS-3 typically synthesizes shorter HA chains <105 Da.Citation70 HAS-2 has however been identified to be the most active isoform during embryonic development.
HA is degraded in vivo by hyaluronidases (HAases), a family of six different enzymes that each differently cleave HA chains by hydrolysis into shorter fragments. These enzymes enable physiologic turnover of HA, which occurs to the extent of nearly 33% of bodily HA content per day.Citation71 However, the extent of HA degradation that occurs under pathological conditions may be greatly enhanced, as well as the size distribution of generated HA fragments significantly altered. Under both physiologic and pathologic scenarios, extremely short-sized (<20 monomers) HA fragments termed “HA oligomers” can by temporarily generated as products of HA degradation by a hyaluronidase isoform HYAL-1.Citation72 Production of these oligomers can be enhanced quite dramatically at sites of injury, inflammation and within tumors, due to increases in HA content itself or synthesis and availability of receptors for HA and HAases,Citation73 sometimes upon generation of reactive oxygen and nitrogen species.Citation74 Unlike native long-chain HA, shorter HA fragments, particularly HA oligomers, are highly bioactive and serve to interact with and influence cellular phenomena (including angiogenesis) in a manner that native long-chain HA does not and cannot.Citation75
Fragment Size-Dependent Biologic Roles of HA
The biologic roles, and cellular interactions of HA with cells, are highly dependent on HA chain length (). Native high molecular weight (HMW) HA (aka long-chain HA) is a largely bioinert molecule, that physically contributes to unique cellular microenvironment and mechanics of tissues, although it can also indirectly influences cell behavior. In tissues, long-chain HA serves to maintain a highly hydrated environment, regulate osmotic balance, acts as a shock-absorber and space-filler, and as a lubricant. Simultaneously, with its numerous functional groups for binding, and highly anionic nature, HMW can sequester and release growth factors and other biologic signaling molecules to impart potent, but localized influence on cell behavior.Citation76 HMW HA has been shown to inhibit EC proliferation and disrupt newly-formed EC monolayers.Citation77 However, HMW HA can also sheath cells to prevent their interaction with other cells, and biologic signaling molecules with their cell-surface receptors ().
Compared to HMW HA, shorter HA fragments and oligomers are more bioactive. Both the long-chain and oligomeric forms of HA are capable of binding to cell-surface HA receptor glycoproteins, such as cluster determinant molecule-44 (CD44) ( and B), although the former is capable of multivalent interactions, while the latter interacts only monovalently. While HMW HA promotes cell quiescence upon such binding, oligomers can cluster and thus activate these receptors to trigger associated intracellular signaling cascades.Citation78 In this manner, HA oligomers can induce inflammatory cytokine release by inflammatory cells, and thereby activate non-inflammatory cell types (e.g., ECs, SMCs) to proliferate, migrate and trigger wound healing or tissue remodeling.Citation74 The glycoprotein receptors that mediate the cellular response to HA fragments are CD44, Receptor for Hyaluronan-Mediated Motility (RHAMM) and Toll-Like-Receptor-4 (TLR-4) (). Through interaction with one or more of these receptors, and the consequent effects of promoting EC proliferation and migration, HA oligomers can induce neo-angiogenesis and sprout formation, which are inherent and critical to cell/tissue viability, growth and to tissue repair and remodeling following injury/trauma.
HA Roles in Physiologic Angiogenesis
A number of in vivo studies and experiments performed on in vitro cell cultures have indicated that native, long-chain HA at physiologic concentrations has anti-inflammatory properties,Citation77 and is potently anti-angiogenic.Citation79 Apparently long-chain HA binds to cellular CD44 receptors and thereupon interrupts key intracellular signaling cascade of events that prompt cell proliferation and thus arrest the cell cycle. In case of ECs, such failure to proliferate can impact angiogenesis.
Differently, HA fragments, particularly oligomers, have been noted to stimulate EC proliferationCitation80 and promote angiogenesis,Citation81 although these effects are often influenced by concomitant signaling by other growth factors, specific to healthy,Citation82 or diseasedCitation83,Citation84 conditions. HA oligomers have been shown to compete with native long-chain HA in binding to the HA receptor CD44 present on ECs. Unlike the long-chain HA, the oligomers triggered intracellular signaling pathways that prompt active cell proliferation. Such promitotic effects on ECs have been demonstrated in simple in vitro cultures,Citation77,Citation81,Citation82,Citation85,Citation86 and in vivo models of rat skin,Citation87,Citation88 synoviumCitation89 and retina.Citation90
The CD44-HA oligosaccharide interactions that primarily cause these pro-mitotic effects have also been shown to stimulate MMP-2 and -9 production and thereby increase cell invasion through ECM barriers to facilitate vessel sprouting and outgrowth.Citation91,Citation92 In addition MMPs can activate endogenous TGF-β to induce angiogenic responses.Citation93 HA/oligomer interaction with RHAMM specifically, appears to induce cytoskeletal changes to cells, including ECs, to promote their migration.Citation94 In wound healing models, HA oligosaccharide-RHAMM interactions rather than those with CD44 have been shown to initiate and enhance both EC proliferation and migration.Citation81 Savani et al.Citation95 demonstrated that RHAMM-ligand interactions primarily dictate EC motility and CD44-ligand interactions, EC proliferation. Both these receptors thus work in tandem to facilitate formation of new blood vessels. A study by Murphy et al.Citation96 and othersCitation97 also showed that engagement of the CD44 by depolymerized HA induces endogenous release of vascular endothelial growth factor (VEGF) which in turn stimulates EC proliferation and blood vessel growth.
Studies by Carson et al.Citation98 showed that hyaluronate biosynthesis is greatly enhanced in growing embryo, with oligosaccharides (1500–5000 Da) forming the most abundant class, followed by HA of sizes in the range of 104 Da and above. While this hyaluronate has been suggested to enable the embryo to attach and spread on the uterine wall, other studies have shown, albeit in vitro, that HA oligomers (3–25 disaccharide units) induce neovascularization by primary ECs, in a manner that was dictated by their interaction with RHAMM.Citation94 Although production of HA is also elevated in inflamed wound sites due to upregulation of HAS1-3, and enhanced expression of HA receptors,Citation99 different from embryogenesis, the HA is rapidly depolymerized by activation of enzymesCitation100,Citation101—hyaluronan-degrading HYALS-1 and -2, and chondroitinases/hexoaminaders and by reactive oxygen and nitrogen species/free radicals generated by the recruited inflammatory cell.Citation74,Citation102,Citation103
Through their interaction with CD44 and RHAMM present on ECs at the wound site, the HA oligosaccharides have been shown to promote angiogenic capillary sprouts that invade the fibrin/fibronectin-rich clot site and establish a microvascular network that nourish the growing, and increasingly remodeled tissue,Citation81 and also stabilize the endothelial tubes as well.Citation104
Angiogenic Role of HA in Diseases
The co-incidence of HA oligosaccharides and angiogenesis in various diseased states strongly suggests a cause-effect relationship between the two. The etiology of a lesion composed of an abnormal growth of cells resulting in unregulated proliferation (tumor), is thought to originate from the genetic mutation of one or more cells. The growth of a solid tumor beyond 2 mm in diameter is “angiogenesis-dependent”Citation105 with growth arresting with translocation into a nonvascular region of the body.Citation106 In many tumor types, long-chain hyaluronan has been shown to be crucial to the progression of cancer either by stimulating malignancy of tumor cells and their poor differentiation, and providing a loose matrix for cell migration,Citation107 while HA breakdown products, including HA oligosaccharides promote tumor spreading by stimulating angiogenesis and creation of a microvascular network.Citation75,Citation108,Citation109 The metastasis of cancer is attributed to HA-cell interactions in mammary carcinoma and other cancers where the formation of blood vessels to nourish the growing tumor also distribute cancer cells to distal body parts.Citation110
Although the HA fragment sizes that contribute to the various mechanisms via which tumors initiate, grow and metastasize are not clearly defined at present, despite the large body of work in the field, it is generally agreed that the HA oligomers/fragment containing between 4–25 disaccharide units are angiogenic and enhance tumor invasiveness.Citation111 Lokeshwar et al.Citation112 showed that tumors contain a specific variant of HYAL, a hyaluronidase, which corresponded with generation of HA oligomers and tumor invasiveness and metastasis.Citation113,Citation114 This was subsequently confirmed in various cancer types. As mentioned previously, the angiogenic effects of HA oligomers is accomplished by its interaction with CD44 and RHAMM on ECs and subsequent stimulation of EC proliferation, motility and tubule formation.
