Abstract
Transplantation of the trachea may become the preferred method for the reconstruction of extensive tracheal defects, however, several unresolved problems must be addressed, such as immunosuppression, preservation, and donor shortage. In this manuscript, the cryopreservation of tracheal grafts is reviewed, which potentially is associated with a lessened immunological response. Cryopreservation may be used clinically for long-term preservation and may solve the donor shortage. It is very important to confirm the immunomodulatory effect of cryopreservation on tracheal allografts in order to expand the potential clinical application of tracheal transplantation in the future. The cartilage as well as the epithelium and lamina propria serve as targets for rejection. However, the effect of cryopreservation on chondrocytes could be associated with reduced allogenicity of the trachea. The long-term cryopreservation of cartilage must be investigated in basic research models of chondrocyte viability. Growth of cryopreserved tracheal allografts is less well understood. Further studies are needed to elucidate the mechanism of synergistic effects of both cryopreservation and adequate immunosuppression for tracheal xenografts.
Introduction
An extensive tracheal resection is often required in patients with malignancies involving the trachea,Citation1,Citation2 or with benign stenosis of the trachea secondary to accidental trauma, or to congenital,Citation3 inflammatoryCitation4,Citation5 or iatrogenic causes.Citation6 When a primary anastomosis is not feasible following an extensive resection of the trachea, a terminal tracheal fistula is created. However, in certain cases it may be preferable to interpose a substitute trachea. While prosthetic and tissue grafts are used in repairing circumferential tracheal defects their use has achieved only limited success. Several experiments on the prosthetic replacement of large tracheal defects have been performed with the prospect of clinical application.Citation7–Citation9 However, most of these prosthetic tracheal grafts failed to reepithelialize.Citation9–Citation11 The repair of tracheal defects with autogenous tissue, such as free periosteal,Citation12 jejunal,Citation13 muscular,Citation14 esophageal,Citation15 bronchialCitation16 and aorticCitation17 grafts also has met with limited success because of difficulties in maintaining a patent airway. In contrast, a tracheal allograft contains native epithelium and has cartilaginous rings to maintain a patent airway. Therefore, a tracheal allograft is an ideal substitute for the defect. However, before the routinge clinical application of the tracheal allograft procedure can become a reality, several questions must be addressed, regarding revascularization, immunosuppression, preservation and donor shortage.
To clarify revascularization for tracheal grafts, the author has already reported several studiesCitation18–Citation23 and a review of the literature.Citation24 In this manuscript, cryopreservation of tracheal grafts is reviewed, which may potentially be used for long-term preservation and may be associated with a low immunological response.
Rejection Reaction and Immunosuppression
Tracheal transplants, in contrast to other organ transplants, induce only a weak graft-rejection reaction even in the presence of major histoincompatibility. Rose and co-workers first demonstrated that a tracheal allotransplant functioned without any evidence of rejection in man.Citation25 However, several investigators have reported that the cartilage as well as the epithelium and lamina propria serve as targets for rejection.Citation26,Citation27 Beigel and colleagues demonstrated the antigenicity of rat tracheal transplants and found that the donor epithelium is rejected and that new epithelium migrated from recipient to the transplant.Citation28–Citation30 Thirteen years later, this was definitively shown to be correct using techniques of molecular biology.Citation31 Human studies have demonstrated Human Leukocyte Antigen (HLA) class II subregion gene to be the antigenic profile of the trachea.Citation32–Citation34
To better characterize the immunobiology of the trachea, Genden and co-workers successfully established an orthotopic tracheal transplant model in the mouse.Citation35 In their experiments using nonimmunosuppressed mice, the allogeneic recipients developed a persistent audible stridor two days after tracheal transplantation and allogeneic grafts demonstrated a loss of normal ciliated respiratory epithelium and a CD8/CD4-positive lymphocytic infiltrate. In nonimmunosuppressed dogs, the author demonstrated that heterotopic tracheal allotransplants display an early rejection reaction similar to that of Genden's murine model and developed a set of criteria for diagnosing rejection. The target for rejection was shown to be epithelium first, followed by lamina propria and cartilage.Citation36
The chronic histopathologic features of tracheal allotransplant rejection remain to be fully elucidated. The author developed a reproducible model of a refractory tracheal allotransplant rejection using Tacrolimus in rats.Citation37 However, it is difficult to develop a reliable chronic rejection model of the tracheal allotransplant because the chronic rejection represents neither just a refractory immunologic reaction nor a repeat of acute rejection.
