Abstract
With the successful testing of the immunosuppressive effects of cyclosporine in transplant patients in 1978, the field of organ transplants began an exponential growth. With that, the field of organ preservation became increasingly important as the need to increase preservation time and improve graft function became paramount. However, for every patient that receives a transplanted organ, there are 4 more on the waiting list. In addition, a patient dies from the lack of a transplant almost every 1½ hour. To alleviate this donor crisis, there is a need to expand the donor pool to marginal donor organs. The main reason these organs are underutilized is because the current method of static preservation, simple cold storage, is ineffective. This article will provide a general review of the methods of preservation including simple cold storage, hypothermic machine perfusion, normothermic machine perfusion, and oxygen persufflation. In addition, the article will provide a review of how these dynamic preservation methods have improved the recovery and preservation of marginal donor organs including donation after cardiac death and fatty livers.
Currently in the U.S. there are over 100,000 patients awaiting kidney or liver transplants with transplant rates of only 20% for kidney and 38% for liver. There is clearly a need to improve both preservation of the organs and to expand the donor pool. The purpose of this article is to review the current methods of preservation for kidneys and livers, including a review of the preservation solutions in clinical use and those undergoing pre-clinical studies (). In addition, this article will provide a review of efforts to expand the current donor pool to include marginal donor organs (Donation after Cardiac Death, Fatty livers).
Methods of Preservation
The main goal in organ preservation is to maintain function of the organ and tissue during storage so that the graft will function at reperfusion. To this end, maintenance of cellular energy must be accomplished. This is done by reducing metabolic demand (and subsequent ATP hydrolysis) by hypothermia. As the temperature of the tissue falls, the metabolic demand drops according to the Arrhenius equation. When cells cool, the ATP utilization falls, as does the work provided by the Na+/K+ ATPase (sodium pump). This pump keeps intracellular sodium low under normothermic conditions. However, when the pump activity falls during hypothermia, the intracellular sodium rises, which pulls water into the cell resulting in lethal cell swelling.Citation1 Southard described the effects of hypothermia on cell swelling in the kidney and how these effects could be mitigated by using simple cell impermeants.Citation2 Molecules such as saccharides and anions are large enough to leave the capillary and enter the interstitial space but too large or too charged to enter the cell, prevent hypothermiainduced lethal cell swelling by osmotically pulling water out of the cell.
There are currently 2 modes of preservation methods for kidneys and livers: static and dynamic. Simple cold storage (SCS) is the main method for static storage while hypothermic machine perfusion (HMP), normothermic machine perfusion (NMP) and oxygen persufflation (OP) comprise the methods for dynamic preservation. Of these 4 methods, only SCS and HMP are approved clinically for kidneys and only SCS for livers. The remaining methods are in various stages of pre-clinical and early clinical studies.
Static preservation method.
Simple cold storage (SCS). Hypothermia was employed for organ preservation to reduce the kinetics of metabolic activities that would otherwise lead to cellular degradation when oxygen is removed from the donor organ. Simple cold storage (SCS) is a process by which the preservation solution is infused into the organ and then stored statically at hypothermic temperatures. References for SCS of kidneys dates to 1955 in the Russian journal, Biulleten' eksperimental'noi biologii i meditsiny, pre-dating M. Collins by 14 years. The development of the Collins' solution in 1969 increased SCS preservation time to 24 hr for kidneys.Citation3,Citation4 Collins' solution reversed the sodium potassium concentration to mimic intracellular-like composition. But it was the high glucose concentration in Collins solution that was shown to reduce cell swelling. Collins created a solution with intracellular ionic composition but the final osmolarity was off by about 140 mM. He made up the difference by adding large amounts of glucose to the solution to balance it osmotically. Later it was shown that the high glucose concentration that was serendipitously added to balance the solution osmotically was the active ingredient by acting as a cell impermeant to preventing cell swelling in the cold. The composition of the preservation solutions described in this review can be seen in .