Despite the complex involvement of HMW HA in lipid accumulation, and inflammatory cell recruitment and vascular cell migration within plaques, again, it is the shorter HA fragments that contribute to intra-plaque angiogenesis. It has also become evident recently that HA is an active modulator of proliferation and inflammation in atherosclerotic plaques.Citation115
Such neo-vascularization of the neointima occurs by sprouting of blood capillaries from vessels in the adventitia, and help nourish cellular metabolism with the plaque and sustain its growth. Though some studies attribute the presence/buildup of angiogenic HA fragments to enhanced expression of HAS-1 and -3 within the plaque,Citation115 others suggest that they are instead generated by enzymatic, oxidative or nitrative degradation of long-chain HA in the inflammatory microenvironment within plaques, to then stimulate new vessel sprouting, plaque growth and rupture. Likewise, in other diseases such as diabetic retinopathy too, long-chain HA is broken down into HA oligomers to locally induce angiogenesis within the normally unvascularized vitreous, and result in hemorrhage and blurred vision.Citation90
HA Cues for Angiogenic Tissue Engineering
The diverse physical and biologic functions of hyaluronan have proven beneficial towards its use in modulating cell behavior by providing biochemical and biomechanical stimuli in a variety of tissue engineering applications. Owing to the demonstrated fragment size-specific modulatory effects of HA on angiogenic phenomena, HA has been increasingly utilized as a tool to direct and control neovascularization of tissue constructs fabricated in vitro or regenerated in vivo. Early work investigating the size-specific impact of HA on cell behavior and neo-capillary formation have been accomplished by simple delivery of exogenous HA cues to cultured cells. Controlled release of HA fragments and other pro-angiogenic growth factors from cell-seeded synthetic/natural biomaterial scaffolds, or alternately fabricating angiogenic scaffolds by chemically derivatizing and crosslinking HA appear to be useful strategies to spatio-temporally direct and control the angiogenic responses of incorporated cell types. These cells may have been isolated from biopsied patient tissues, then expanded and seeded within scaffolds in vitro, or spontaneously recruited from host tissues to the developing construct following its implantation in vivo.
Exogenous HA cues.
Delivery of exogenous HA to cell cultures has been useful to identify the HA fragment sizes that specifically induce EC proliferation, migration and tube formation. In 2002, Slevin et al. showed that over a period of several hours, angiogenic oligosaccharides of HA (3 to 10-mers) induced multiple signaling pathways enhancing bovine vascular EC proliferation and migration.Citation86 Ibrahim and RamamurthiCitation116 comprehensively studied the effects of exogenous high molecular weight (>1000 kDa) and oligomeric (0.75–10 kDa) ranges, on various phenotypic and functional aspects of rat aortic ECs cultured over a period of 3 weeks. HA digests (HA-o) containing HA 6mers and 12mers, and pure HA 6mers in particular stimulated EC proliferation and EC tube formation (angiogenesis) on matrigel substrates, much more than non-HA controls, while high molecular weight HA (HA 1500) had a definite, but more modest effect ( and ). Long-chain HA induced formation of thicker, and longer individual tubes but showed few laterally branching tubes resulting in low tube numbers relative to that formed by control ECs (no HA addition). Lengths of individual tubes in TNFα-, HA 6mer-, and HA-oligomer mixture-stimulated cultures were similar to that in the control cultures but the numbers of tubes were significantly higher, resulting in a greater cumulative tube length. These tube lengths were in the order TNFα > HA 6mer > HA oligomer digest > HMW HA. In all cultures, except those that received suramin (inhibitor of various growth factors) and TNFα, tubes appeared complete and free of any breaks, and extended between clusters of cells. The long-chain HA reduced cumulative capillary production but increased the length of each individual tube, likely by physically limiting EC migration, thus inhibiting capillary branching. ECs cultured in presence of HA oligomer digests or pure HA 6mers showed a greater expression of the pro-angiogenic cytokines VEGF, leptin and TNFα than those cultured with high molecular weight HA (), suggesting that HA-induced enhancement of angiogenic response was mediated by endogenous release of pro-angiogenic cytokines.Citation116 In a parallel study Gao et al. likewise showed that pooled fractions of HA fragments between 4 to 20-mers long induced increases in proliferation of cultured pig ECs relative to non-digested high molecular weight HA;Citation117 this effect was particularly significant at low HA oligomer concentrations (∼10 mg/ml). In an in vitro EC culture model of wounding, Gao et al. showed that over 40 hour period following injury, HA oligomers induced EC migration and almost complete EC coverage of a lesion area through mechanisms involving , not CD44.Citation81
HA scaffolds.
Following indications that uncrosslinked HA oligomers and to some extent, high molecular weight HA stimulate EC proliferation, migration and tube formation, follow-up studies have explored their incorporation and controlled release from scaffolding substrates. Among other studies, earlier work by Deed et al. suggested that despite being able to broadly predict cell responses to biomolecular cues, exogenous supplements only transiently interact with cells, and thus may elicit cellular responses that are muted relative to that elicited by the same cues when they are immobilized on a 2D substrate, or 3D scaffold in intimate contact with cells.Citation118 Such studies can also be useful to investigate the effects of chemical derivatization and crosslinking, essential to impart ease of handling and degradation-resistance to the formed biomaterials, on retention of the innate biologic properties of native HA/oligomers, including their ability to spontaneously recruit angiogenic cell types in vivo, or support viability and proliferation of cells seeded within in vitro, and stimulate neo-vascularization and capillary in-growth.
