Abstract
Cryopreservation of human blood vessels may become an important tool in bypass surgery and peripheral vascular reconstruction. Ideally cryopreservation of a blood vessel should preserve functional characteristics comparable to those of fresh controls. The key advantage of cryopreservation is the fact that storage at deep subzero temperatures allows storage of structurally intact living vascular tissues for virtually infinite time. Originally developed for long-time storage of isolated cells, the techniques of cryopreservation of tissues are challenged by the fact that these are complex multicellular systems containing diverse types of cells with differing requirements for optimal preservation. Therefore, the post-thaw functional activity of vascular tissues is determined by the type of blood vessel and, in addition, by the cell packing effect. Moreover, evidence from pharmacological studies suggests that cryopreservation induces tissue specific changes in transmembrane signaling and the mechanisms coupling intracellular calcium release, sensitivity and calcium entry into the smooth muscle cells.
Introduction
Cryopreserved human blood vessels may become important tools in pharmacological research, in bypass surgery and peripheral vascular reconstruction in patients without sufficient autologous graft material. While prosthetic material can be used for bypass of larger diameter vessels, its patency is poor when used as conduit to replace small diameter vessels. In these cases banked human blood vessels, are useful alternatives. In addition, promising applications in development are the use of cryopreserved blood vessel cells and scaffolds for tissue-engineered constructs.Citation1–Citation5 The key advantage of cryopreservation is the fact that storage at deep subzero temperatures allows preservation of vascular tissues for virtually infinite timeCitation6 ensuring long-term banking of tissues for elective use in reconstructive vascular surgery.Citation7
However, freezing and thawing of blood vessels can cause substantial injury to cells and tissues. Cryopreservation of vascular tissues leads to certain loss of both smooth muscle contractility and endothelial function which is a key regulator of vascular homeostasis. Endothelial cells interact continuously with blood components and with the structure of the vessel itself, maintaining both vascular tone and antithrombotic properties. The adequate preservation of the endothelial layer plays a crucial role in the mid- and long-term viability of arterial grafts. The rates of cooling to and rewarming from the storage temperature, the selection of the vehicle solution and cryoprotectant agents (CPA), the manner of CPA addition to and removal from the tissue, all these steps may influence the degree of injury sustained by the tissue during cryopreservation.
Cryopreservation methods were originally developed for isolated cells. For each cell type there exists a set of conditions that is optimal for its preservation and can be taken into consideration. Tissues, however, are complex multicellular systems containing diverse types of cells with differing requirements for optimal preservation. In a cryopreservation procedure all cell types within a tissue will be subjected to the same cooling and thawing parameters. Moreover, cells within a tissue are subject to additional factors such as cell-to-cell and cell-to-matrix interactions, temperature and solute gradients within the tissue, and restricted exchange of water across cell membranes which may exacerbate freezing injury. Extracellular ice, which is harmless to a cell suspension, is still within the tissue that is to preserved and can disrupt its structure when tissues are cryopreserved. Therefore, densely packed cells within a tissue, e.g., in an artery, are more likely to be damaged by cryopreservation-induced mechanical stresses than cells in loosely packed tissues such as veins.Citation8 The post-thaw functional activity of a cryopreserved blood vessel is determined by both the cell packing effect and the wall thickness, i.e., cryopreservation efficacy improves with fewer and less packing of cells. For example, in contrast to a nearly complete cryopreservation of both smooth muscle and endothelial function of small and thin intramyocardial resistance arteries only partial preservation of post-thaw vascular function of the larger epicardial coronary arteries can be obtainedCitation9 and cryopreservation of the thick-walled humanCitation10,Citation11 and porcineCitation12,Citation13 aortae revealed no or only negligible post-thaw preservation of smooth muscle and endothelial function.
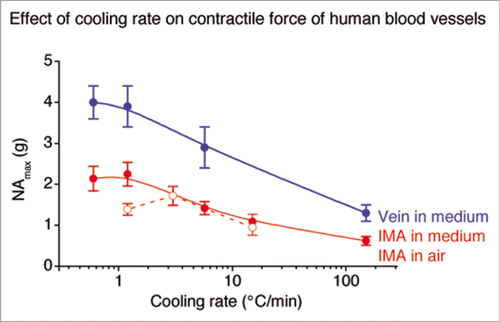
Freezing of living mammalian cells without cryoprotective additives generally induces severe cell damage and only few if any cells survive. During cooling to subzero temperatures water tends to flow out of the cell to freeze externally and the cell shrinks during this process (). If cooled too fast, cells are injured by the formation of intracellular ice crystals; if cooled too slowly water will freeze externally, and the cells will be injured by the “solution-effect,” i.e., by changes in the composition of extra- and intracellular solutions since the concentration of extracellular salts in the residual unfrozen medium increases as ice is formed.Citation8,Citation14 As a consequence of changes in both temperature and concentration of extracellular salts, cell membranes will be injured, i.e., they become leaky and permeable to substances that normally do not enter the cell.Citation8
Cryomedia
To reduce freezing injury during cryopreservation tissues must be equilibrated with cryoprotectant solutes consisting of a vehicle solution containing the cryoprotecting agent(s) (CPA). Optimal protection against cryoinjury will be obtained by the combined action of a permeating and a non-permeating CPA. Most commonly used vehicle solutions for cryomedia are RPMI culture medium, Krebs-Henseleit (KH) solution and Dulbecco's modified Eagle's medium (DMEM). Comparative experiments with rat aorta suggest that KH solution with reduced calcium content (1.25 mM) provides better post-thaw contractile and endothelial function than a solution with 2.5 M or without calcium (unpublished). In addition, there exist studies using fetal calf serum (FCS) as the vehicle to cryopreserve canine saphenous veinsCitation15–Citation17 and arteries,Citation17 canine basilar arteries,Citation15 porcine coronary arteries,Citation17–Citation20 rabbit ear arteries,Citation21 human saphenous veinsCitation22 and human pulmonary arteries.Citation23 Indeed, experimental evidence suggests that for many cell types, e.g., for endothelial cells in canine coronary arteries, it seems to be important to have at least 20% serum in the cryomedium.Citation24 However, comparative studies on various vascular tissues revealed that neither rabbit carotid arteriesCitation25 nor human saphenous veins,Citation6,Citation26 human coronary and mesenteric arteriesCitation27 require FCS for optimal preservation of smooth muscle and post-thaw endothelial function.