For the liver, prior to the discovery of the University of Wisconsin (UW) solution, preservation by SCS was limited to 6 hrs. With the UW solution, preservation improved to 16 hrs and allowed long distance procurement of the donor organ. The formulation of a new completely synthetic perfusate containing cell impermeant such as lactobionic acid, raffinose and anions like phosphate and sulfate prevented the lethal hypothermia-induced cell swelling problem.Citation1,Citation2,Citation5 It was determined that the chloride and glucose content in the Euro-Collins solution permeated the hepatocytes and increased the intracellular osmolarity. This led to cell swelling and damage to the cells. Lactobinate, which is an anion, replaced chloride, and raffinose (594 MW) replaced the smaller glucose (180 MW) molecule. The UW solution also prevented edema formation during perfusion by using hydroxyethyl starch (HES) as a colloid, which was a replacement for albumin. Additional improvements included the use of adenine, ribose and phosphate to increase the synthesis of adenine nucleotides during perfusion preservationCitation6–Citation9 and free radical scavengers such as allopurinol and reduced glutathioneCitation10–Citation12 to prevent oxidative stress. The UW solution is now used for other donor organs including kidneys, hearts, pancreas, intestine and lungs.
Since the development of the UW solution, other preservation solutions have entered the market including Histidine-Tryptophane-Ketoglutarate (HTK) and Celsior. HTK developed by Bretschneider,Citation13 has shown to be a comparable preservation solution for both kidneys and livers when preservation times were shorter and when the organs were from standard criteria donors.Citation14–Citation17 It was developed originally as a non-high potassium cardioplegic solution.Citation13 The three main ingredients are beneficial in that histidine was thought to be a superior buffer compared to phosphate in the UW solution and tryptophane and ketoglutarate exhibited membrane protecting effects and were substrates for intermediary metabolism. Celsior developed in 1993 by Menasche et al.Citation18 was intended as a cardioplegic/preservation solution. It contains components in common with both the UW and HTK solutions.
Recently, works by McAnulty et al.Citation19–Citation21 have focused on trophic factors as additives to improve simple cold storage. Their group showed that with the addition of an antimicrobial peptide (bovine neutrophil peptide-1, 1 mg/L), a neurotrophin (nerve growth factor, 20 g/L), a neuropeptide (substance P, 2.5 mg/L), and two polypeptide growth factors (epidermal and insulin-like growth factors, 10 g/L for both) to the UW solution successful 6 day kidney preservation in a canine model was achieved. In addition, improvements in both livers and hearts were also observed. There is evidence to suggest that the trophic factors improve mitochondrial function and prevent early apoptotic activities.Citation22
Recently, Rauen et al.Citation23,Citation24 have improved the HTK solution, now known as Custodiol-N, by substituting a non-toxic buffer N-acetylhistidine for histidine. In addition, glycine, alanine and iron chelators, Deferoxamine and a membrane permeable iron chelator LK 614, were added. Improvements in mouse heart transplant models were observed with 7/8 grafts still beating 14 days postop after 24 hr preservation.Citation25 Currently pre-clinical transplant studies are being conducted.
Dynamic preservation methods.
The next three methods require some dynamic movement of either fluid or gas to facilitate preservation. Hypothermic machine perfusion was one of the earliest methods of preservation while normothermic machine perfusion has shown promise in recent years. Venous oxygen persufflation is a method by which gaseous oxygen is bubbled via the blood vessels through the organ and the gas escapes via pin holes at the surface of the organ. The advantage of these methods over simple cold storage is that they all have been shown to improve recovery of Donation after Cardiac Death organs. These organs have the potential to increase the donor pool by 20–40%.
Hypothermic machine perfusion (HMP) preservation. Hypothermic machine perfusion was developed for kidneys to extend both preservation time and preservation quality. The concept of hypothermic perfusion as a mode of organ preservation was first developed by Alexis Carrel in the early 20th century.Citation26 As a transplant surgeon, Carrel understood the need for preserving organs once they were removed from the body. He cultured chick embryo myocardial cells and coined the term organ culture to refer to an analogous technique for whole organs that he hoped to develop by using vascular perfusion.Citation26 Carrel recruited Charles Lindbergh who helped with the engineering aspects of developing a perfusion pump. Lindbergh developed a glass perfusion pump that could support kidneys by maintaining oxygen delivery through perfusion. They later conducted experiments with hypothermia and the earlier works were later redone and published by Lindbergh in 1966 after Carrel's death.Citation27 This work and work in limb preservation in the Soviet Union by LapchinskyCitation28 was the basis for experiments by LilleheiCitation29 and HoffmanCitation30 who focused on the role of hypothermia in preserving abdominal organs for transplantation. The two techniques of hypothermia and perfusion were combined and studied extensively by Belzer in the 1960s.Citation31,Citation32 Belzer's lab would evolve these techniques into modern day hypothermic perfusion preservation by using both systematic scientific testing and by capitalizing on a bit of serendipity.