Since the pro-angiogenic HA oligomers cannot by themselves be crosslinked to yield solid biomaterials, and can also potentially incite inflammatory/activated cell responses,Citation119,Citation120 they must be presented on other scaffolding biomaterials, either synthetic or natural in origin, or must be complexed with the more bioinert long-chain HA in such a manner as to fulfill the physical and mechanical requirements of the biomaterial, elicit the desired biologic responses, and yet deter excessive and long-term inflammation. Ibrahim et al.Citation120 developed a surface-tethered model of HA to test EC responses to chemically-derivatized, yet uncrosslinked HA fragments of singular defined sizes, and compared the outcomes to that obtained with exogenous HA supplements containing identical fragment sizes. These studies showed that uncrosslinked HA 6mers and 12mers immobilized onto aminated glass surfaces via their carboxyl groups, stimulated EC proliferation and tube formation in a manner similar to that when they were provided exogenously to cells. These results suggested that the carboxyl functional groups on the HA chains are not particularly involved in biologic signaling. Larger HA fragments, immobilized in a likewise manner, exhibit high charge densities and smooth surface topographies and were thus expectably found to deter cell adhesion.Citation120 In recent unpublished work, this author has shown that the above HA oligomer digests continue to stimulate EC proliferation and tube formation when mixed with high molecular weight HA and crosslinked with divinyl sulfone (DVS), which links the hydroxyl groups on the HA chains. Not surprisingly, the extent of EC adherence and tube formation increased as a function of HA oligomer content. However, the highly anionicity and hydrophilicity of HA, particularly long-chain HA within these crosslinked hydrogels, deterred cell adherence and spreading and thus tempered angiogenic EC responses to the HA oligomers.
Chemical derivatization and crosslinking are useful to modulate the properties of HA such as to create scaffolds that satisfy physical (e.g., charge, hydrophilicity, surface topography, pore structure) and mechanical requirements specific to the target tissue regenerative application, and also either temper or exaggerate angiogenic and other biologic responses inherently elicited by interaction of incorporated HA/fragments with contacting cells. While many natural and synthetic biomaterials have few functional groups available for chemical modification, HA chains present numerous available functional groups, which may be of three kinds, namely hydroxyl, carboxyl and acetamido. These reaction sites render HA amenable to a wide variety of derivatizations and chemical crosslinking strategies, to tethering of HA onto other materials or tethering of other bio active moieties (e.g., growth factors) to HA. In an early study, Collier et al.Citation121 developed composite biomaterials composed of the electrically conductive polymer polypyrrole, and HA for use as scaffolds for tissue engineering and wound-healing applications that may benefit from both electrical stimulation and enhanced vascularization. Though the incorporated was of high molecular weight (>2 MDa), it was found to stimulate a two-fold increase in vascularization when implanted sub-cutaneously in rats, compared to that elicited by polypyrrole-poly-4-styrene sulfonate composites. This was attributed to rapid enzymatic degradation of the uncrosslinked long-chain HA in vivo, which generated pro-angiogenic HA fragments, assumedly, HA oligomer. Baier Leach et al.Citation122 also showed that photocrosslinked hydrogels containing glycidyl methacrylate-derivatized long-chain HA (GMHA) retained the intrinsic bioactivity of HA when implanted sub-dermally in rats. The gels recruited host ECs, and promoted a degree of vascularization at the edge of the implant that was comparable to fibrin gel controls. However, it was not determined if vascularization was an outcome of EC interactions with long-chain GMHA or the fragmented products of its biodegradation in vivo. Other studiesCitation123 showed that esterified HA biomaterials (HYAFF-11), when implanted sub-cutaneously in rats, prompted an immediate, yet prolonged (up to 12 weeks) angiogenic response wherein the formation of mature blood vessels preceded degradation of the scaffold by phagocytosis (starting at 3 weeks); depending on the extent of esterification however, it is likely, that some degree of hydrolytic degradation of HYAFF occurred even earlier, with the generated HA fragments stimulating the angiogenic response. In a very recent study, Flynn et al.Citation124 compared vascularization of unseeded and adipose stem cell-seeded (ASC) placental decellularized matrices (PDS) and polyethylene-glycol diacrylate-crosslinked thiolated HA-composited PDS, when these scaffolds were implanted in a murine sub-cutaneous pouch. Inclusion of the ASC promoted implant vascularization, quite possibly due to their secretion of factors that stimulate endothelial cell migration and in vivo vascularization. Compositing the PDS with derivatized and crosslinked HA was found to enhance angiogenic responses, although this was modest; limited detection of the crosslinked HA after prolonged periods of implantation (8 weeks) suggested biodegradation of the HA, which quite possibly resulted in generation of pro-angiogenic HA fragments. The biodegradation of HA itself was attributed to enhanced hyaluronidase expression by the seeded cells as they approached terminal differentiation, a phenomenon observed by others.Citation125 Regardless, the study suggested that chemically derivatized and crosslinked HA can undergo biodegradation in vivo to yield HA fragments that retain pro-angiogenic activity, and that these cues alone may be insufficient to establish an extensive neo-vascular network; presence of additional cues, such as pro-angiogenic factors released by cells could however, together with the generated HA oligosaccharides quite significantly improve the angiogenic response. Similar deductions were made by Tonello et al.Citation126 who investigated the formation of microvascular networks by human dermal fibroblasts and human umbilical vein ECs, either cultured alone or together on esterified HA scaffolds (HYAFF) in vitro. The findings suggested that interactions between fibroblasts and HUVEC are necessary to prompt formation of microcapillary structures, with the HYAFF providing the baseline stimuli for EC proliferation and tube formation, and the fibroblasts enhancing the phenomenon via the production of angiogenic extracellular molecules (e.g., collagen) and other angiogenic factors, as others have shown previously.Citation127,Citation128 Such outcomes as described above, prompted the investigation of controlled delivery of pro-angiogenic growth factors from HA biomaterials in order to further the HA-elicited angiogenic outcomes.
HA as passive/active delivery vehicles for angiogenic growth factors.
As an example of reverse thinking relative that described above, Fournier and DoillonCitation129 and Montesano et al.Citation82 independently suggested that the activity of angiogenic growth factors released from biomaterials incorporating HA could be potentiated by the oligosaccharide products of HA biodegradation. Paettie et al.Citation130 examined the validity of this hypothesis by comparing microvessel density between rat pinna tissues implanted with hydrogels composed of adipic-dyhydrazide-functionalized, PEG-diald-crosslinked HA, and in which in cases incorporated release of VEGF or bFGF. Microvessel formation as stimulated by ADH-HA gels incorporating VEGF release was much greater than those tissues that received aqueous VEGF or HA gels alone, suggesting strong and specific synergistic interaction between HA and VEGF in stimulating in vivo angiogenesis; such synergy was not observed between HA and bFGF. Possibly, a combination of VEGF-induced, MMP-mediated physical disruption of the ECMCitation131 and HA-mediated ECM enhancement may have conditioned the ECM so as to produce a favorable environment capable of sustaining a prolonged angiogenic response. In other variants of this work, the same authors later showed tremendous synergy between HA (provided as a scaffold, as above) and two cytokine growth factors, namely VEGF and keratinocyte growth factor (KGF)Citation132 or angiopoietin-1,Citation133 released simultaneously therefrom, in stimulating micro-capillary in-growth in a murine pinna implantation model. As described above, it is quite possible that the fragmented products of HA degradation, and each of the released cytokines may initiate complementary intracellular signaling pathways within ECs, or either agent might induce upregulation of receptors for the other, with net outcomes of enhanced angiogenesis.Citation82 However, the open network structure within the hydrated HA biomaterials may also serve to store the cytokines mimicking sequestration of growth factors in native ECM. Thus, both exogenous and endogenous VEGF and KGF/angiopoietin could have been sequestered within the construct/tissue and made available to ECs, to stimulate prolonged capillary formation. Further, it was suggested that interactions between the pro-angiogenic cytokines and HA could augment binding affinities for their cell surface receptors through allosteric modulations to potentiate the angiogenic response. Lai et al.Citation76 provided independent evidence that HA inherently present in acellular tissues could be used for binding and sustained release of bFGF to enhance angiogenesis and tissue regeneration. When implanted sub-cutaneously in rats, the density and the depth of neo-vessels infiltrated into acellular bovine pericardium tissue was higher in samples loaded with greater amounts of bFGF, which in turn corresponded to the lowest GAG content, specifically of HA. It was however not apparent from these studies, as to whether the HA merely serves as a passive reservoir for storage and sustained release of angiogenic growth factors, or assumes a more active role, in itself stimulating angiogenesis through its interactions with host ECs and fibroblasts, or alternately, by potentiating the angiogenic effects of the loaded growth factors.