Taking the maximal contractile responses to noradrenaline of canine saphenous vein strips as parameter for the preservation of smooth muscle cell function various permeating CPA including dimethyl sulfoxide (DMSO), polyethylene glycol and glycerol and various freezing procedures have been tested. In those studies best recovery of post-thaw contractile function was obtained with venous segments that had been slowly frozen while immersed in FCS containing 1.8 M DMSO and rapidly thawed.Citation28–Citation30 Today for cryopreservation of vascular tissues the most commonly used permeating CPA is DMSO.
Non-permeating CPA such as sucrose, trehalose and chondroitin sulfate, are suggested to stabilize the cell volume by retaining more liquid water at low temperatures, thereby reducing the external electrolyte concentration.Citation14 Both types of CPA act directly at the level of the cell membrane, but their protective actions are synergistic. The beneficial effect of sucrose as additive to a DMSO-containing cryomedium has been studied on canine saphenous veins and arteries and on pig coronary arteries. In all vessels the addition of sucrose to the DMSO-containing cryomedium enhanced not only post-thaw contractile responses but improved also endothelium-dependent relaxant responses to substance P of porcine coronaries.Citation17 Increasing the sucrose concentration in a DMSO-containing cryomedium to 0.2 M does not improve contractile or endothelial function in rat aortic rings (unpublished), and replacement of sucrose by trehalose in a DMSO-containing cryomedium also failed to improve significantly the post-thaw contractile function of human saphenous veins.Citation6
The use of chondroitin sulphate as CPA for cryopreservation of venous tissue was originally stimulated by its employment in preservation solutions for corneas.Citation31 When canine saphenous veins are cooled at 0.5°C/min while immersed in Hepes-buffered DMEM containing 1 M DMSO, 2.5% chondroitin sulphate and 10% FCS followed by immersion in liquid nitrogen, post-thaw contractile functions are well preserved, though the contractile force is reduced by up to 50% compared to unfrozen veins.Citation32,Citation33
Comparative studies on veins preserved by the chondroitin sulphate method (DMEM containing 2.5% chondroitin sulphate and 1 M DMSO) and the sucrose method (RPMI solution containing 0.1 M sucrose and 1.8 M DMSO) have not been performed up to now. However, comparison of endothelium-dependent relaxant responses to acetylcholine of rings from rat aorta revealed no significant differences between both methods (, unpublished).
Exposure to CPA Without Freezing
It is important, that cryomedia at concentrations exhibiting cryoprotection are not toxic. For DMSO the optimal concentration for cryoprotection has been demonstrated to be around 1.5 to 1.8 M in rabbit carotid arteriesCitation25 and in canine saphenous veins.Citation28,Citation30 Exposure to these DMSO concentrations without freezing for up to 60 min changes neither contractile function of canine femoral arteriesCitation34 nor contractile and endothelial function of human IMA,Citation35,Citation36 whereas higher DMSO concentrations (2.4 M) induce significant attenuation of contractile and relaxant responses.Citation25,Citation28,Citation30 The same applies for canine jugular veins.Citation37 Exposure of rabbit carotid arteries for 30 min to 10% ethylene glycol (EG) without freezing is well tolerated whereas exposure to the higher concentration of 40% EG reduces endothelial function by 50% though it does not change contractile responses to noradrenaline.Citation38
These functional data are in line with light and electron microscopy examinations showing only minimal alterations of the endothelium in vascular tissues from various species. After exposure of human IMA rings to a DMSO-containing cryomedium without freezing, the endothelial cells eventually appear slightly flattened, but the integrity of the layer seems intact.Citation36 Similar observation have been reported for porcine aortaeCitation39 and iliac arteriesCitation2,Citation40 and for rabbit carotid arteries after exposure to DMSOCitation41 and EG.Citation38
As observed with arteries, exposure of human saphenous veinsCitation6 and canine jugular veins tolerate exposure to a cryomedium containing DMSOCitation37,Citation42,Citation43 and glycerolCitation43 as demonstrated by preserved fibrinolytic activity,Citation42 contractile functionCitation6,Citation37 and morphology.Citation43
Addition of CPA and Pre-Freezing Equilibration Rate
To keep cell shrinkage within safe limits, eventually the CPA is added gradually over a period of 20 to 30 min. This procedure can be successful with isolated cells where permeability parameters of the particular cells to both water and the cryoprotectant can be determined and taken into consideration.Citation5,Citation8 For tissues, however, stepwise addition of the cryoprotectant DMSO implies that the tissue is transferred into different cryomedia containing progressively increasing DMSO concentrations since the addition of DMSO to the vehicle solution induces considerable changes in temperature () which could exacerbate cryoinjury. The procedure of stepwise addition of DMSO has been applied for iliac arteries from minipigsCitation2 and human femoral arteries.Citation44 In a study with human IMA pre-freezing exposure of arterial rings to cryomedia containing gradually increasing DMSO concentrations failed to improve the post-thaw contractile responses compared to controls which had been immersed directly into the cryomedium containing the final DMSO concentration.Citation36 Experimental evidence for a beneficial effect on the endothelial function of a stepwise increase of the CPA before cryopreservation is lacking.