HMP is able to supply oxygen to the tissue for ATP synthesis via perfusion of fluids that can carry oxygen. The oxygen requirements of cold tissues are low so the demand for oxygen is also low. This allows for slow flow rates during hypothermia and the relatively low oxygen carrying capacity of most crystalloid perfusates are adequate at low temperatures. However, perfusing organs at low temperatures cause side effects from both hypothermia and perfusion per se that must be mitigated for successful preservation. Mitigating these side effects is a main function of most modern day perfusion solutions. Belzer and Southard worked at perfecting HMP preservation of kidneys in the 70s and 80s, mainly by overcoming the side effects to hypothermia and perfusion on kidney function.
An additional problem during perfusion at low temperatures is the accumulation of fluid in the interstitial space (edema). This is caused by both increased capillary hydraulic conductivity due to hypothermiaCitation33 and the maintenance of relatively high capillary pressures during perfusion, which are necessary to drive perfusate flow through the system. Thus, perfusion preservation in the cold effectively maintains cellular energy storesCitation8 but causes both lethal intracellular swelling and extracellular edema formation. Unattended edema and cell swelling during hypothermic perfusion preservation will later cause reduced capillary perfusion due to compression of the micro vessels by the high interstitial fluid pressures generated by the cell and tissue swelling. This leads to high resistance to flow, low perfusion and preservation injury. The early experiments by Belzer would test for preservation injury on the pump by measuring perfusion pressure changes over time. A spike would indicate the onset of these problems.Citation34 To solve the edema problem, he used serum that contained albumin. The large albumin molecule (68 KDa) oncotically holds water in the capillary and retards movement into the interstitial space to cause edema. The human plasma was later modified when it was accidentally discovered that serum lipoproteins precipitated out of solution and embolized the microcirculation during perfusion. This was solved by prior cryoprecipitation of plasma lipoproteins and this was a major advancement in hypothermic perfusion preservation of kidneys. This allowed for the successful preservation of canine kidneys for 24–72 hoursCitation31 and the first successful human preservation of a kidney for 17 hours.Citation32
This new synthetic perfusate was then shown effective in canine kidney transplants perfused in the cold for up to 72 hours.Citation35 This formulation () is the current Belzer-MPS solution that is used today for HMP preservation of human kidneys world-wide (marketed under many different names now). The old handmade perfusion machines are no longer used but have been replaced by new automated devices produced by Waters or Organ Recovery Systems. One attribute of the machine that must be maintained is the pulsatility of the duty cycle of the pump motor to simulate pressure waves created by the cardiac cycle. Without pulsatile flow, the kidneys swell and do not preserve well. This response is believed to be due to reduced mean capillary pressures during pulsatile perfusion, compared to non-pulsatile perfusion and by activation of various gene products by the pulsatile forces and sheer stress on the vessel wall.Citation36–Citation38
The clinical effects of HMP preservation in kidney transplantation have been largely anecdotal until recently. Many centers preferred to use simple cold storage because of the low cost and ease of use, but those that used perfusion claimed it was better. Recently, a multi-center international randomized trial using either cold storage or hypothermic machine perfusion preservation was undertaken. In a well designed study, one kidney of a pair removed from cadaver donors was assigned to cold storage and the contralateral kidney was assigned to perfusion preservation. The graft survival and other preservation read-outs were followed for 1 year. The study clearly showed that machine perfusion produces less frequent and less severe delayed graft function and the 1 year graft survival was significantly higher compared to the cold store group ().Citation39
HMP preservation has proven to be a reliable method for preserving good renal function in explanted cadaveric kidneys for transplantation. The Belzer-MPS solution continues to be the predominant perfusion solution. Although countless studies and reports have attempted to alter the composition of MPS to improve its performance, it has remained commercially unchanged throughout the years. New and significant advancements in perfusion preservation will likely come about only by an increased understanding of the basic and root causal molecular mechanisms of hypothermic preservation injury in kidneys and other transplanted organs.