Many researchers have presented immunosuppressive therapies for acute rejection of tracheal allografts. Delaere and colleagues demonstrated that tracheal allografts undergoing continuous immunosuppression with 10 mg/kg per day of Cyclosporin-A showed no rejection and remained functional with preservation of mucociliary activity.Citation38,Citation39 The author advocated that an appropriate dose of 15 mg/kg/day of Cyclosporin-A may be used to maintain rat tracheal allograft viability.Citation40 Davreux and co-workers demonstrated that 2 mg/kg/day of methylprednisolone in addition to 15 mg/kg/day of Cyclosporin-A may improve epithelial viability in rat tracheal allografts.Citation41 With regard to other agents, Moriyama and co-workers reported successful tracheal allotransplantation using 0.1 mg/kg/day of Tacrolimus in dogs.Citation42
The author and colleagues made an attempt to minimize the dose of immunosuppressive agent in order to avoid side effects such as organ toxicity, carcinogenesis and infectious complications. A short-course of immunosuppression, such as 1.5 mg/kg/day of Tacrolimus administered for only 3 consecutive days after surgery in rats,Citation43 5 mg/kg/day of Mizoribine for 21 days in dogs,Citation44 and 150 to 225 mg/kg/day of Multiglycosidorum tripterygii for 3 days in rats, maintained the morphology of tracheal allografts.Citation37,Citation45 A short-course and a large dose of immunosuppressive agent seem to induce immunologic unresponsiveness.Citation46 Acceptable efficacy of those agents was shown in animals although such methods cannot be applied directly to humans.
Other immunosuppression methods include denervation of the spleen,Citation47 high dose radiation therapy,Citation48 photodynamic therapy,Citation49 medication (Cialit and Merthiolate,Citation50 and t-octylphenoxy-polyethoxyethanolCitation51) used in the treatment of reduction of graft antigenicity, or interleukin-10 gene transfer therapy to restrain an immune response of T cells.Citation52 It is necessary to wait for future reports on the long-term results of those methods. However, Yokomise and co-workers have reported that tracheal allografts do not survive long term following high dose irradiation therapy.Citation48,Citation53 Recently, several researchers have reported that cryopreservation reduces antigenicity of the tracheal allografts and controls a rejection reaction in a large animal model. These will be reviewed below.
Long-Term Preservation
As transplantation of the trachea becomes a more preferred method for the reconstruction of extensive tracheal defects in the future, the shortage of donor trachea will remain a major obstacle. A long-term period of preservation is one of the methods to help solve this problem. In regard to cold ischemia time of the trachea, Macchiarini and associates demonstrated that the trachea may be safely preserved for as long as 15 hours of static storage in Euro-Collins solution at 4 degrees Celsius, as assessed by histology and mucous-secreting functions.Citation54 However, the period of the preservation in this study is too short to solve the donor shortage. A longer-term preservation method is required.
The first experimental study on cryopreservation of the trachea was reported in 1951.Citation55,Citation56 Experimental work intensified after Deschamps and co-workers reported the functional and hisotological changes of the cryopreserved canine trachea.Citation55 Yokomise and colleagues showed that structural reliability of a cryopreserved trachea was maintained for one month with a new solution including trehalose in addition to dimethyl sulfoxide.Citation57 The author and associates assessed the maximal period of cryopreservation using an easy and cheap slow-freezing instrument for viable tracheal isograft in rats. As a result, the effect of cryopreservation on the trachea has been recognized as the degeneration of both the cartilage and the epithelium. The cartilage showed irreversible damage while the epithelium partially remained after cryopreservation for 6 months. The permissible period of cryopreservation to maintain the isograft viability was 3 months.Citation58 This period of cryopreservation could help clinicians to greatly relieve the shortage of donors.
If the trachea can be retrieved for transplantation late after circulatory arrest, the shortage of donors may be further alleviated. The combined use of non beating-heart cadaveric donors and a long period of cryopreservation for tracheal grafting offers great hope for their eventual clinical use in humans. The author and associates therefore tried to elucidate the permissible period of warm ischemia before cryopreservation in rat tracheal isografts. The maximum permissible period of warm ischemia before a 3-month cryopreservation which maintained isograft viability was 18 hours.Citation59 Concerning the method of cryopreservation, several researchers have reported the usefulness of lyophilization for tracheal allografts.Citation56,Citation60 Such a technique could expand the human tracheal donor pool. When a longer period of cryopreservation is required, investigators and clinicians may face decreased viability, but this may be balanced by the decreased antigenicity that results from cryopreservation.
Effect of Cryopreservation on Immunologic Reaction
The effect of cryopreservation on tissue allografts has been studied in basic research models for many years. However, its effect on immune responses remains controversial. Several investigators have reported that cryopreservation decreases tracheal allogenicity and prolongs survival of allografts in canine experimental models.Citation61–Citation63 Similar experiments using rats have suggested that denuded epithelium caused by cryopreservation is closely related to decreased antigenicity.Citation64,Citation65 However, the author and associates found that the epithelium does not always disappear after thawing of cryopreserved tracheal grafts. In each cryopreserved graft that we observed, approximately 10 to 40% of the epithelium remained immediately after thawing, although longer periods of cryopreservation resulted in more denuded epithelium. The remaining epithelium underwent degeneration, consisting of a single layer of non-ciliated epithelium ().Citation58,Citation66 After transplantation of the cryopreserved tracheal grafts, the remaining epithelium was gradually regenerated in the isografts. In the allografts, however, the remaining epithelium was rejected whereas other components of the trachea were maintained.Citation66 These facts refute the speculation that the denuded epithelium caused by cryopreservation is related to decreased antigenicity, and suggest that the remaining epithelium after cryopreservation may possess antigenicity.