The time required for 95% equilibration of rabbit carotid arteries exposed to 1 M DMSO is temperature-dependent being 13 min at room temperature and 18 min at 2°C.Citation45 This implies that vascular tissues should equilibrate at least 10–20 min with a DMSO-containing cryomedium before starting the cooling process. Experiments with canine femoral arteries, however, revealed a progressive reduction in contractility of the arterial smooth muscle with increasing periods of pre-freezing exposure to the cryomedium (KH solution containing 2 M DMSO, 0.1 M sucrose and 50% FCS). In these studies contractile responses to noradrenaline were attenuated progressively with increasing pre-freezing periods of exposure to the DMSO-containing cryomedium. Optimal preservation of post-thaw contractility was observed with 10 min pre-freezing equilibration.Citation34 By contrast, human IMA tolerate pre-freezing equilibration periods of 10–120 min equally well and endothelium-dependent relaxation in response to acetylcholine can still be evoked after pre-freezing equilibration for 60 min.Citation35 Similarly, the post-thaw contractile function of human saphenous veins is equally well preserved after pre-freezing equilibration periods of 10–120 min.Citation6
Freezing Rate and the Effect of Surrounding Medium
The optimal cooling rate differs from cell to cell and is dependent on both the water permeability of the cell membrane and the surface area to volume ratio of the cell.Citation8 In porcine aorta the optimum cooling rate for smooth muscle cells is 0.3°C/min but that for endothelial cells 10°C/min.Citation5 Yet an unique freezing rate (usually about 1°C/min) must be applied for cryopreservation of vascular tissues. Under these conditions biochemical viability indices of cryopreserved porcine aortae are similar to those of unfrozen controlsCitation39 but morphologic changes suffer from great variationsCitation46 and functional studies reveal marked attenuation of contractility and no or only negligible endothelium-dependent relaxation in response to acetylcholine.Citation11,Citation13
Using a high-potassium Tes-buffered solutionCitation47 as vehicle in rabbit carotid arteries optimal preservation of post-thaw contractile function and endothelium-dependent relaxation with a survival rate of 70% for endothelial cells was obtained at the freezing rate of 0.69°C/min.Citation41 It seems, however, that a potassium-rich vehicle solution is not required since experiments with KH solution gave similar results. Cryopreservation of rabbit carotid arteries after freezing at 1°C/min in KH solution containing 1.2 or 1.8 M DMSO retain about 40% of their contractility and 60% of their capacity to produce endothelium-dependent relaxation.Citation25 Even better preservation of both parameters was obtained with rabbit carotid arteries cryopreserved after draining off the surrounding surplus but leaving the intraluminal fluid before starting the freezing process.Citation48
In 1987 Fong et al. discovered that rabbit corneas sustained the least degree of damage when equilibrated with the cryomedium containing 1 M DMSO and frozen without rather than surrounded by medium during the cryopreservation process.Citation49 Experiments with canine jugular veins revealed best preservation of endothelial layer in air filled veins that had been soaked in cryomedium and rapidly frozen.Citation43 Though cellular junctions were not well delimited electron microscopy examination of porcine aorta indicated that the endothelial layer was better preserved when freezing and cryopreservation were performed after removal of surplus cryomedium.Citation39 One contributory factor to the better preservation of endothelial layers after removal of surplus medium might be the observation that freezing in air leads to the formation of numerous small ice crystals throughout the tissue whereas freezing of a tissue immersed in medium gives rise to fewer but much larger ice crystals which may disrupt cell-to-cell contacts.Citation50 Theoretical calculation using a two-compartments model showed that thermal stresses occurring during cryopreservation “in air” are much smaller than during cryopreservation “in medium.”Citation55
When human IMA are cryopreserved in 2 ml liquid nitrogen ampoules filled with the DMSO-containing cryomedium, optimal freezing rate for post-thaw contractile function is around 1°C/min. Under these conditions, however, preservation of the endothelial function is only marginal accompanied by marked exfoliation of the endothelial layer in scanning electron microscopy. Significantly better preservation of the post-thaw endothelial function and its layer in scanning electron microscopy is obtained when the surplus cryomedium is removed and the IMA frozen at 3°C/min.Citation36 Under these conditions, however, the contractile function is reduced supporting the contention, that for optimal post-thaw function smooth muscle cells require a lower freezing rate than endothelial cells. For human saphenous veins the cooling rate for optimal post-thaw contractile function is 0.6°C/min ().Citation6
Thus, for mammalian vascular tissue the optimal cooling rate is between 0.6–3°C/min, but once a sample is cooled to about −70°C, it can be transferred directly into liquid nitrogen and stored there at −196°C for virtually infinite time until use. Although storage at higher temperatures (−70 to −85°C) of vascular tissues has been shown to allow short-term storage for 3–4 weeks,Citation9,Citation15 this temperature range does not provide truly long-term survival of mammalian cells.Citation15
Storage during Cryopreservation
Vascular samples supposed for clinical use are usually cryopreserved in cryoresistant bags filled with or without cryomedium and stored in the vapour phase of liquid nitrogen. By contrast, small vascular samples supposed for pharmacological experiments are usually frozen in liquid nitrogen ampoules containing the cryomedium and stored either in the vapour phase or in the liquid phase of the liquid nitrogen tank. Cryopreservation of porcine femoral arteries in 2 ml liquid nitrogen ampoules abolished completely post-thaw contractile responses of these arteries whereas cryopreservation in plastic bags filled with 100 ml cryomedium revealed 50% preservation of the contractile and 90% of the endothelial function.Citation51 In human IMA the post-thaw endothelial function is reduced to about 16–26% when cryopreservation is performed in liquid nitrogen ampoulesCitation35,Citation36 whereas 80% of the acetylcholine-induced relaxation can be preserved when cryopreservation is performed in bags containing 100 ml cryomedium, (personal communication).Citation52 Finally, cryopreservation of human femoral arteries in liquid nitrogen ampoules eliminated completely the endothelial function,Citation44 while after cryopreservation in bags about 90% of endothelium-dependent relaxation responses were maintained.Citation53,Citation54 Both storage methods, however, reduced the post-thaw contractile activity of the femoral arteries to about 40%.
The reason for these discrepancies is not clear but differences during the thawing process cannot be excluded. Bags are usually stored in the vapour phase, while liquid nitrogen ampoules are often plunged into the liquid phase. It might be possible, that the improved endothelial function of arteries which had been cryopreserved without medium was due merely to the fact that these samples were stored in the vapour phase of liquid nitrogen. It never can be ruled out completely that during the freezing process liquid nitrogen enters the liquid nitrogen ampoules and causes damage of the tissue during the thawing process.