For livers, HMP has not made the transition from laboratory experiments to clinical practice. The earliest study that showed successful long term preservation of livers was conducted by Kamada.Citation40 These studies showed that with the use of fluorocarbon, 25 hr preservation of rat livers in a transplant model was possible. Tamaki et al.Citation41 showed that 48 hr HMP preservation in the rat liver transplant model was possible using a Haemaccel isotonic citrate solution. Their solution also contained a fluorocarbon. No successful long-term (>24 hr) transplant studies in the rat has been shown without the addition of fluorocarbon. It is not clear whether it is the oxygen carrying capacity of the fluorocarbon or the increased viscosity of the solution that is beneficial during HMP. Recently, Bessems et al.Citation42 have conducted extensive studies on a new HMP solution, Polysol, which contains amino acids, histidine, glutamine, and tryptophan and vitamins, ascorbic acid and α-tocopherol. Their studies show that the new solution improved 24 hr preservation compared to Belzer's MPS with lower enzyme release and increased bile production. However, a main concern for HMP of livers is the susceptibility of the endothelial cells to shear stress at hypothermic temperatures.Citation43–Citation45 The cause of this damage remains unclear.
In large animals studies, Piennar et al.Citation46 showed that HMP with the UW solution could be used for 72 hr preservation of the liver in the canine transplant model. They provided oxygenated perfusate through the portal vein only with pulsatile flow (30 BPM) at pressures varying between 16–18 mm Hg. Guarrera et al.Citation47 showed that using Vasosol, a new HMP solution, 12 hr preservation in a swine transplant model was possible. Their solution, using Belzer's MPS as a base, adds α-ketoglutarate, L-arginine, N-acetylcysteine, nitroglycerin and prostanglandin E1. The rationale for these additional substrates was to provide energy substrates, improve micro-circulation, and support the anti-oxidant systems.
Normothermic machine perfusion (NMP). In 2001, Friend et al.Citation48 showed that normothermic machine perfusion livers (NMP) in a swine isolated perfusion model had improved 24 hr preservation compared to SCS. The system they developed utilized autologous blood with infused total parental nutrition and prostacyclin. The flows and pressures through the portal vein and hepatic artery were maintained at physiological levels. During reperfusion, their results showed a near 10 fold decrease in alanine aminotransferase release while maintaining a two-fold increase in bile production. In addition, glucose levels were halved and urate levels were near one-tenth of SCS. Subsequent studiesCitation49–Citation52 have shown that NMP could be extended to 72 hrs and also for recovery of DCD livers. Recently, Tolboom et al.Citation53 showed in a rat model that NMP livers can be transplanted after 6 hr perfusion with good survival after 1 month. A main advantage for NMP is that viability assessment is possible prior to implantation thus reducing the incidence of primary non-function of the graft.
Oxygen persufflation. Oxygen Persufflation is a technique for organ preservation that was first reported by Isselhard et al.Citation54,Citation55 This technique utilizes gaseous oxygen that is bubbled through the vasculature and the gas escapes through small perforations in the organ's surface. This technique was first tested on canine kidneysCitation55 and has undergone a pilot clinical study.Citation56 This technique was shown by Minor et al.Citation57 to be effective in the liver with global homogenous distribution of the gaseous oxygen.Citation58,Citation59 It is typical however to include free radical scavengers such as super oxide dismutase to the bathing solution to reduce and prevent oxidation damage.Citation57,Citation60 This method has shown excellent results in recovering DCD organs.
Marginal Donor Organs
For every organ transplanted in the US, there are 4 more patients on the waiting list (28). Similar numbers exist for Europe and Asia. This profound shortage of donor organs can only be met by expanding the donor pool. Unfortunately, the donor pools available are those donors from which we currently do not obtain grafts because of their status. However, efforts to utilize marginal donor organs are on the increase. In particular, we will review Donation after Cardiac Death organs and steatotic (fatty) livers. The main reason that these donor organs are underutilized is because SCS is an ineffective method of preservation leading to higher incidence of primary graft non-function. The dynamic methods of preservation described above have been shown to be effective in reclaiming and preserving these classes of donor organs. Currently, only HMP has been approved as a method to preserve DCD kidneys. The other methods have shown excellent results in animal and some limited clinical studies.
Donation after cardiac death organs.