If the denudation of the mucosa including the epithelium and the mixed glandular tissue holds the key of treatment for immunologic rejection in the tracheal allotransplantation, allografts could maintain their structure without immunologic rejection in artificial mucosa denudation models. Liu and associates showed that the antigenicity of canine tracheal allografts could be reduced by removing the epithelium and mixed glands from the graft by detergent treatment.Citation51 However, the detergent treatment using t-octylphenoxypolyethoxyethanol may reduce the antigenicity of the cartilage adventitia as well as the mucosa. The author found that fresh tracheal allografts undergoing an artificial removal of the epithelium could not maintain their patent structure in rats because of immunologic rejection (unpublished data) (). Therefore, loss of the epithelium only does not necessarily hold the key for treatment of immunologic rejection.
It is very important to confirm the immunomodulatory effect of cryopreservation on tracheal allografts in order to expand the potential clinical applications of tracheal transplantation in the future. The author and colleagues assessed second-set allograft rejection to demonstrate the credibility of the immunomodulatory effect of cryopreservation. In this study, second-set rejection occurred only in rats undergoing fresh primary allografting, not in rats undergoing cryopreserved primary allografting ().Citation67 Thus, cryopreservation prevents enhanced allograft rejection and potentially has an immunomodulatory effect on the allograft. The mechanism for this immunomodulatory effect potentially includes delayed antigen sensitization and reduced antigenicity of the trachea. The antigen sensitization could be weakly induced by cryopreservation until the blood supply is restored to the transplanted graft. However, our experimental results showed that the cryopreserved allografts survived for a long period after transplantation and did not show delayed rejection.Citation67 Therefore, cryopreservation does not seem to delay the antigen sensitization of tracheal allografts but probably decreases the antigenicity itself.
Similar effects of cryopreservation have been reported in other tissues. In skin flaps transplanted via microvascular anastomosis, Hirase and co-workers demonstrated a decrease in the antigenicity of dermis in cryopreserved allografts without immunosuppression.Citation68 The history of cryopreserved cardiac valve allotransplantation has suggested that the denuded endothelium after cryopreservation may be associated with decreased antigenicity although the immunologic rejection is inevitable.Citation69,Citation70 The immunomodulatory effect of cryopreservation on tissue allografts is considered to be credible.
Other factors as well as reduced epithelium may be associated with decreased antigenicity in the cryopreserved tracheal allografts. Although the mucosa including the epithelium and the mixed glandular tissue is reported to express HLA class II subregion gene products,Citation32 the chondrocytes are also thought to have a tissue-specific surface antigen.Citation27 The author and associates demonstrated that a longer period of cryopreservation helps to maintain a better patency of tracheal allografts despite the decreased viability of chondrocytes.Citation66 Messineo and co-workers also showed that cryopreserved tracheal allografts without immunosuppression have no viable chondrocytes, but the patency of the lumen can be adequately preserved.Citation71 The effect of cryopreservation on chondrocytes may be associated with reduced allogenicity of the trachea. On the other hand, Ohlendorf and colleagues showed that the beneficial effect of cryoprotectant is confined to the chondrocytes in the superficial layer of the cartilage.Citation72 The degeneration of the chondrocytes occurring in a major part of the cartilage in cryopreserved allografts may lead to decreased antigenicity because the cryoprotectant does not penetrate deeply into the cartilage. The chondrocytes could determine the fate of the cryopreserved tracheal allografts. The long-term cryopreservation of cartilage must therefore be investigated in basic research models of chondrocyte viability.
Although Murakawa and colleagues showed in vitro that cryopreservation does not downregulate the allogenicity of human fibroblasts,Citation73 they did however demonstrate the successful allotransplantation of cryopreserved tracheal grafts in nonhuman primates.Citation74 They reported that cryopreservation potentially reduces antigenicity by the downregulation of HLA class II antigen presentation of airway epithelial cells and also infiltration of various kinds of antigen presenting cells.Citation74,Citation75 The effect of cryopreservation on antigen presenting cells remains controversial, and further investigation is required to clarify the mechanism.
The growth potential of tracheal transplants is another important, unresolved issue. In an experimental study using autografts, Kubota and co-workers demonstrated the growth potential of tracheal autografts in a growing puppy model.Citation76 On the other hand, Hisamatsu and colleagues studied growth of cryopreserved tracheal allotransplant using rabbits for the treatment of congenital tracheal stenosis, and demonstrated that patency of the cryopreserved tracheal allografts was favorable, but no growth occurred even with the use of an immunosuppressant.Citation77 This issue will also be the subject of future investigated.