Thawing Procedure
Maximum thermal stress during cryopreservation occurs in the thawing process.Citation55 In addition, because cells are more sensitive to swelling than to shrinkage, removal of CPA can be more hazardous than their addition. With isolated cells in general a rapid thawing rate is applied, which limits the growth of ice crystals in the frozen samples. Thawing of isolated tissues at a high warming rate, however, may lead to fractures as a consequence of mechanical stresses.Citation8 Rapidly thawed carotid arteries from rabbits show a high incidence of circumferential fractures a phenomenon that is not affected by the presence or absence of surrounding medium if rapid thawing is applied.Citation56 These fractures occur when during rewarming the temperature range of −150 to −100°C is transversed and can be prevented when warming to −100°C is performed at a slow rate.Citation48 Similar damages have been observed after rapid thawing of minipig iliac arteries showing accumulation of liquid in the subelastica, and increased expression of wall-degradative enzymes.Citation57 Such changes may lead to severe damages of both the extracellular matrix and the living cells, and affect the post-thaw function of the tissue. While fractures can provide serious problems to the surgeon these changes may be of less relevance if tissues are used for pharmacological experiments. Though the improvement did not reach significance, contractile responses of human saphenous veinsCitation6 and human IMA to noradrenaline have been shown to be better preserved when a slow thawing rate (15° instead of 100°C/min) was applied.Citation35 It might be possible that a slower thawing also contributes to improve the post-thaw endothelial function of arteries. Morphologic evidence for this has been provided by microscopic examinations of the endothelial surface of frozen-thawed pig iliac arteries and by a modified TUNEL technique detecting damaged endothelial cells. In those studies, a slow thawing at 1°C/min resulted in markedly improved morphological features of the endothelial surface and a lower proportion of damaged endothelial cell compared to rapidly thawed arteries.Citation2,Citation40,Citation57,Citation58
Reversibility of Cryoinjury
Because cells are generally more sensitive to swelling than to shrinkage, often a stepwise dilution protocol is used after thawing, in order to avoid osmotic shock when returned to isotonic solution.Citation8 In isolated vascular tissues such as human IMA, canine saphenous and rat portal veins, which had been frozen in a medium containing 1.8 M DMSO this procedure did not improve the post-thaw contractile function (unpublished). In addition, organ cultures aimed to study cell ability to recover cryopreservation injury revealed irreversible damage of a significant fraction of arterial smooth muscle and endothelial cells from human aortaCitation58 and rat iliac arteries.Citation59 Nevertheless, post-thaw incubation of human saphenous veins for 24 h in DMEM at 37°C produced a small but significant improvement by 15% of the contractile force.Citation6
Methods for Cryopreservation of Human Vessels
Based on these findings some suggestions for optimal post-thaw functional activity of cryopreserved human blood vessels can be provided. Compared to liquid nitrogen ampoules cryoresistant bags and storage in the vapour phase may give better post-thaw functional activity. The cryomedium should contain both a permeating and non-permeating CPA in nontoxic concentrations, usually RPMI culture medium containing 1.8 M DMSO and 0.1 M sucrose. If KH solution is used as the vehicle, the calcium concentration should be reduced to 1.25 M. In most cases the addition of FCS is not required and neither addition nor removal of CPA must be performed in a stepwise manner. The rate of pre-freezing equilibration depends upon the wall thickness of the tissue and the working temperature but should be at least 10 to 20 min. Human arteries and veins tolerate a pre-freezing equilibration period with the cryomedium for 10–120 min equally well. Freezing and thawing should be performed slowly preferably in a programmable freezer at 1°C/min for arteries and 0.6°C/min for veins. Freezing of arteries without surrounding medium changes the optimal freezing speed requiring faster cooling rate, but may provide improved preservation of endothelium. Once a sample is cooled to about −70°C, it can be transferred directly into liquid nitrogen and stored there at −196°C for virtually indefinite time. To improve post-thaw functional activity and reduce cryopreservation-induced damage of the tissues thawing before use should be performed slowly.
Pharmacological Changes in Frozen/Thawed Blood Vessels
Drug potencies.
Despite considerable post-thaw reduction of contractile force by about 50% there is always a fairly good correlation between the potencies in terms of apparent pD2 values for most receptor-mediated contractile and relaxant agonists in unfrozen and frozen/thawed blood vessels from various species.Citation15,Citation18,Citation22,Citation23,Citation27,Citation60,Citation61 The same is true for the blocking potencies of various antagonists tested against 5-HT and noradrenaline.Citation18,Citation22
Adrenergic nerve endings.
After cryopreservation adrenergic nerve endings appear to be well preserved. Contractile responses to electrical field stimulation of frozen/thawed rabbit ear arteries were similar to those elicited in unfrozen controls.Citation21 Similarly, after pre-incubation of canine saphenous vein strips with 3H-noradrenaline sustained electrical stimulation elicited the same absolute tritium overflow as observed with unfrozen controls, yet the basal outflow from frozen/thawed vein strips was slightly higher than that from unfrozen veins.Citation29
Endothelium-derived substances.
Depending on the tissue and stimuli endothelial cells generate and release at least 3 mayor endothelium-derived relaxant factors, namely the endothelium-derived relaxing factor (EDRF), which has been identified as nitric oxide (NO),Citation62,Citation63 the prostacyclin PGI2Citation64 and the endothelium-derived hyperpolarizing factor(s).Citation65 An increased accumulation of guanosine 3′,5′-cyclic monophosphate (cGMP) within the vascular smooth muscle cells is the common mechanism by which acetylcholine, substance P, bradykinin and nitrovasodilators such as sodium nitroprusside (SNP) elicit at least partially smooth muscle relaxation.Citation66 However, while SNP acts directly through activation of soluble guanylate cyclase,Citation67 acetylcholine, substance P and bradykinin first interact with specific receptors on the vascular endothelium to trigger the formation of NO which then activates soluble guanylate cyclase in smooth muscle cells, leading to increased intracellular concentration of cGMP.Citation68 Maintained post-thaw relaxant response to SNP while endothelium-dependent relaxant responses of arterial tissues to acetylcholine,Citation9,Citation21,Citation24,Citation25,Citation35,Citation44,Citation48,Citation51–Citation54,Citation69 substance PCitation18,Citation23,Citation27,Citation69 or bradykininCitation27 are diminished indicate, therefore, cryoinjury of the endothelium. Experiments on various animal arteries have demonstrated that activation of protein kinase C (PKC) attenuates the endothelium-dependent relaxation.Citation70–Citation72 However, no such mechanism seems to contribute to the post-thaw diminished EDRF-induced response of cryopreserved human IMA.Citation36
Under normal conditions contractile responses of human mesenteric and coronary arteries to PGF2α are counteracted by endogenous relaxing prostanoids released by both smooth muscle and the endothelium.Citation73,Citation74 Blockade of the endogenous prostaglandin synthesis by indomethacin enhances responses to PGF2α of mesenteric arteries before but inhibits PGF2α responses after cryopreservation.Citation27 Moreover, on human IMA the post-thaw inhibitor effect of indomethacin against PGF2α increases with prolonged pre-freezing equilibration periods with the DMSO-containing cryomedium. These data suggest that damage of the vascular tissue may occur already before freezing, i.e., during exposure to the cryomedium.Citation35
In contrast to arteries, the role of NO in venous relaxation is of less importance than that of certain metabolites of arachidonic acid.Citation75,Citation76 Fibrinolytic activity has been suggested as a quantitative criterion of venous endothelial integrity.Citation42,Citation77 However, because the basal production of 6-keto-prostaglandin F1α, a stable breakdown product of PGI2, is significantly higher in cryopreserved than in unfrozen humanCitation78,Citation79 and canine saphenous veinsCitation80,Citation81 and because the action of enzymes is generally well preserved during cryopreservation,Citation15,Citation22,Citation78,Citation79,Citation82 maintained fibrinolytic activity is not indicative of endothelial integrity and cell viability.Citation78
Calcium metabolism.