Donation after Cardiac Death or DCD organs and formerly known as non-heart beating donor organs are a pool of potential donor organs that are under-utilized. These organs retrieved after cardiac death of the donor can experience various periods of warm ischemia and thus varying degree of warm ischemic injury. Kootstra et al.Citation61 categorized DCD organs known as the Maastricht Categories which range from I–IV. Maastricht Category I donors were found dead at the scene and resuscitation was deemed pointless. Category II donors died as a result of sudden cardiac arrest while on route to emergency departments with attempts of resuscitation. Category III donors have controlled removal of life support with procurement usually occurring within 20 mins of death. Category IV donors suffer unexpected cardiac death after being declared brain dead. Estimates for the pool of donors in Categories II and III range from 20–40% of the current donor pool.Citation62–Citation64 The reason these donor organs are underutilized is because SCS becomes ineffective as a preservation method once the warm ischemic period is beyond 20 mins. Local donor laws and policies also can restrict early intervention in these patients and reduce the chances of a successful harvest. The methods of hypothermic and normothermic machine perfusion and oxygen persufflation have been shown to be effective in resuscitating these donor organs.
Successful preservation of DCD kidneys can be accomplished with hypothermic machine perfusion. In a recent clinical trial, 114 kidneys from DCD donors were preserved by machine per- fusion while a control group of 27 were cold stored. Despite longer cold preservation times, the perfusion group still had significantly less delayed graft function (11%) compared to the cold stored group (34%) over 2 years.Citation65 In another study using canine kidneys, prior severe warm ischemia (60 minutes) before preservation resulted in significantly higher patient survival when the kidneys were perfused with cold MPS solution (100%) compared to cold storage in Viaspan-UW solution (40%).Citation66 This same study also demonstrated that perfusion preservation with UW solution is better for kidneys from DCD donors compared to perfusion with MPS solution ().
Experimental studies to reanimate severely injured kidneys from warm ischemia have used near normothermic perfusion. Dog kidneys with 2 hours of warm ischemia, which are considered not usable, were reanimated by perfusing them at 32°C for 18 hours with a complex acellular perfusate containing substrates, co-factors, oxygen carriers and oxygenCitation67–Citation69 (32–34). While the process sounds exciting for harvesting terminally injured kidneys, the complexity and supervision required for the process may not be conducive for clinical use. Perhaps a compromise between near normothermic temperatures and the typical 4°C perfusion would be best for clinical use. Here the warmer temperatures at midthermia (15–20°C) may promote metabolic repair but remain cool enough to suppress metabolism and slow reactions and flow rates so that machine automation can be practical for clinical use. In conclusion, preservation of warm, ischemically injured kidneys requires perfusion preservation and warmer temperatures of perfusion than what is currently used may provide better ex-vivo reanimation. This is vital to expand the donor pool of kidneys and alleviate the donor shortage.
Currently, studies have shown that the HMP has the potential to revive DCD livers. In the rat transplant model, Lee et al.Citation70 showed that after 30 mins warm ischemia, HMP livers after 5 hr preservation were transplantable while SCS livers were not (). In addition Stegman and MinorCitation71 showed that mitochondrial function and energy stores could be replenished after HMP. Bessems et al.Citation72 showed that Polysol improved recovery of DCD livers in the rat isolated perfused model after 24 hr preservation compared with Belzer's MPS. Increased bile production and ammonia clearance and decreased enzyme released were observed. Currently, pre-clinical transplant studies are being conducted in large animals to determine whether the results seen in small animal models are translatable.
Normothermic machine perfusion has also shown excellent promise in recovering DCD livers.Citation51,Citation73,Citation74 Schon et al.Citation73 were the first to demonstrate successful porcine DCD liver transplant after 1 hr warm ischemia and 4 hr preservation. Their system utilized a dialysis system to remove metabolites and balance electrolytes during the preservation period. St. Peter et al.Citation51 showed that livers could be preserved for 24 hrs with excellent function following 1 hr of warm ischemia. Their study showed superior recovery compared to SCS based on enzyme release (80% less), increased bile production, and glucose uptake. Recently, Tolboom et al.Citation75 demonstrated that this technique could also be used following cold storage of the ischemic liver. Their study showed that following 45 min warm ischemia , 2 hr SCS and 4 hr NMP, 100% of the rats receiving these livers survived for the study period of 4 weeks. This suggests that NMP can be used in conjunction with simple cold storage.