Xenotransplantation
As a possible solution to the donor shortage, xenotransplation of the trachea has been examined as an option to tracheal transplantation. Humoral immunity is always seen early after concordant xenogeneic transplantation, followed by T-cell mediated cellular immunity.Citation78 In discordant xenogeneic transplantation, the hyperacute rejection reaction is caused by activation of antibodies and complement. Even if this reaction is averted, the delayed xenogeneic rejection occurs within five days after transplantation, followed by cellular rejection by T cell.Citation79,Citation80 Therefore, it is very difficult to control the course of xenogeneic rejection. However, several investigators have shown that immunosuppressive agents can maintain the morphology of xenogeneic transplants. Valdivia and associates reported the effectiveness of tacrolimus in suppressing antibody production in hamster-to-rat liver xenotransplantation.Citation81 Reichenspurner and co-workers presented Leflunomide to potentially maintain the epithelial viability in hamster-to-rat heterotopic tracheal xenotransplantation.Citation82 An appropriate immunosuppressive regimen could prolong xenograft survival.
The author and colleagues combined the effects of reduced immunogenicity by cryopreservation and inhibited T cell-derived rejection by immunosuppressive agents, and studied the feasibility of cryopreserved xenografts with the use of short-course immunosuppression. An observed synergistic effect of cryopreservation and adequate dose of tacrolimus therapy enabled tracheal xenografts to remain viable over a long period ().Citation83 In tissue xenotransplantation, hyperacute rejection between discordant species may not occur because of low natural antibody titers.Citation84 A primitive immunoreaction may be associated with tracheal xenotransplantation, as evidenced by the effect of immunosuppression.Citation83 Further studies are needed to elucidate the mechanism of tracheal xenograft rejection.
Clinical Application
Rose and co-workers reported the first successful human tracheal allotransplantation in 1979.Citation25 In 1993, one-staged allotransplantation of the trachea was performed in a patient with marked tracheal stenosis caused by idiopathic fibrosing mediastinitis, and successful results were achieved with adequate immunosuppression and omental revascularization.Citation85 Thereafter, several investigators working with Herberhold reported successful tracheal transplantation using chemically treated allografts for pediatric patients with congenital tracheal stenosis.Citation86–Citation88 Such a treatment is now an established option for congenital tracheal reconstruction.
The study of tracheal transplantation using non-chemically treating allografts is proceding, as described above.Citation89 Tracheal transplantation using cryopreserved and irradiated homograft for human laryngotracheal reconstruction has been reported to be a viable alternative.Citation90 As the tracheal transplantation was used in the treatment of glottic cancer,Citation91 an immunosuppressant-free allotransplantation was required. Although the clinical applications for cryopreserved allografts are limited at present, they will almost certainly expand with further advances in immunosuppression and preservation techniques.
Figures and Tables
Figure 1 Histologic findings of cryopreserved rat tracheal allograft immediately after thawing. The epithelium shows degeneration but not denuded. (Hematoxylin and eosin; original magnification, ×200).
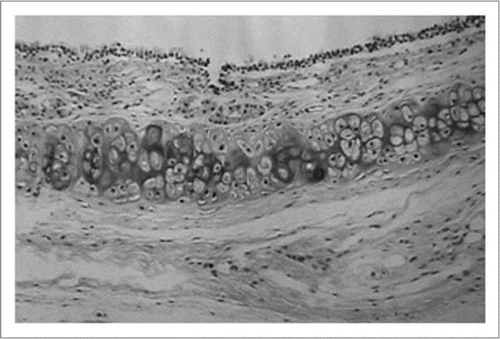
Figure 2 Histologic findings of fresh rat tracheal allograft 3 months after transplantation, which was denuded of the epithelium. The graft shows narrowed patency because of thickened mucosa with mononuclear cell infiltration. (Hematoxylin and eosin; original magnification, ×25).
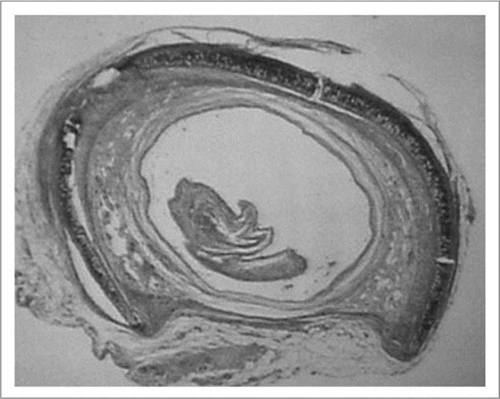
Figure 3 Histologic findings of rat tracheal allografts 28 days after transplantation. (A) a second allograft after transplantation of fresh allograft. The allograft is severely rejected. (B) a second allograft after transplantation of cryopreserved allograft. The patency of the allograft is narrow but the structure is maintained. (Hematoxylin and eosin; original magnification, ×25).

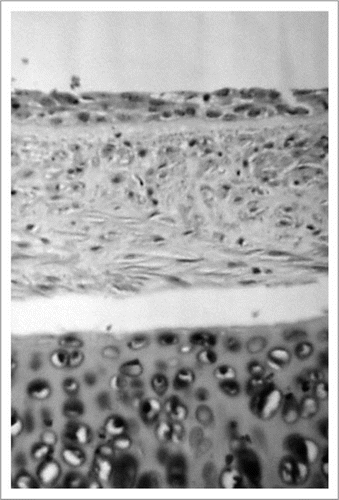
Figure 4 Histologic findings of cryopreserved tracheal xenograft 28 days after transplantation, which received short-course immunosuppression with 3.5 mg/kg/day of Tacrolimus. The epithelium shows degeneration but the cartilage is well maintained. (Hematoxylin and eosin; original magnification, ×200).