Vascular smooth muscle cells are receptive to physical forces which occur during the freezing/thawing procedures and may modify both transmembrane signal transduction and intracellular pathways, that are common to pharmacological agonists. Under normal conditions stimulation of α1-adrenoceptorsCitation83 and endothelin-1 receptorsCitation84 activates a pertussis toxin-sensitive G-protein which stimulates phospholipase C (PLC). PLC then catalyses the breakdown of phosphoinositide 4,5-bisphosphate to inositol 1,4,5-triphosphate (IP3) and diacylglycrol (DAG) triggering the release of Ca2+ from intracellular stores and activating PKC respectively. In addition, DAG can be hydrolyzed to form monoacylglycerol which is further hydrolyzed to release arachidonic acid, the precursor of prostanoids.Citation68
Experiments on human IMA demonstrated that the freezing/thawing procedures besides inducing activation of PKC cause an increase in calcium-influx into the arterial smooth muscle.Citation35,Citation85 Evidence for cryopreservation-induced enhanced Ca2+-influx comes from the observation that in cryopreserved IMA (1) activation of PKC through direct stimulation of DAG by phorbol dibutyrate (PDBu) occurs at significantly lower concentrations than in unfrozen controls and (2) that this phenomenon can be completely reversed by the dihydropyridine-sensitive Ca2+-channel blocker nifedipine.Citation35 Similar observations have been made with endothelin-1, the effect of which is resistant to nifedipine before but highly sensitive to blockade of dihydropyridine-sensitive Ca2+-channels after cryopreservation.Citation85 Evidence for activation of PKC in cryopreserved arteries was produced by staurosporine, a potent though nonspecific inhibitor of PKC.Citation86,Citation87 Staurosporine proved to be significantly more potent at inhibiting responses to PDBu and endothelin-1 when tested in frozen/thawed IMA as compared to unfrozen controls.Citation35,Citation85
Enhanced post-thaw Ca2+-influx and/or Ca2+ sensitivity could also explain the frequent observation that after thawing of cryopreserved human and animal blood vessels the compliance is diminishedCitation15,Citation20,Citation34 while at the same time the sensitivity to contractile stimuli of smooth muscle cells may be rather enhanced.Citation27,Citation35
Pharmacological experiments with canine saphenous veins suggested than cryopreservation has no deleterious effect on the mechanisms coupling Ca2+ entry in this tissue.Citation16 While in canine veins the Ca2+ channel activating (+)-(−) (S) enantiomer of SDZ 202–791 was equally effective in unfrozen and cryopreserved tissuesCitation16 the same compound was significantly less effective at enhancing contractile responses to depolarization in cryopreserved human saphenous veins compared to unfrozen controls suggesting that increased basal concentration of intracellular Ca2+ ([Ca2+]i) in cryopreserved human saphenous veins modifies the post-thaw pharmacological responses.Citation61
In contrast to that observed with human IMA,Citation85 blockade of PKC by staurosporine failed completely to modify contractile responses of cryopreserved human saphenous veins to endothelin-1 and KCl. Moreover, attempts to counteract a putative depolarization-induced increase in [Ca2+]i by the potassium channel opener pinacidil resulted in only partial reversal.Citation61 However, in human saphenous veins the Rho kinase inhibitor HA-1077 proved to be considerably more efficacious after cryopreservation than in unfrozen veins indicating that in human venous smooth muscle the cryopreservation procedures induce Ca2+ sensitization through activation of Rho kinase.Citation61
In summary, pharmacological evidence suggests that cryopreservation of human IMA induces enhanced Ca2+ release from intracellular Ca2+ stores followed by Ca2+ influx through dihydropyridine-sensitive Ca2+ channels evoked by activation of IP3 and PLCCitation35,Citation85 whereas cryopreservation of human saphenous veins induces Ca2+ sensitization evoked by activation of Rho kinase.Citation61
Abbreviations
| [Ca2+]i | = | intracellular calcium concentration |
| cGMP | = | guanosine 3′,5′-cyclic monophosphate |
| CPA | = | cryoprotecting agents |
| DAG | = | diacylglycerol |
| DMEM | = | dulbecco's modified eagle's medium |
| DMSO | = | dimethyl sulfoxide |
| EDRF | = | endothelium-derived relaxing factor(s) |
| EG | = | ethylene glycol |
| FCS | = | fetal calf serum |
| 5-HT | = | 5-hydroxytryptamine |
| IMA | = | internal mammary artery |
| IP3 | = | inositol 1,4,5-triphosphate |
| KH | = | Krebs-Henseleit |
| NO | = | nitric oxide |
| pD2 | = | negative logarithm of the molar concentration of an agonists producing 50% of maximum effect |
| PKC | = | protein kinase C |
| PLC | = | phospholipase C |
| SNP | = | sodium nitroprusside |
Figures and Tables
Figure 1 During cooling to subzero temperature water tends to flow out of the cell and the cell shrinks during this process. Ice nucleation is initiated at around −5°C outside the cell. If cooling is performed rapidly, small ice crystals form inside the cell. These small ice crystals will fuse to form larger crystals and damage the cell membrane during re-warming. If cooling is performed slowly, ice crystals are formed outside the cell, the environment of the cell becomes hyperosmotic and the cells will be injured by the ‘solution-effect’.
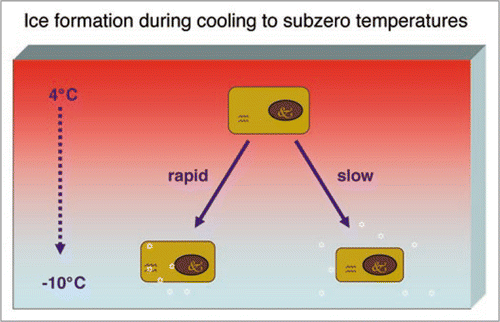
Figure 2 Cumulative relaxation-response curves to ACH on rings from unfrozen (•) and cryopreserved rat aorta stimulated with 3 µM PGF2α in the presence of 1 µM indomethacin. Cryopreservation and storage at −196°C was performed in 2 ml liquid nitrogen ampoules filled with KH solution containing 1.8 M DMSO plus 0.1 M sucrose (○), 1.8 M DMSO plus 2.5% chondroitin sulphate (▪) and 1.0 M DMSO plus 2.5% chondroitin sulphate (□). Tissues were frozen at about 1°C/min and thawed rapidly in a 37°C water bath. Responses are expressed in percentages of the SNP-induced relaxation, for each curve n = 6, vertical bars represent mean ± SEM.