Perspectives and Challenges
As will be apparent from the above review, early studies have provided significant evidence as to the inductive benefits of HA to neo-vascularization of tissue constructs engineered in vitro, or regenerated in vivo. Specifically, HA oligosaccharides appear to trigger angiogenesis through their stimulation of endothelial cell proliferation, migration and tube formation, their induction of MMP release by cells, which facilitates capillary ingrowth, and their contribution to stability of formed microvessels by prompting ECM (e.g., collagen) deposition by fibroblasts. On the other hand, in a hydrated state, native HA chains form open network structures that can serve to sequester endogenously generated or exogenous angiogenic growth factors and facilitate their sustained release and action. In the light of the potential of HA oligosaccharides to stimulate prolonged inflammation in vivo, or adverse cell activation in vitro, there is a need to temper the adverse effects of these biomolecules, by (1) identifying their threshold safe doses required for angiogenic stimulation, (2) ensuring their controlled and localized delivery at these doses to the intended site of vascularization via novel delivery mechanisms (e.g., nanoparticulate-, microbead-based delivery), (3) capitalizing on the synergistic enhancement of their angiogenic effects when provided together with other pro-angiogenic growth factors in order to further reduce delivery doses, and (4) delivering them from biomaterials containing predominantly bioinert, long-chain HA that can additionally serve to potentiate the elicited angiogenic response, by sequestration and slow release of endogenous/exogenous growth factors. With rapid advances in technologies In utilizing HA as a tool to tissue engineer microvascular networks, the next generation of studies must also address the need to modulate the process to be able to mimic the tissue-specific structural aspects of the microvasculature (e.g., continuous or fenestrated capillaries, sinusoids etc.) and concurrently ensure their early maturation and stabilization to ensure physiologic perfusion. Perhaps the greatest challenge to functional vascularization of engineered constructs is and will continue to be our ability to exercise spatio-temporal control over the process to faithfully mimic the extent and tissue-specific spatial organization of microvessels, and prevent repercussions of exaggerated angiogenesis. In vivo, the rate of formation and the direction of propagation of neo-vessels are controlled by temporal and spatial gradients of both diffusible and matrix-based cell-signaling molecules.Citation134 Therefore, logically, modulating the spatial gradient of the ECM-based angiogenic cues may enable one to directionally guide newly forming vascular sprouts within 3-dimensional tissue constructs. Future studies will have to build up on the limited work that has been performed to date in this regard; Borselli et al.Citation134 showed that opposed to using the ECM merely as a tool to provide gradients of soluble pro-angiogenic factors for directing angiogenesis, the gradient of macromolecular components of the ECM itself can provide directional guidance for vessel growth within tissue constructs in vitro. This was exemplified in a test system of three-dimensional collagen-HA semi-interpenetrated networks wherein by creating HA gradients, sprouting rate of new microvessels and the architecture of vessel growth could be controlled and directed. Studies such as this also increasingly emphasize the need to concurrently provide multiple cellular macromolecular cues, each with different roles in angiogenesis, so as to be able to more closely replicate the process as it occurs naturally, and exert more fine control over the angiogenic outcomes. Since the ECM represents a natural reservoir of angiogenic macromolecules, spatially presented to evoke natural patterns of microvessel sprouting and in-growth, future studies will likely focus on utilizing organ-specific acellular matrices for engineering vascularized tissue constructs. The work performed by Lai et al.Citation76 mentioned previously, wherein HA within acellular bovine pericardium was used to sequester and release bFGF for angiogenic stimulation, represents this line of thinking. With rapid advances in technologies to genetically modify cells, a future strategy to utilize HA macromolecules in the ECM as angiogenic stimuli, would be to engineer cells to over express specific hyaluronidases, as do tumor cells, to generate HA oligosaccharides of defined sizes, capable of inducing tissue neo-vascularization. Though many of the mentioned technologies are at the cutting edge of basic biomedal research today today, in the coming decades, their application to regeneration of functional, vascularized tissue constructs for clinical use is inevitable.
Figures and Tables
Figure 1 The chemical structure of HA. The HA polymer may contain up to 25,000 repeats of the disaccharide units of N-acetyl-D-glucosamine (GlcNAc) and D-glucuronic acid (GlcUA) linked together by alternating 1 → 3 and 1 → 4 glycosidic bonds. Each repeating disaccharide unit has one carboxylate group, four hydroxyl groups and an acetamido group. The negative charge of the molecule is due to ionization of the carboxyl groups of the glucuronic acid constituents at physiological pH.
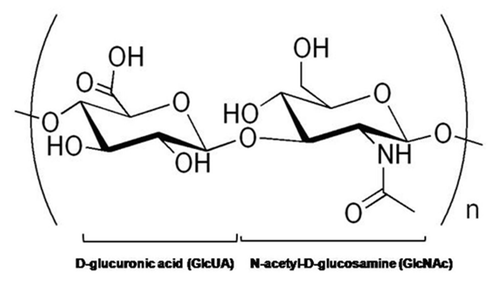
Figure 2 MW-dependant interactions of HA with cells. (A) High MW HA binds cells and extracellular hyaladherins in a polyvalent manner to protect and maintain the structure and integrity of tissues with few stimulatory effects on cells. The HA also tends to form pericellular sheaths that prevent cell-cell and cell-growth factor interactions. (B) In contrast, HA oligomers interact with cellular receptors in a monovalent manner, and may cause clustering of cell surface receptors (e.g., CD44) to evoke a multitude of intracellular signaling cascades.