References
- Regnard JF, Fourquier P, Levasseur P. The French Society of Cardiovascular Surgery. Pairolero P. Results and prognostic factors in resection of primary tracheal tumors: a multicenter retrospective study. J Thorac Cardiovasc Surg 1996; 111:808 - 814
- Maziak DE, Todd TRJ, Keshavjee SH, Winton TL, Nostrand PV, Pearson FG. Adenoid cystic carcinoma of the airway: thirty-two-year experience. J Thorac Cardiovasc Surg 1996; 112:1522 - 1532
- Grillo HC. Slide tracheoplasty for long-segment congenital tracheal stenosis. Ann Thorac Surg 1994; 58:613 - 621
- Grillo HC. Terminal or mural tracheostomy in the anterior mediastinum. J Thorac Cardiovasc Surg 1966; 51:422 - 427
- Weber TR, Connors RH, Tracy TF Jr. Acquired tracheal stenosis in infants and children. J Thorac Cardiovasc Surg 1991; 102:29 - 35
- Grillo HC. Postintubation tracheal stenosis. Treatment and results. J Thorac Cardiovasc Surg 1995; 109:486 - 493
- Leake D, Habal M, Pizzoferrato A, Vespucci A. Prosthetic replacement of large defects of the cervial trachea in dog. Biomaterials 1985; 6:17 - 22
- Mendak SH, Jensik RJ, Haklin MF, Roseman DL. The evaluation of various bioabsorbable materials on the titanium fiber metal tracheal prosthesis. Ann Thorac Surg 1984; 38:488 - 493
- Neville WE, Bolanowski PJP, Soltanzadeh H. Prosthetic reconstruction of the trachea and carina. J Thorac Cardiovasc Surg 1976; 72:525 - 538
- Pearson FG, Henderson RD, Gross AE, Ginsberg RJ, Stone RM. The reconstruction of circumferential tracheal defects with a porous prosthesis: an experimental and clinical study using heavy Marlex mesh. J Thorac Cardiovasc Surg 1968; 55:605 - 616
- Nelson RJ, Goldberg L, White RA, Shors E, Hirose FM. Neovascularity of a tracheal prosthesis/tissue complex. J Thorac Cardiovasc Surg 1983; 86:800 - 808
- Cohen RC, Filler RM, Konuma K, Bahoric A, Kent G, Smith C. A new model of tracheal stenosis and its repair with free periosteal grafts. J Thorac Cardiovasc Surg 1986; 92:296 - 304
- Jones RE, Morgan RF, Marcella KL, Mills SE, Kron IL. Tracheal reconstruction with autogenous jejunal microsurgical transfer. Ann Thorac Surg 1986; 41:636 - 638
- Papp C, McCraw JB, Arnold PG. Experimental reconstruction of the trachea with autogenous materials. J Thorac Cardiovasc Surg 1985; 90:13 - 20
- Kato R, Onuki AS, Watanabe M, Hashizume T, Kawamura M, Kikuchi K, et al. Tracheal reconstruction by esophageal interposition: an experimental study. Ann Thorac Surg 1990; 49:951 - 954
- Murakami S, Sato H, Yokoi K, Tunezuka N, Hayashi Y, Shimizu J, Watanabe Y. An experimental study of tracheal reconstruction using a freed piece of the right bronchus. Thorac Cardiovasc Surg 1994; 42:76 - 80
- Carbognani P, Spaggiari L, Solli P, Corradi A, Cantoni AM, Barocelli E, et al. Experimental tracheal transplantation using a cryopreserved aortic allograft. Eur Surg Res 1999; 31:210 - 215
- Nakanishi R, Shirakusa T, Takachi T. Omentopexy for tracheal autografts. Ann Thorac Surg 1994; 57:841 - 845
- Nakanishi R, Shirakusa T, Mitsudomi T. Maximum length of tracheal autografts in dogs. J Thorac Cardiovasc Surg 1993; 106:1081 - 1087
- Takachi T, Shirakusa T, Shiraishi T, Okabayashi K, Inutsuka K, Kawahara K, Nakanishi R. Experimental carinal autotransplantation and allotransplantation. J Thorac Cardiovasc Surg 1995; 110:762 - 767
- Nakanishi R, Nagaya N, Yoshimatsu T, Hanagiri T, Yasumoto K. Optimal dose of basic fibroblast growth factor for long-segment orthotopic tracheal autografts. J Thorac Cardiovasc Surg 1997; 113:26 - 36
- Nakanishi R, Hashimoto M, Yasumoto K. Improved airway healing using basic fibroblast growth factor in a canine tracheal autotransplantation model. Ann Surg 1998; 227:446 - 454
- Nakanishi R, Hashimoto M, So T, Sugaya S, Yasumoto K. Successful tracheo-carinal transplantation. J Cardiovasc Surg 1999; 40:591 - 596
- Nakanishi R. Revascularization of trachea in lung and tracheal transplantation. Clin Transplant 2007; 21:668 - 674
- Rose KG, Sesterhenn K, Wustrow F. Tracheal allotransplantation in man (Letter). Lancet 1979; 1:433