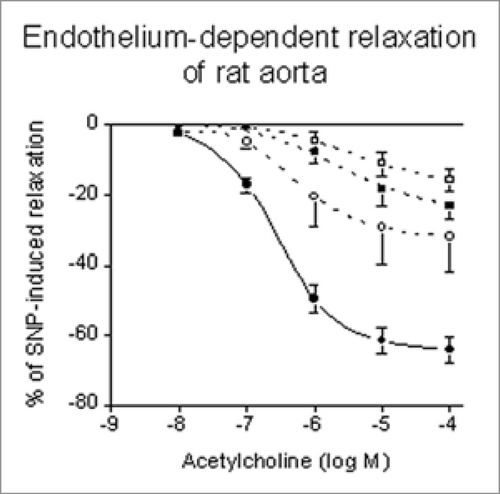
Figure 3 Temperature changes within a 2 ml liquid nitrogen ampoules during 1-step (black), 2-step (red) and 3-step (green) addition of DMSO to the cryomedium to reach the final concentration of 1.8 M DMSO. Measurements were performed at room temperature with type du3s of ellab a-s (Ellab Instruments, Copenhagen, Denmark). Addition of DMSO is indicated by arrows.
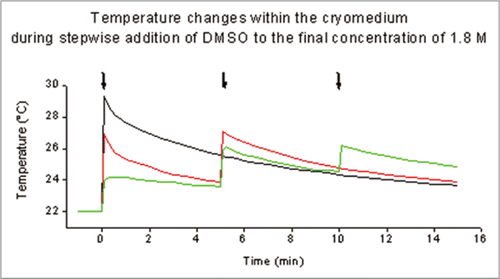
Figure 4 Maximal responses to noradrenaline of human saphenous veins (blue) and IMA cryopreserved in medium (red) and in air (broken line) (reviewed in refs. Citation6 and Citation36).
References
- Gulbins H, Pritisanac A, Dauner M, Petzold R, Goldemund A, Doser M, et al. Seeding of human vascular cells onto small diameter polyurethane vascular grafts. Thorac Cardiovasc Surg 2006; 54:102 - 107
- Pascual G, Rodriguez M, Corrales C, Turegano F, Garcia-Honduvilla N, Bellon JM, et al. New approach to improving endothelial preservation in cryopreserved arterial substitutes. Cryobiology 2004; 48:62 - 71
- Kuleshova LL, Gouk SS, Hutmacher DW. Vitrification as a prospect for cryopreservation of tissue-engineered constructs. Biomaterials 2007; 28:1585 - 1596
- Hoenicka M, Lehle K, Jacobs VR, Schmid FX, Birnbaum DE. Properties of the human umbilical vein as a living scaffold for a tissue-engineered vessel graft. Tissue Eng 2007; 13:219 - 229
- Wusteman MC, Pegg DE. Differences in the requirements for cryopreservation of porcine aortic smooth muscle and endothelial cells. Tissue Eng 2001; 7:507 - 518
- Muller-Schweinitzer E, Striffeler H, Grussenmeyer T, Reineke DC, Glusa E, Grapow MT. Impact of freezing/thawing procedures on the post-thaw viability of cryopreserved human saphenous vein conduits. Cryobiology 2007; 54:99 - 105
- Wusteman MC, Pegg DE, Warwick RM. The banking of arterial allografts in the United kingdom. A technical and clinical review. Cell Tissue Bank 2000; 1:295 - 301
- Pegg DE. Principles of cryopreservation. Methods Mol Biol 2007; 368:39 - 57
- Ku DD, Liu Q, Norton P, Caulfield JB. Cryopreservation of coronary endothelium and endothelial-mediated responses. Cryobiology 1994; 31:82 - 89
- Langerak SE, Groenink M, van der Wall EE, Wassenaar C, Vanbavel E, van Baal MC, et al. Impact of current cryopreservation procedures on mechanical and functional properties of human aortic homografts. Transpl Int 2001; 14:248 - 255
- Rendal Vazquez ME, Rodriguez Cabarcos M, Martinez Santos MV, Fernandez Mallo RO, Sanchez Ibanez J, Segura Iglesias R, et al. Functional assessment of cryopreserved human aortas for pharmaceutical research. Cell Tissue Bank 2004; 5:119 - 123
- Rendal Vazquez ME, Rodriguez Cabarcos M, Martinez Santos MV, Fernandez Mallo RO, Sanchez Ibanez J, Segura Iglesias R, et al. Functional assessment of cryopreserved pig aortas for pharmaceutical research. Cell Tissue Bank 2004; 5:111 - 118
- Rendal E, Rodriguez M, Martinez MV, Fernandez RO, Sanchez J, Segura R, et al. Function of cryopreserved pig aortas. J Surg Res 2004; 120:304 - 311
- Mazur P. Slow-freezing injury in mammalian cells. Ciba Found Symp 1977; 19 - 48
- Muller-Schweinitzer E, Tapparelli C. Pharmacological studies on frozen stored canine saphenous veins and basilar arteries. Naunyn Schmiedebergs Arch Pharmacol 1986; 332:74 - 78
- Ebeigbe AB, Muller-Schweinitzer E, Vogel A. Effects of calcium channel blockade in canine saphenous veins after storage at −190°C. Br J Pharmacol 1988; 94:381 - 388
- Muller-Schweinitzer E, Ellis P. Sucrose promotes the functional activity of blood vessels after cryopreservation in DMSO-containing fetal calf serum. Naunyn Schmiedebergs Arch Pharmacol 1992; 345:594 - 597
- Schoeffter P, Muller-Schweinitzer E. The preservation of functional activity of smooth muscle and endothelium in pig coronary arteries after storage at −190°C. J Pharm Pharmacol 1990; 42:646 - 651
- Muller-Schweinitzer E. Bevan RD, Bevan JA. Vascular tissue preservation techniques. The Human Brain Circulation: Functional Changes in Disease 1994; Totowa, New Jersey The Humana Press Inc 319 - 331
- Muller-Schweinitzer E. Applications for cryopreserved blood vessels in pharmacological research. Cryobiology 1994; 31:57 - 62
- Thompson L, Duckworth J, Bevan J. Cryopreservation of innervation, endothelial and vascular smooth muscle function of a rabbit muscular and resistance artery. Blood Vessels 1989; 26:157 - 164
- Muller-Schweinitzer E, Tapparelli C, Victorzon M. Functional studies on human veins after storage at −190°C. Br J Pharmacol 1986; 88:685 - 687
- Ellis P, Muller-Schweinitzer E. Maintenance of functional activity of human pulmonary arteries after cryopreservation. Br J Pharmacol 1991; 103:1377 - 1380
- Ku DD, Willis WL, Caulfield JB. Retention of endothelium-dependent vasodilatory responses in canine coronary arteries following cryopreservation. Cryobiology 1990; 27:511 - 520
- Bateson EAJ, Pegg DE. Cryopreservation of arteries. Cryo Letters 1994; 15:15 - 26
- Schilling A, Glusa E, Muller-Schweinitzer E. Nature of the vehicle solution for cryopreservation of human peripheral veins: preservation of reactivity to pharmacological stimuli. Cryobiology 1995; 32:109 - 113