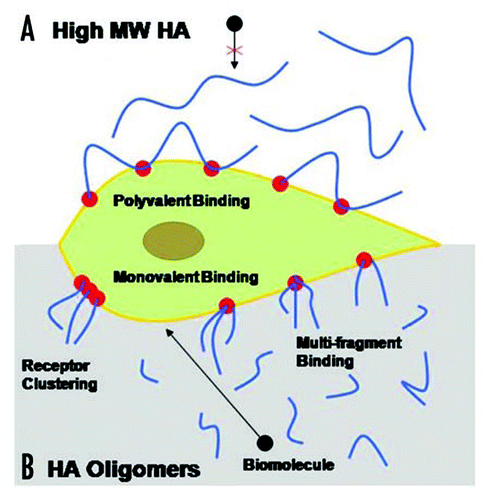
Figure 3 Proliferation of EC cultures in response to exogenous HA supplements (2 micrograms/ml). The proliferation ratios of ECs treated with pure HA 6mers and digests (HA-o) containing HA 6mers (33.3 ± 2.4% w/w) and 12mers (39.2 ± 2.7% w/w) proliferated to a greater degree than cells cultured with HA of MW = 1500 KDa or no HA. Morphology of ECs cultured with HA supplements remained similar to HA-free control cells. *denotes a p-value < 0.05 in comparison to the no HA control. Results adapted from reference Citation116.
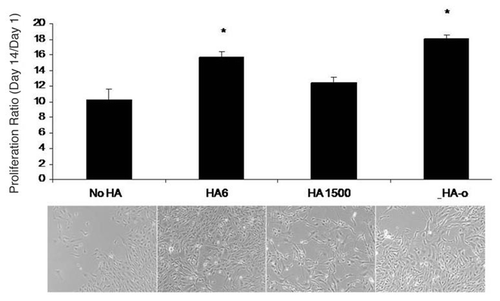
Figure 4 Impact of HA and HA oligomers on EC tube formation on matrigel. HA of MW = 1500 kDa increased individual tube lengths but decreased tube densities resulting in a cumulative tube length similar to the non-HA control cultures. Lengths of individual tubes in TNF-α HA 6mer- and HA-o- (see composition in ) stimulated cultures were similar to that in the controls but the number of tubes were significantly greater, resulting in a greater cumulative tube length. Suramin drastically inhibited tube formation and tubes in TNFα-supplemented cultures seemed to be incomplete, with gaps between junctions. Tube formation images were labeled with calcein AM *denotes a p-value < 0.05 in comparison to the no HA control. All HA supplements were added at a dose of 2 µg/ml. Results adapted fromCitation116.
Figure 5 Impact of HA and HA oligomers on production of angiogenic cytokines by cultured rat aortic ECs. Of the 19 cytokines tested in each cytokine array, here we report three that are specifically known to influence angiogenesis. HA-oligomers (HA-o) upregulated production of pro-angiogenic TNFα, Leptin, and VEGF but also enhanced production of tissue inhibitor of matrix metalloproteinases (TIMP-1), an inhibitor of angiogenesis in vivo. HMW HA (HA 1500) had no effect on VEGF, Leptin, and TNFα production, but enhanced TIMP-1 synthesis, suggesting anti-angiogenic effects. HA dose was 2 micrograms/ml. *denotes a p-value < 0.05 in comparison to the no HA control. Results adapted from reference Citation116.
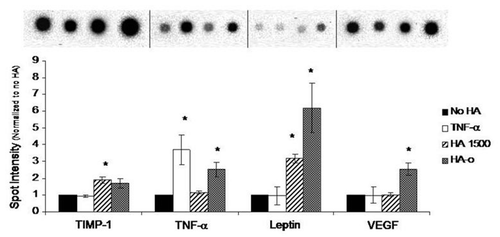
Table 1 Angiogenic stimulating and inhibiting factors
Table 2 Size-dependent biologic interactions of HA
Table 3 HA-receptor interactions
References
- Folkman J. Toward an understanding of angiogenesis: search and discovery. Perspect Biol Med 1985; 29:10 - 36
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996; 86:353 - 364
- Laschke MW, Harder Y, Amon M, et al. Angiogenesis in tissue engineering: breathing life into constructed tissue substitutes. Tissue Eng 2006; 12:2093 - 2104
- Arnold F, West DC. Angiogenesis in wound healing. Pharmacol Ther 1991; 52:407 - 422
- Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alphavbeta3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994; 79:1157 - 1164
- Re F, Zanetti A, Sironi M, Polentarutti N, Lanfrancone L, Dejana E, Colotta F. Inhibition of anchorage-dependent cell spreading triggers apoptosis in cultured human endothelial cells. J Cell Biol 1994; 127:537 - 546
- Isner JM. Cancer and atherosclerosis: the broad mandate of angiogenesis. Circulation 1999; 99:1653 - 1655
- Colton CK. Implantable biohybrid artificial organs. Cell Transplant 1995; 4:415 - 436
- Folkman J, Hochberg M. Self-regulation of growth in three dimensions. J Exp Med 1973; 138:745 - 753
- Kyzas PA, Stefanou D, Batistatou A, Agnantis NJ. Hypoxia-induced tumor angiogenic pathway in head and neck cancer: an in vivo study. Cancer Lett 2005; 225:297 - 304
- Distler O, Neidhart M, Gay RE, Gay S. The molecular control of angiogenesis. Int Rev Immunol 2002; 21:33 - 49
- Karamysheva AF. Mechanisms of angiogenesis. Biochemistry (Mosc) 2008; 73:751 - 762
- Ratajska A, Czarnowska E, Kolodzinska A, Kluzek W, Lesniak W. Vasculogenesis of the embryonic heart: origin of blood island-like structures. Anat Rec A Discov Mol Cell Evol Biol 2006; 288:223 - 232
- Masuda H, Asahara T. Post-natal endothelial progenitor cells for neovascularization in tissue regeneration. Cardiovasc Res 2003; 58:390 - 398
- Tepper OM, Capla JM, Galiano RD, Ceradini DJ, Callaghan MJ, Kleinman ME, Gurtner GC. Adult vasculogenesis occurs through in situ recruitment, proliferation and tubulization of circulating bone marrow-derived cells. Blood 2005; 105:1068 - 1077
- Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA 2000; 97:3422 - 3427
- Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bonemarrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001; 7:430 - 436
- Kawamoto A, Asahara T, Losordo DW. Transplantation of endothelial progenitor cells for therapeutic neovascularization. Cardiovasc Radiat Med 2002; 3:221 - 225
- Enciso JM, Hirschi KK. Nutrient regulation of tumor and vascular endothelial cell proliferation. Curr Cancer Drug Targets 2007; 7:432 - 437
- Stupack DG, Cheresh DA. Apoptotic cues from the extracellular matrix: regulators of angiogenesis. Oncogene 2003; 22:9022 - 9029
- Polverini PJ, Leibovich SJ. Induction of neovascularization in vivo and endothelial proliferation in vitro by tumor-associated macrophages. Lab Invest 1984; 51:635 - 642
- Canfield AE, Schor AM, Schor SL, Grant ME. The biosynthesis of extracellular-matrix components by bovine retinal endothelial cells displaying distinctive morphological phenotypes. Biochem J 1986; 235:375 - 383
- Niedbala MJ. Cytokine regulation of endothelial cell extracellular proteolysis. Agents Actions Suppl 1993; 42:179 - 193
- Madri JA, Pratt BM, Tucker AM. Phenotypic modulation of endothelial cells by transforming growth factor-beta depends upon the composition and organization of the extracellular matrix. J Cell Biol 1988; 106:1375 - 1384
- Maragoudakis ME, Sarmonika M, Panoutsacopoulou M. Inhibition of basement membrane biosynthesis prevents angiogenesis. J Pharmacol Exp Ther 1988; 244:729 - 733
- Zhang YW, Su Y, Volpert OV, Vande Woude GF. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation. Proc Natl Acad Sci USA 2003; 100:12718 - 12723
- Rastinejad F, Polverini PJ, Bouck NP. Regulation of the activity of a new inhibitor of angiogenesis by a cancer suppressor gene. Cell 1989; 56:345 - 355