- Lane BP, Habicht GS, Jasper GS. Lymphocyte-epithelium interaction during rejection of nonisogeneic rat tracheal grafts. Am J Pathol 1977; 86:71 - 77
- Gertzbein SD, Tait JH, Devlin SR, Argue S. The antigenicity of chondrocytes. Immunology 1977; 33:141 - 145
- Beigel A, Muller-Ruchholtz W. Tracheal transplantation I. The immunogenic effect of rat tracheal transplants. Arch Otorhinolaryngol 1984; 240:185 - 192
- Beigel A, Muller-Ruchholtz W. Tracheal transplantation II. Influence of genetic difference and degree of sensitization on reaction to the tracheal transplants. Arch Otorhinolaryngol 1984; 240:217 - 225
- Beigel A, Steffens-Knutzen R, Muller B, Schumacher U, Stein H. Tracheal transplantation III. Demonstration of transplantation antigens on the tracheal mucosa of inbred rat strains. Arch Otorhinolaryngol 1984; 241:1 - 8
- Mukaida T, Shimizu N, Aoe M, Andou A, Date H, Moriyama S. The origin of regenerated epithelium in cryopreserved tracheal allotransplantation. Ann Thorac Surg 1998; 66:205 - 208
- Bujia J, Wilmes E, Hammer C, Kastenbauer E. Tracheal transplantation: demonstration of HLA class II subregion gene products on human trachea. Acta Otolaryngol 1990; 110:149 - 154
- Kalb TH, Chuang MT, Marom Z, Mayer L. Evidence for accessory cell function by class II MHC antigen-expressing airway epithelial cells. Am J Respir Cell Mol Biol 1991; 4:320 - 329
- Shaari CM, Farber D, Brandwein MS, Gannon PJ, Urken ML. Characterizing the antigenic profile of the human trachea: implications for tracheal transplantation. Head Neck 1998; 20:522 - 527
- Genden EM, Boros P, Liu J, Bromberg JS, Mayer L. Orthotopic tracheal transplantation in the murine model. Transplantation 2002; 73:1420 - 1425
- Nakanishi R, Shirakusa T, Hanagiri T. Early histopathologic features of tracheal allotransplantation rejection. A study in nonimmunosuppressed dogs. Transplant Proc 1994; 26:3715 - 3718
- Nakanishi R, Yasumoto K. Multiglycosidorum tripterygii versus Tacrolimus for rat tracheal allografts. Eur J Cardiothorac Surg 2005; 28:588 - 593
- Delaere PR, Liu ZY, Hermans R, Sciot R, Feenstra L. Experimental tracheal allograft revascularization and transplantation. J Thorac Cardiovasc Surg 1995; 110:728 - 737
- Delaere PR, Liu Z, Sciot R, Welvaart W. The role of immunosuppression in the long-term survival of tracheal allografts. Arch Otolaryngol Head Neck Surg 1996; 122:1201 - 1208
- Nakanishi R, Yasumoto K. Minimal dose of cyclosporin A for tracheal allografts. Ann Thorac Surg 1995; 60:635 - 639
- Davreux CJ, Chu NH, Waddell TK, Mayer E, Patterson GA. Improved tracheal allograft viability in immunosuppressed rats. Ann Thorac Surg 1993; 55:131 - 134
- Moriyama S, Shimizu N, Teramoto S. Experimental tracheal allotransplantation using omentopexy. Transplant Proc 1989; 21:2596 - 2600
- Hashimoto M, Nakanishi R, Muranaka H, Umesue M, Eifuku R, Yasumoto K. Short-course immunosuppression using FK506 for rat tracheal allografts. J Cardiovasc Surg 2000; 41:487 - 492
- Nakanishi R, Yasumoto K, Shirakusa T. Short course immunosuppression after tracheal allotransplantation in dogs. J Thorac Cardiovasc Surg 1995; 109:910 - 917
- Nakanishi R, Yasumoto K. Efficacy of Multiglycosidorum tripterygii for rat tracheal allografts. J Heart Lung Transplant 2005; 24:289 - 295
- Thompson AW. FK506. How much potential?. Immunol Today 1989; 10:6 - 9
- Toivio I, Siirala U, Lauerma S, Rapo S, Tallberg T, Mahlberg K, et al. Homotransplantation of canine trachea after denervation of the spleen. Acta Otolaryngol 1973; 75:127 - 131
- Yokomise H, Inui K, Wada H, Goh T, Yagi K, Hitomi S, Takahashi M. High-dose irradiation prevents rejection of canine tracheal allografts. J Thorac Cardiovasc Surg 1994; 107:1391 - 1397
- LaMuraglia GM, Adili F, Schmitz-Rixen T, Michaud NA, Flotte TJ. Photodynamic therapy inhibits experimental allograft rejection. Circulation 1995; 92:1919 - 1926
- Bujia J, Wilmes E, Hammer C, Kastenbauer E. A comparison of class II antigenicity of human tracheal allografts stored in Cialit and in Merthiolate. Laryngoscope 1990; 100:1337 - 1340