- Muller-Schweinitzer E, Mihatsch MJ, Schilling M, Haefeli WE. Functional recovery of human mesenteric and coronary arteries after cryopreservation at −196°C in a serum-free medium. J Vasc Surg 1997; 25:743 - 750
- Muller-Schweinitzer E. Cryopreservation: a useful technique for storing tissues for pharmacological investigations. Trends Pharmacol Sci 1988; 9:221 - 223
- Muller-Schweinitzer E, Tapparelli C. Nobin A, Owman C, Arneklo-Nobin B. Cryopreservation of isolated blood vessels for pharmacological studies: experiments on canine and human veins. Neuronal messengers in Vascular Function 1987; Amsterdam—New York—Oxford Elsevier Science Publishers (Biomedical Division) 105 - 110
- Muller-Schweinitzer E. Cryopreservation of isolated blood vessels. Folia Haematol Int Mag Klin Morphol Blutforsch 1988; 115:405 - 409
- Brockbank KG. Effects of cryopreservation upon vein function in vivo. Cryobiology 1994; 31:71 - 81
- Brockbank KG, Donovan TJ, Ruby ST, Carpenter JF, Hagen PO, Woodley MA. Functional analysis of cryopreserved veins. Preliminary report. J Vasc Surg 1990; 11:94 - 100
- Brockbank KGM. Brockbank KGM. Effects of cryopreservation upon venous functions. Principles of Autologous, Allogeneic and Cryopreserved Venous Transplantation 1995; Austin, Texas USA Landes Company 113 - 119
- Muller-Schweinitzer E. Arterial smooth muscle function after prolonged exposure to a medium containing dimethyl sulfoxide (Me2SO) and storage at −196°C. Cryobiology 1994; 31:330 - 335
- Muller-Schweinitzer E, Stulz P, Striffeler H, Haefeli WE. Functional activity and transmembrane signaling mechanisms after cryopreservation of human internal mammary arteries. J Vasc Surg 1998; 27:528 - 537
- Muller-Schweinitzer E, Grapow M, Konerding MA, Zerkowski HR. Freezing without surrounding cryomedium preserves the endothelium and its function in human internal mammary arteries. Cryobiology 2005; 51:54 - 65
- Weber TR, Dent TL, Lindenauer SM, Allen E, Weatherbee L, Spencer HH, et al. Viable vein graft preservation. J Surg Res 1975; 18:247 - 255
- Wusteman M, Busza A, Boylan S, Hayes A, Pegg D. Ethylene glycol permeation and toxicity in the rabbit common carotid artery. Cryobiology 1995; 32:428 - 435
- Arnaud F. Endothelial and smooth muscle changes of the thoracic and abdominal aorta with various types of cryopreservation. J Surg Res 2000; 89:147 - 154
- Pascual G, Garcia-Honduvilla N, Rodriguez M, Turegano F, Bujan J, Bellon JM. Effect of the thawing process on cryopreserved arteries. Ann Vasc Surg 2001; 15:619 - 627
- Song YC, Pegg DE, Hunt CJ. Cryopreservation of the common carotid artery of the rabbit: optimization of dimethyl sulfoxide concentration and cooling rate. Cryobiology 1995; 32:405 - 421
- Malone JM, Moore WS, Kischer CW, Keown K, Conine R. Venous cryopreservation: endothelial fibrinolytic activity and histology. J Surg Res 1980; 29:209 - 222
- Gottlob R, Stockinger L, Gestring GF. Conservation of veins with preservation of viable endothelium. J Cardiovasc Surg (Torino) 1982; 23:109 - 116
- Stanke F, Riebel D, Carmine S, Cracowski JL, Caron F, Magne JL, et al. Functional assessment of human femoral arteries after cryopreservation. J Vasc Surg 1998; 28:273 - 283
- Bateson EA, Busza AL, Pegg DE, Taylor MJ. Permeation of rabbit common carotid arteries with dimethyl sulfoxide. Cryobiology 1994; 31:393 - 397
- Rendal E, Santos MV, Rodriguez M, Sanchez J, Segura R, Matheu G, et al. Effects of cryopreservation and thawing on the structure of vascular segment. Transplant Proc 2004; 36:3283 - 3287
- Taylor MJ, Hunt CJ. A new preservation solution for storage of corneas at low temperatures. Curr Eye Res 1985; 4:963 - 973
- Pegg DE, Wusteman MC, Boylan S. Fractures in cryopreserved elastic arteries. Cryobiology 1997; 34:183 - 192
- Fong LP, Hunt CJ, Pegg DE. Cryopreservation of the rabbit cornea: freezing with dimethyl sulphoxide in air or in medium. Curr Eye Res 1987; 6:569 - 577
- Hunt CJ, Taylor MJ, Pegg DE. Freeze-substitution and isothermal freeze-fixation studies to elucidate the pattern of ice formation in smooth muscle at 252 K (-21°C). J Microsc 1982; 125:177 - 186
- Rigol M, Heras M, Martinez A, Zurbano MJ, Agusti E, Roig E, et al. Changes in the cooling rate and medium improve the vascular function in cryopreserved porcine femoral arteries. J Vasc Surg 2000; 31:1018 - 1025
- Pompilio G, Polvani GL, Antona C, Rossoni G, Guarino A, Porqueddu M, et al. Retention of endothelium-dependent properties in human mammary arteries after cryopreservation. Ann Thorac Surg 1996; 61:667 - 673
- Rendal Vazquez ME, Rodriguez Cabarcos M, Martinez Santos MV, Fernandez Mallo RO, Sanchez Ibanez J, Segura Iglesias R, et al. Functional assessment of cryopreserved human femoral arteries for pharmaceutical research. Cell Tissue Bank 2004; 5:105 - 110
- Rendal Vazquez ME, Rodriguez Cabarcos M, Fernandez Mallo RO, Sanchez Ibanez J, Segura Iglesias R, Bermudez Gonzalez T, et al. Functional assessment of human femoral arteries after cryopreservation. Cryobiology 2004; 49:83 - 89
- Zhang A, Cheng S, Gao D, Xu LX. Thermal stress study of two different artery cryopreservation methods. Cryo Letters 2005; 26:113 - 120