- Polverini PJ. The pathophysiology of angiogenesis. Crit Rev Oral Biol Med 1995; 6:230 - 247
- Bauland CG, van Steensel MA, Steijlen PM, Rieu PN, Spauwen PH. The pathogenesis of hemangiomas: a review. Plast Reconstr Surg 2006; 117:29 - 35
- Heidenreich R, Röcken M, Ghoreschi K. Angiogenesis: the new potential target for the therapy of psoriasis?. Drug News Perspect 2008; 21:97 - 105
- Velasco P, Lange-Asschenfeldt B. Dermatological aspects of angiogenesis. Br J Dermatol 2002; 147:841 - 852
- Simó R, Carrasco E, García-Ramírez M, Hernández C. Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr Diabetes Rev 2006; 2:71 - 98
- Laschke MW, Menger MD. In vitro and in vivo approaches to study angiogenesis in the pathophysiology and therapy of endometriosis. Hum Reprod Update 2007; 13:331 - 342
- Fearon U, Veale DJ. Angiogenesis in arthritis: methodological and analytical details. Methods Mol Med 2007; 135:343 - 357
- Sluimer JC, Gasc JM, van Wanroij JL, Kisters N, Groeneweg M, Sollewijn Gelpke MD, Cleutjens JP, van den Akker LH, Corvol P, Wouters BG, Daemen MJ, Bijnens AP. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol 2008; 51:1258 - 1265
- Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for antiangiogenesis and normalization. Microvasc Res 2007; 74:72 - 84
- Jayasankar V, Woo YJ, Pirolli TJ, Bish LT, Berry MF, Burdick J, Gardner TJ, Sweeney HL. Induction of angiogenesis and inhibition of apoptosis by hepatocyte growth factor effectively treats postischemic heart failure. J Card Surg 2005; 20:93 - 101
- Hausman MR, Rinker BD. Intractable wounds and infections: the role of impaired vascularity and advanced surgical methods for treatment. Am J Surg 2004; 187:44 - 55
- Langer R, Vacanti JP. Tissue engineering. Science 1993; 260:920 - 926
- Nomi M, Miyake H, Sugita Y, Fujisawa M, Soker S. Role of growth factors and endothelial cells in therapeutic angiogenesis and tissue engineering. Curr Stem Cell Res Ther 2006; 1:333 - 343
- Steffens L, Wenger A, Stark GB, Finkenzeller G. In vivo engineering of a human vasculature for bone tissue engineering applications. J Cell Mol Med 2008; in press
- Finkenzeller G, Torio-Padron N, Momeni A, Mehlhorn AT, Stark GB. In vitro angiogenesis properties of endothelial progenitor cells: a promising tool for vascularization of ex vivo engineered tissues. Tissue Eng 2007; 13:1413 - 1420
- Jang JH, Kim SK, Choi JE, Kim YJ, Lee HW, Kang SY, Park JS, Choi JH, Lim HY, Kim HC. Endothelial progenitor cell differentiation using cryopreserved, umbilical cord blood-derived mononuclear cells. Acta Pharmacol Sin 2007; 28:367 - 374
- Bouis D, Kusumanto Y, Meijer C, Mulder NH, Hospers GA. A review on pro- and antiangiogenic factors as targets of clinical intervention. Pharmacol Res 2006; 53:89 - 103
- Soker S, Machado M, Atala A. Systems for therapeutic angiogenesis in tissue engineering. World J Urol 2000; 18:10 - 18
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature 2000; 407:242 - 248
- Morishita R, Aoki M, Hashiya N, et al. Therapeutic angiogenesis using hepatocyte growth factor (HGF). Curr Gene Ther 2004; 4:199 - 206
- Vashi AV, Abberton KM, Thomas GP, et al. Adipose tissue engineering based on the controlled release of fibroblast growth factor-2 in a collagen matrix. Tissue Eng 2006; 12:3035 - 3043
- Kontos CD, Annex BH. Engineered transcription factors for therapeutic angiogenesis. Curr Opin Mol Ther 2007; 9:145 - 152
- Landman KA, Cai AQ. Cell proliferation and oxygen diffusion in a vascularising scaffold. Bull Math Biol 2007; 69:2405 - 2428
- Butler MJ, Sefton MV. Poly(butyl methacrylate-co-methacrylic acid) tissue engineering scaffold with pro-angiogenic potential in vivo. J Biomed Mater Res A 2007; 82:265 - 273
- Druecke D, Langer S, Lamme E, Pieper J, Ugarkovic M, Steinau HU, Homann HH. Neovascularization of poly(ether ester) block-copolymer scaffolds in vivo: long-term investigations using intravital fluorescent microscopy. J Biomed Mater Res A 2004; 68:10 - 18
- Bakshi A, Fisher O, Dagci T, Himes BT, Fischer I, Lowman A. Mechanically engineered hydrogel scaffolds for axonal growth and angiogenesis after transplantation in spinal cord injury. J Neurosurg Spine 2004; 1:322 - 329
- Sung HJ, Meredith C, Johnson C, Galis ZS. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials 2004; 25:5735 - 5742
- Rücker M, Laschke MW, Junker D, Carvalho C, Tavassol F, Mülhaupt R, Gellrich NC, Menger MD. Vascularization and biocompatibility of scaffolds consisting of different calcium phosphate compounds. J Biomed Mater Res A 2008; 86:1002 - 1011
- Laurencin CT, Pierre-Jacques HM, Langer R. Toxicology and biocompatibility considerations in the evaluation of polymeric materials for biomedical applications. Clin Lab Med 1990; 10:549 - 570
- Peter SJ, Miller ST, Zhu G, Yasko AW, Mikos AG. In vivo degradation of a poly(propylene fumarate)/beta-tricalcium phosphate injectable composite scaffold. J Biomed Mater Res 1998; 41:1 - 7
- Narmoneva DA, Oni O, Sieminski AL, Zhang S, Gertler JP, Kamm RD, Lee RT. Self-assembling short oligopeptides and the promotion of angiogenesis. Biomaterials 2005; 26:4837 - 4846
- Callegari A, Bollini S, Iop L, et al. Neovascularization induced by porous collagen scaffold implanted on intact and cryoinjured rat hearts. Biomaterials 2007; 28:5449 - 5461
- Hall H. Modified fibrin hydrogel matrices: both, 3D-scaffolds and local and controlled release systems to stimulate angiogenesis. Curr Pharm Des 2007; 13:3597 - 3607
- Jeon O, Ryu SH, Chung JH, Kim BS. Control of basic fibroblast growth factor release from fibrin gel with heparin and concentrations of fibrinogen and thrombin. J Control Release 2005; 105:249 - 259
- Daamen WF, Nillesen ST, Wismans RG, et al. A biomaterial composed of collagen and solubilized elastin enhances angiogenesis and elastic fiber formation without calcification. Tissue Eng Part A 2008; 14:349 - 360
- Doi K, Ikeda T, Marui A, et al. Enhanced angiogenesis by gelatin hydrogels incorporating basic fibroblast growth factor in rabbit model of hind limb ischemia. Heart Vessels 2007; 22:104 - 108