- Liu Y, Nakamura T, Yamamoto Y, Matsumoto K, Sekine T, Ueda H, Shimizu Y. Immunosuppressant-free allotransplantation of the trachea: the antigenicity of tracheal grafts can be reduced by removing the epithelium and mixed glands from the graft by detergent treatment. J Thorac Cardiovasc Surg 2000; 120:108 - 114
- Boehler A, Chamberlain D, Xing Z, Slutsky AS, Jordana M, Gauldie J, et al. Adenovirus-mediated interleukin-10 gene transfer inhibits post-transplant fibrous airway obliteration in an animal model of bronchiolitis obliterans. Hum Gene Ther 1998; 9:541 - 551
- Yokomise H, Inui K, Wada H, Hitomi S. The infeasibility of using ten-ring irradiated grafts for tracheal allotransplantation even with omentopexy. Surg Today 1996; 26:427 - 430
- Macchiarini P, Mazmanian GM, Montpreville VT, Dulmet EM, Chapelier AR, Dartevelle PG. Maximal preservation time of tracheal allografts. Ann Thorac Surg 1995; 60:1597 - 1604
- Deschamps C, Trastek VF, Ferguson JL, Martin WJ, Colby TV, Pairolero PC, Payne WS. Cryopreservation of canine trachea: functional and histological changes. Ann Thorac Surg 1989; 47:208 - 212
- Marangonni AG. Homotransplantation of tracheal segments preserved by lyophilization: an experimental study. J Thorac Surg 1951; 21:398 - 401
- Yokomise H, Inui K, Wada H, Hasegawa S, Ohno N, Hitomi S. Reliable cryopreservation of trachea for one month in a new trehalose solution. J Thorac Cardiovasc Surg 1995; 110:382 - 385
- Nakanishi R, Hashimoto M, Muranaka H, Umesue M, Kohno H, Yasumoto K. Maximal period of cryopreservation using Bicell biofreezing vessel for rat tracheal isografts. J Thorac Cardiovasc Surg 1999; 117:1070 - 1076
- Nakanishi R, Umesue M, Hashimoto M, Muranaka H, Hachida M, Yasumoto K. Limit of warm ischemia time before cryopreservation in rat tracheal isografts. Ann Thorac Surg 2000; 70:1880 - 1884
- Inutsuka K, Kawahara K, Takachi T, Okabayashi K, Shiraishi T, Shirakusa T. Reconstruction of trachea and carina with immediate or cryopreserved allografts in dogs. Ann Thorac Surg 1996; 62:1480 - 1484
- Tojo T, Niwaya K, Sawabata N, Nezu K, Kawachi K, Kitamura S. Tracheal allogenic immunoresponse is reduced by cryopreservation: canine experiment. Transplant Proc 1996; 28:1814 - 1815
- Yokomise H, Inui K, Wada H, Ueda M, Hitomi S. Long-term cryopreservation can prevent rejection of canine tracheal allografts with preservation of graft viability. J Thorac Cardiovasc Surg 1996; 111:930 - 934
- Mukaida T, Shimizu N, Aoe M, Andou A, Date H, Okabe K, et al. Experimental study of tracheal allotransplantation with cryopreserved grafts. J Thorac Cardiovasc Surg 1998; 116:262 - 266
- Tojo T, Kitamura S, Gojo S, Kushibe K, Nezu K, Taniguchi S. Epithelial regeneration and preservation of tracheal cartilage after tracheal replacement with cryopreserved allograft in the rat. J Thorac Cardiovasc Surg 1998; 116:624 - 627
- Aoki T, Yamato Y, Tuchida M, Souma T, Yoshiya K, Watanabe T, Hayashi J. Successful tracheal transplantation using cryopreserved allografts in a rat model. Eur J Cardiothoracic Surg 1999; 16:169 - 173
- Nakanishi R, Hashimoto M, Muranaka H, Yasumoto K. Effect of cryopreservation period on rat tracheal allografts. J Heart Lung Transplant 2001; 20:1010 - 1015
- Nakanishi R, Onitsuka T, Shigematsu Y, Hashimoto M, Muranaka H, Yasumoto K. The immunomodulatory effect of cryopreservation in rat tracheal allotransplantation. J Heart Lung Transplant 2002; 21:890 - 898
- Hirase Y, Kojima T, Takeshi M, Hwang KH, Tanaka M. Transplantation of long-term cryopreserved allocutaneous tissue by skin graft or microsurgical anastomosis: experimental studies in the rat. Plast Reconstr Surg 1993; 91:492 - 501
- Hoekstra FM, Witvliet M, Knoop CY, Wassenaar C, Bogers AJ, Weimar W, Claas FH. Immunogenic human leukocyte antigen class II antigens on human cardiac valves induce specific alloantibodies. Ann Thorac Surg 1998; 66:2022 - 2026
- Doty JR, Salazar JD, Liddicoat JR, Flores JH, Doty DB. Aortic valve replacement with cryopreserved aortic allograft: ten-year experience. J Thorac Cardiovasc Surg 1998; 115:371 - 380