- Hunt CJ, Song YC, Bateson EA, Pegg DE. Fractures in cryopreserved arteries. Cryobiology 1994; 31:506 - 515
- Bujan J, Pascual G, Garcia-Honduvilla N, Gimeno MJ, Jurado F, Carrera-San Martin A, et al. Rapid thawing increases the fragility of the cryopreserved arterial wall. Eur J Vasc Endovasc Surg 2000; 20:13 - 20
- Pasquinelli G, Foroni L, Buzzi M, Tazzari PL, Vaselli C, Mirelli M, et al. Smooth muscle cell injury after cryopreservation of human thoracic aortas. Cryobiology 2006; 52:309 - 316
- Bellon JM, Gimeno MJ, Pascual G, Garcia-Honduvilla N, Dominguez B, Bujan J. Arterial damage induced by cryopreservation is irreversible following organ culture. Eur J Vasc Endovasc Surg 1999; 17:136 - 143
- Muller-Schweinitzer E. Cryopreserved human tissue in pharmacological research. Pharmacol Res 1992; 25:103 - 111
- Muller-Schweinitzer E, Reineke DC, Glusa E, Ebeigbe AB, Grapow MT, Carrel TP. Activated Rho/Rho kinase and modified calcium sensitivity in cryopreserved human saphenous veins. Cryobiology 2008; 57:37 - 45
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980; 288:373 - 376
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987; 327:524 - 526
- Moncada S, Gryglewski R, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature 1976; 263:663 - 665
- Taylor SG, Weston AH. Endothelium-derived hyperpolarizing factor: a new endogenous inhibitor from the vascular endothelium. Trends Pharmacol Sci 1988; 9:272 - 274
- Ignarro LJ, Kadowitz PJ. The pharmacological and physiological role of cyclic GMP in vascular smooth muscle relaxation. Annu Rev Pharmacol Toxicol 1985; 25:171 - 191
- Holzmann S, Kukovetz WR, Windischhofer W, Paschke E, Graier WF. Pharmacologic differentiation between endothelium-dependent relaxations sensitive and resistant to nitro-L-arginine in coronary arteries. J Cardiovasc Pharmacol 1994; 23:747 - 756
- Berridge MJ. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem 1987; 56:159 - 193
- Ku DD, Winn MJ, Grigsby T, Caulfield JB. Human coronary vascular smooth muscle and endothelium-dependent responses after storage at −75°C. Cryobiology 1992; 29:199 - 209
- Murphy TV, Cross KM, Dunning PM, Garland CJ. Phorbol esters impair endothelium-dependent and independent relaxation in rat aortic rings. Gen Pharmacol 1994; 25:581 - 588
- Lewis MJ, Henderson AH. A phorbol ester inhibits the release of endothelium-derived relaxing factor. Eur J Pharmacol 1987; 137:167 - 171
- Rubanyi GM, Desiderio D, Luisi A, Johns A, Sybertz EJ. Phorbol dibutyrate inhibits release and action of endothelium-derived relaxing factor(s) in canine blood vessels. J Pharmacol Exp Ther 1989; 249:858 - 863
- Hadhazy P, Nagy L, Juhasz F, Malomvolgyi B, Magyar K. Effects of indomethacin and prostaglandins I2 and E2 on the tone of human isolated mesenteric arteries. Eur J Pharmacol 1983; 91:477 - 484
- Needleman P, Kulkarni PS, Raz A. Coronary tone modulation: formation and actions of prostaglandins, endoperoxides and thromboxanes. Science 1977; 195:409 - 412
- Miller VM, Vanhoutte PM. Endothelium-dependent contractions to arachidonic acid are mediated by products of cyclooxygenase. Am J Physiol 1985; 248:432 - 437
- Illiano S, Marsault R, Descombes JJ, Verbeuren T, Vanhoutte PM. Regulation of nitric oxide-like activity by prostanoids in smooth muscle of the canine saphenous vein. Br J Pharmacol 1996; 117:360 - 364
- Sachs SM, Ricotta JJ, Scott DE, DeWeese JA. Endothelial integrity after venous cryopreservation. J Surg Res 1982; 32:218 - 227
- Louagie YA, Legrand-Monsieur A, Lavenne-Pardonge E, Remacle C, Delvaux P, Maldague P, et al. Viability of long-term cryopreserved human saphenous veins. J Cardiovasc Surg (Torino) 1990; 31:92 - 100
- Passani SL, Angelini GD, Breckenridge IM, Newby AC. Endothelial function can be preserved during cryostorage of human saphenous vein. Eur J Cardiothorac Surg 1988; 2:233 - 236
- Elmore JR, Gloviczki P, Brockbank KG, Miller VM. Functional changes in canine saphenous veins after cryopreservation. Int Angiol 1992; 11:26 - 35
- Elmore JR, Gloviczki P, Brockbank KG, Miller VM. Cryopreservation affects endothelial and smooth muscle function of canine autogenous saphenous vein grafts. J Vasc Surg 1991; 13:584 - 592
- Showalter D, Durham S, Sheppeck R, Berceli S, Greisler H, Brockbank K, et al. Cryopreserved venous homografts as vascular conduits in canine carotid arteries. Surgery 1989; 106:652 - 658
- Nichols AJ, Motley ED, Ruffolo RR Jr. Effect of pertussis toxin treatment on postjunctional alpha-1 and alpha-2 adrenoceptor function in the cardiovascular system of the pithed rat. J Pharmacol Exp Ther 1989; 249:203 - 209
- Pollock DM, Keith TL, Highsmith RF. Endothelin receptors and calcium signaling. FASEB J 1995; 9:1196 - 1204
- Muller-Schweinitzer E, Brett W, Zerkowski HR, Haefeli WE. The mechanism of cryoinjury: In vitro studies on human internal mammary arteries. Br J Pharmacol 2000; 130:636 - 640
- Ruegg UT, Burgess GM. Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol Sci 1989; 10:218 - 220
- Tamaoki T, Nomoto H, Takahashi I, Kato Y, Morimoto M, Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun 1986; 135:397 - 402