- Ishihara M, Obara K, Nakamura S, et al. Chitosan hydrogel as a drug delivery carrier to control angiogenesis. J Artif Organs 2006; 9:8 - 16
- Perets A, Baruch Y, Weisbuch F, Shoshany G, Neufeld G, Cohen S. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J Biomed Mater Res A 2003; 65:489 - 497
- Flynn L, Prestwich GD, Semple JL, Woodhouse KA. Adipose tissue engineering in vivo with adipose-derived stem cells on naturally derived scaffolds. J Biomed Mater Res A 2008; in press
- Schoenfelder M, Einspanier R. Expression of hyaluronan synthases and corresponding hyaluronan receptors is differentially regulated during oocyte maturation in cattle. Biol Reprod 2003; 69:269 - 277
- Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 2004; 4:528 - 539
- Itano N, Kimata K. Mammalian hyaluronan synthases. IUBMB Life 2002; 54:195 - 199
- Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delisser H, Savani RC, Kumar S. Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol 2007; 26:58 - 68
- Stern R. Hyaluronan catabolism: a new metabolic pathway. Eur J Cell Biol 2004; 83:317 - 325
- West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science 1985; 228:1324 - 1326
- Slevin M, West D, Kumar P, Rooney P, Kumar S. Hyaluronan, angiogenesis and malignant disease. Int J Cancer 2004; 109:793 - 794
- Noble PW. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol 2002; 21:25 - 29
- Rooney P, Kumar S, Ponting J, Wang M. The role of hyaluronan in tumour neovascularization. Int J Cancer 1995; 60:632 - 636
- Lai PH, Chang Y, Chen SC, Wang CC, Liang HC, Chang WC, Sung HW. Acellular biological tissues containing inherent glycosaminoglycans for loading basic fibroblast growth factor promote angiogenesis and tissue regeneration. Tissue Eng 2006; 12:2499 - 2508
- West DC, Kumar S. The effect of hyaluronate and its oligosaccharides on endothelial cell proliferation and monolayer integrity. Exp Cell Res 1989; 183:179 - 196
- Evanko SP, Johnson PY, Braun KR, Underhill CB, Dudhia J, Wight TN. Platelet-derived growth factor stimulates the formation of versican-hyaluronan aggregates and pericellular matrix expansion in arterial smooth muscle cells. Arch Biochem Biophys 2001; 394:29 - 38
- Deed R, Rooney P, Kumar P, et al. Early-response gene signalling is induced by angiogenic oligosaccharides of hyaluronan in endothelial cells. Inhibition by non-angiogenic, high-molecular-weight hyaluronan. Int J Cancer 1997; 71:251 - 256
- Genasetti A, Vigetti D, Viola M, Karousou E, Moretto P, Rizzi M, Bartolini B, Clerici M, Pallotti F, De Luca G, Passi A. Hyaluronan and human endothelial cell behavior. Connect Tissue Res 2008; 49:120 - 123
- Gao F, Yang CX, Mo W, Liu YW, He YQ. Hyaluronan oligosaccharides are potential stimulators to angiogenesis via RHAMM mediated signal pathway in wound healing. Clin Invest Med 2008; 31:106 - 116
- Montesano R, Kumar S, Orci L, Pepper MS. Synergistic effect of hyaluronan oligosaccharides and vascular endothelial growth factor on angiogenesis in vitro. Lab Invest 1996; 75:249 - 262
- Chajara A, Raoudi M, Delpech B, Levesque H. Inhibition of arterial cells proliferation in vivo in injured arteries by hyaluronan fragments. Atherosclerosis 2003; 171:15 - 19
- Chajara A, Raoudi M, Delpech B, Levesque H. The fibroproliferative response of arterial smooth muscle cells to balloon catheter injury is associated with increased hyaluronidase production and hyaluronan degradation. Atherosclerosis 2001; 157:293 - 300
- Slevin M, Krupinski J, Kumar S, Gaffney J. Angiogenic oligosaccharides of hyaluronan induce protein tyrosine kinase activity in endothelial cells and activate a cytoplasmic signal transduction pathway resulting in proliferation. Lab Invest 1998; 78:987 - 1003
- Slevin M, Kumar S, Gaffney J. Angiogenic oligosaccharides of hyaluronan induce multiple signaling pathways affecting vascular endothelial cell mitogenic and wound healing responses. J Biol Chem 2002; 277:41046 - 41059
- Sattar A, Rooney P, Kumar S, et al. Application of angiogenic oligosaccharides of hyaluronan increases blood vessel numbers in rat skin. J Invest Dermatol 1994; 103:576 - 579
- Lees VC, Fan TP, West DC. Angiogenesis in a delayed revascularization model is accelerated by angiogenic oligosaccharides of hyaluronan. Lab Invest 1995; 73:259 - 266
- Sattar A, Kumar S, West DC. Does hyaluronan have a role in endothelial cell proliferation of the synovium?. Semin Arthritis Rheum 1992; 22:37 - 43
- West DC, Kumar S. Endothelial cell proliferation and diabetic retinopathy. Lancet 1988; 1:715 - 716
- Ohno-Nakahara M, Honda K, Tanimoto K, Tanaka N, Doi T, Suzuki A, Yoneno K, Nakatani Y, Ueki M, Ohno S, Knudson W, Knudson CB, Tanne K. Induction of CD44 and MMP expression by hyaluronidase treatment of articular chondrocytes. J Biochem 2004; 135:567 - 575
- Zhang Y, Thant AA, Machida K, Ichigotani Y, Naito Y, Hiraiwa Y, Senga T, Sohara Y, Matsuda S, Hamaguchi M. Hyaluronan-CD44s signaling regulates matrix metalloproteinase-2 secretion in a human lung carcinoma cell line QG90. Cancer Res 2002; 62:3962 - 3965
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGFbeta and promotes tumor invasion and angiogenesis. Genes Dev 2000; 14:163 - 176
- Lokeshwar VB, Selzer MG. Differences in hyaluronic acid-mediated functions and signaling in arterial, microvessel and vein-derived human endothelial cells. J Biol Chem 2000; 275:27641 - 27649
- Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, DeLisser HM. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J Biol Chem 2001; 276:36770 - 36778
- Murphy JF, Lennon F, Steele C, Kelleher D, Fitzgerald D, Long AC. Engagement of CD44 modulates cyclooxygenase induction, VEGF generation and proliferation in human vascular endothelial cells. FASEB J 2005; 19:446 - 448
- Rodgers LS, Lalani S, Hardy KM, et al. Depolymerized hyaluronan induces vascular endothelial growth factor, a negative regulator of developmental epithelial-to-mesenchymal transformation. Circ Res 2006; 99:583 - 589
- Carson DD, Dutt A, Tang JP. Glycoconjugate synthesis during early pregnancy: hyaluronate synthesis and function. Dev Biol 1987; 120:228 - 235