- Messineo A, Filler RM, Bahoric A, Smith CR. Repair of long tracheal defects with cryopreserved cartilaginous allografts. J Pediatr Surg 1992; 27:1131 - 1135
- Ohlendorf C, Tomford WW, Mankin HJ. Chondrocyte survival in cryopreserved osteochondral articular cartilage. J Orthop Res 1996; 14:413 - 416
- Murakawa T, Nakajima J, Ono M, Murakami A, Suematsu Y, Takamoto S. Allogenicity of cryopreserved human fibroblasts: cryopreservation does not downregulate the allogenicity of fibroblasts making up the matrices of allografts. J Thorac Cardiovasc Surg 2000; 120:712 - 719
- Murakawa T, Nakajima J, Motomura N, Murakami A, Takamoto S. Successful allotransplantation of cryopreserved traceal grafts with preservation of the pars membranacea in nonhuman primates. J Thorac Cardiovasc Surg 2002; 123:153 - 160
- Nakajima J, Ono M, Kobayashi J, Takeda M, Kawauchi M, Takamoto S, Takizawa H. Effect of cryopreservation on the allogenicity of an airway epithelial cell line. Transplant Proc 1998; 30:3366 - 3367
- Kubota I, Shibata K, Onitsuka T, Koga Y. Growth of tracheal autografts in puppies. Surg Today 1997; 27:321 - 329
- Hisamatsu C, Maeda K, Tanaka H, Okita Y. Transplantation of the cryopreserved tracheal allograft in growing rabbits: effect of immunosuppressant. Pediatr Surg Int 2006; 22:881 - 885
- Braidley P, White DJG. Concordant organ xenotransplantation. Xenobiotica 1994; 2:25 - 30
- Blakely ML, Van der Werf WJ, Berndt MC, Dalmasso AP, Bach FH, Hancock WW. Activation of intragraft endothelial and mononuclear cells during discordant xenograft rejection. Transplantation 1994; 58:1059 - 1066
- Dorling A, Riesbeck K, Lechler RI. The T cell response to xenografts: molecular interactions and graft specific immunosuppression. Xenotransplantation 1996; 4:68 - 76
- Valdivia LA, Demetris AJ, Fung JJ, Celli S, Murase N, Starzl TE. Successful hamster-to-rat liver xenotransplantation under FK506 immunosuppression induces unresponsiveness to hamster heart and skin. Transplantation 1993; 55:659 - 661
- Reichenspurner H, Soni V, Nitschke M, Berry GJ, Brazelton TR, Shorthouse R, et al. Obliterative airway disease after heterotopic tracheal xenotransplantation: pathogenesis and prevention using new immunosuppressive agents. Transplantation 1997; 64:373 - 383
- Hashimoto M, Nakanishi R, Umesue M, Muranaka H, Hachida M, Yasumoto K. Feasibility of cryopreserved tracheal xenotransplants with the use of short-course immunosuppression. J Thorac Cardiovasc Surg 2001; 121:241 - 248
- Tze WJ, Tai J, Cheung SSC. Transplantation of discordant pig islet xenografts in diabetic rats. Diabetes Res Clin Pract 1992; 15:197 - 204
- Levashov YuN, Yablonsky PK, Cherny SM, Orlov SV, Shafirovsky BB, Kuznetzov IM. One-staged allotransplantation of thoracic segment of the trachea in a patient with idiopathic fibrosing mediastinitis and marked tracheal stenosis. Eur J Cardiothorac Surg 1993; 7:383 - 386
- Jacobs JP, Elliot MJ, Haw MP, Bailey CM, Herberhold C. Pediatric tracheal homograft reconstruction: a novel approach to complex tracheal stenosis in children. J Thorac Cardiovasc Surg 1996; 112:1549 - 1560
- Elliot MJ, Haw MP, Jacobs JP, Bailey CM, Herberhold C. Tracheal reconstruction in children using cadaveric homograft trachea. Eur J Cardiothorac Surg 1996; 10:707 - 712
- Jacobs JP, Quintessenza JA, Andrews T, Burke PP, Spektor Z, Delius RE, et al. Tracheal allograft reconstruction: the total North American and worldwide pediatric experiences. Ann Thorac Surg 1999; 68:1043 - 1051
- Ueda M, Yokomise H, Wada H, Hitomi S. Experimental tracheal transplantation for possible clinical application. Transplant Proc 1997; 29:871 - 873
- Kunachak S, Kulapaditharom B, Vajaradul Y, Rochanawutanon M. Cryopreserved, irradiated tracheal homograft transplantation for laryngotracheal reconstruction in human beings. Otolaryngol Head Neck Surg 2000; 122:911 - 916
- Delaere P, Vander Poorten V, Guelinckx P, Van Den Hof B, Hermans R. Progress in larynx-sparing surgery for glottic cancer through tracheal transplantation. Plast Reconstr Surg 1999; 104:1635 - 1641