- Lovvorn HN 3rd, Cass DL, Sylvester KG, et al. Hyaluronan receptor expression increases in fetal excisional skin wounds and correlates with fibroplasia. J Pediatr Surg 1998; 33:1062 - 1069
- Dechert TA, Ducale AE, Ward SI, Yager DR. Hyaluronan in human acute and chronic dermal wounds. Wound Repair Regen 2006; 14:252 - 258
- Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem 2004; 279:17079 - 17084
- Greenwald RA, Moak SA. Degradation of hyaluronic acid by polymorphonuclear leukocytes. Inflammation 1986; 10:15 - 30
- Yamazaki K, Fukuda K, Matsukawa M, et al. Reactive oxygen species depolymerize hyaluronan: involvement of the hydroxyl radical. Pathophysiology 2003; 9:215 - 220
- Cao G, Savani RC, Fehrenbach M, et al. Involvement of endothelial CD44 during in vivo angiogenesis. Am J Pathol 2006; 169:325 - 336
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971; 285:1182 - 1186
- Folkman J, Cole P, Zimmerman S. Tumor behavior in isolated perfused organs: in vitro growth and metastases of biopsy material in rabbit thyroid and canine intestinal segment. Ann Surg 1966; 164:491 - 502
- Fjeldstad K, Kolset SO. Decreasing the metastatic potential in cancers—Targeting the heparan sulfate proteoglycans. Curr Drug Targets 2005; 6:665 - 682
- Koyama H, Hibi T, Isogai Z, et al. Hyperproduction of hyaluronan in neu-induced mammary tumor accelerates angiogenesis through stromal cell recruitment: possible involvement of versican/PG-M. Am J Pathol 2007; 170:1086 - 1099
- West DC, Kumar S. Hyaluronan and angiogenesis. Ciba Found Symp 1989; 143:187 - 201
- Itano N, Sawai T, Miyaishi O, Kimata K. Relationship between hyaluronan production and metastatic potential of mouse mammary carcinoma cells. Cancer Res 1999; 59:2499 - 2504
- Lokeshwar VB, Obek C, Soloway MS, Block NL. Tumor-associated hyaluronic acid: a new sensitive and specific urine marker for bladder cancer. Cancer Res 1997; 57:773 - 777
- Lokeshwar VB, Young MJ, Goudarzi G, et al. Identification of bladder tumor-derived hyaluronidase: its similarity to HYAL1. Cancer Res 1999; 59:4464 - 4470
- Hautmann SH, Schroeder GL, Civantos F, et al. [Hyaluronic acid and hyaluronidase. 2 new bladder carcinoma markers]. Urologe A 2001; 40:121 - 126
- Paiva P, Van Damme MP, Tellbach M, Jones RL, Jobling T, Salamonsen LA. Expression patterns of hyaluronan, hyaluronan synthases and hyaluronidases indicate a role for hyaluronan in the progression of endometrial cancer. Gynecol Oncol 2005; 98:193 - 202
- Bot PT, Hoefer IE, Piek JJ, Pasterkamp G. Hyaluronic Acid: targeting immune modulatory components of the extracellular matrix in atherosclerosis. Curr Med Chem 2008; 15:786 - 791
- Ibrahim S, Ramamurthi A. Hyaluronic acid cues for functional endothelialization of vascular constructs. J Tissue Eng Regen Med 2008; 2:22 - 32
- Gao F, Cao M, Yang C, He Y, Liu Y. Preparation and characterization of hyaluronan oligosaccharides for angiogenesis study. J Biomed Mater Res B Appl Biomater 2006; 78:385 - 392
- Deed R, Rooney P, Kumar P, Norton JD, Smith J, Freemont AJ, Kumar S. Early-response gene signalling is induced by angiogenic oligosaccharides of hyaluronan in endothelial cells. Inhibition by non-angiogenic, high-molecular-weight hyaluronan. Int J Cancer 1997; 71:251 - 256
- Joddar B, Ibrahim S, Ramamurthi A. Impact of delivery mode of hyaluronan oligomers on elastogenic responses of adult vascular smooth muscle cells. Biomaterials 2007; 28:3918 - 3927
- Ibrahim S, Joddar B, Craps M, Ramamurthi A. A surface-tethered model to assess size-specific effects of hyaluronan (HA) on endothelial cells. Biomaterials 2007; 28:825 - 835
- Collier JH, Camp JP, Hudson TW, Schmidt CE. Synthesis and characterization of polypyrrole-hyaluronic acid composite biomaterials for tissue engineering applications. J Biomed Mater Res 50:574 - 584
- Baier Leach J, Bivens KA, Patrick CW Jr, Schmidt CE. Photocrosslinked hyaluronic acid hydrogels: natural, biodegradable tissue engineering scaffolds. Biotechnol Bioeng 2003; 82:578 - 589
- Jansen K, van der Werff JF, van Wachem PB, Nicolai JP, de Leij LF, van Luyn MJ. A hyaluronan-based nerve guide: in vitro cytotoxicity, subcutaneous tissue reactions, and degradation in the rat. Biomaterials 2004; 25483 - 25489
- Flynn L, Prestwich GD, Semple JL, Woodhouse KA. Adipose tissue engineering in vivo with adipose-derived stem cells on naturally derived scaffolds. J Biomed Mater Res A 2008; in press
- Allingham PG, Brownlee GR, Harper GS, Pho M, Nilsson SK, Brown TJ. Gene expression, synthesis and degradation of hyaluronan during differentiation of 3T3-L1 adipocytes. Arch Biochem Biophys 2006; 452:83 - 91
- Tonello C, Zavan B, Cortivo R, Brun P, Panfilo S, Abatangelo G. In vitro reconstruction of human dermal equivalent enriched with endothelial cells. Biomaterials 2003; 24:1205 - 1211
- Hartlapp I, Abe R, Saeed RW, Peng T, Voelter W, Bucala R, Metz CN. Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J 2001; 15:2215 - 2224
- Pinney E, Liu K, Sheeman B, Mansbridge J. Human three-dimensional fibroblast cultures express angiogenic activity. J Cell Physiol 2000; 183:74 - 82
- Fournier N, Doillon CJ. Biological molecule-impregnated polyester: an in vivo angiogenesis study. Biomaterials 1996; 17:1659 - 1665
- Peattie RA, Nayate AP, Firpo MA, Shelby J, Fisher RJ, Prestwich GD. Stimulation of in vivo angiogenesis by cytokine-loaded hyaluronic acid hydrogel implants. Biomaterials 2004; 25:2789 - 2798
- Kim MH. Flavonoids inhibit VEGF/bFGF-induced angiogenesis in vitro by inhibiting the matrix-degrading proteases. J Cell Biochem 2003; 89:529 - 538
- Peattie RA, Rieke ER, Hewett EM, Fisher RJ, Shu XZ, Prestwich GD. Dual growth factor-induced angiogenesis in vivo using hyaluronan hydrogel implants. Biomaterials 2006; 27:1868 - 1875
- Riley CM, Fuegy PW, Firpo MA, Shu XZ, Prestwich GD, Peattie RA. Stimulation of in vivo angiogenesis using dual growth factor-loaded crosslinked glycosaminoglycan hydrogels. Biomaterials 2006; 27:5935 - 5943
- Borselli C, Oliviero O, Battista S, Ambrosio L, Netti PA. Induction of directional sprouting angiogenesis by matrix gradients. J Biomed Mater Res A 2007; 80:297 - 305