The benefit of oxygen persufflation (OP) for reviving DCD organs was recognized early by Fischer et al.Citation76 Their study showed that retrograde OP of a warm ischemic canine kidney displayed comparable inulin and p-aminohirruate clearance to hypothermic machine perfusion. Rolles et al.Citation77 showed that oxygen as oppose to air or an inert gas was needed for reviving the warm ischemic kidney. This process was first tested by Minor et al.Citation78 in a rat DCD liver model and subsequently in a porcine DCD liver transplant model by Saad et al.Citation79 They showed that after 60 mins of warm ischemia and 4 hr storage with OP, 100% 5-day survival was achieved, comparable to controls with no warm ischemia and persufflation. In addition, this group showed that including an antioxidant (i.e., superoxide dismutase)Citation80 was important in reducing lipid peroxidation, enzyme release and vascular resistance. This technique has since undergone a pilot clinical study with excellent results whereby 5 patients receiving livers with 20–60 mins of warm ischemia are alive after 2 years. Current work on this methodology has progressed to studies with new preservation solutions including Custodiol-N for improved and prolong storage of warm ischemic livers.Citation81
Fatty livers.
Steatotic or fatty livers, with greater than 30% macrovesicular steatosis, are typically rejected for transplant due to poor preservation by SCS. Although new additivesCitation82,Citation83 to the preservation solution are being introduced no consensus has been reached. The main reason for this is that the mechanism of damage remains unclear with several studies showing increased necrapoptosis,Citation82 ultrastructuralCitation84 and oxidative phosphorylation impairment,Citation85,Citation86 microcirculation disruptionCitation86,Citation87 and decreased plasma membrane fluidity.Citation88 There are preliminary studies to show that HMP and oxygen persufflation have the potential to improve preservation of steatotic (fatty) livers. Bessems et al.Citation89 showed that HMP improved both hepatocellular and endothelial function while reducing damage after 24 hr preservation in a diet-induced rat fatty liver model. Minor et al.Citation84 showed that oxygen persufflation reduced Kupffer cell activation, mitochondrial enzyme release, and improved structural integrity of both mitochondria and endothelial cells. These studies are in their early stages and more are needed to determine their potential impact.
In the fifty plus years since the first successful organ transplantation, organ preservation has made incredible advances both in increasing preservation time and improving function. In spite of these advances, there is still a great disparity between the number of patients on waiting lists and the number of donor organs available. The next challenge for organ preservation will be to improve the recovery and resuscitation of marginal donor organs especially the Donation after Cardiac Death organs. Given the ineffectiveness of SCS to restore these organs, the advancement of the dynamic methods of preservation, HMP, NMP and oxygen persufflation will be key in improving the donor crisis.
Abbreviations
| UW | = | university of wisconsin |
| MPS | = | machine perfusion solution |
| SCS | = | simple cold storage |
| HMP | = | hypothermic machine perfusion |
| NMP | = | normothermic machine perfusion |
| DCD | = | donation after cardiac death |
| HTK | = | histidine-tryptophaneketoglutarate |
Figures and Tables
Figure 1 Graft survival comparison of paired kidneys preserved by simple cold storage or hypothermic machine perfusion.
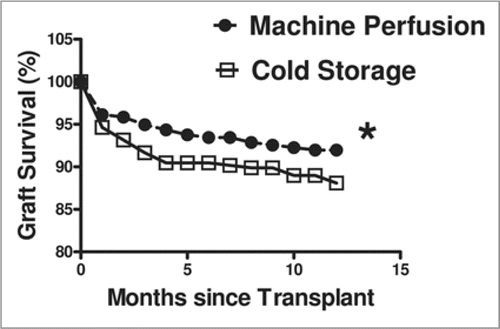
Figure 2 Levels of serum creatinine and survival percentage following transplant of canine kidneys that experienced 60 mins of warm ischemia and 24 hr preservation by simple cold storage or hypothermic machine perfusion.
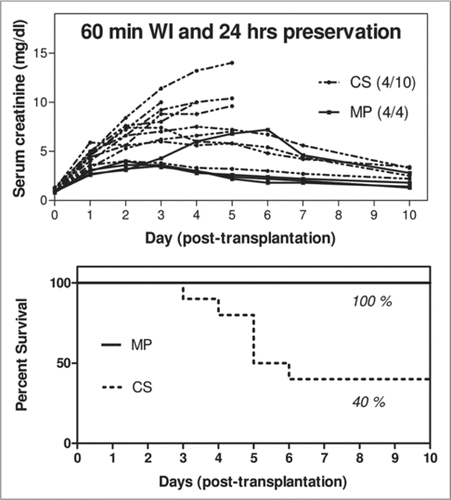
Figure 3 Rat liver transplant survival plot following 30 mins warm ischemia and 5 hr preservation by simple cold storage or hypothermic machine perfusion. Controls did not experience warm ischemia or preservation.
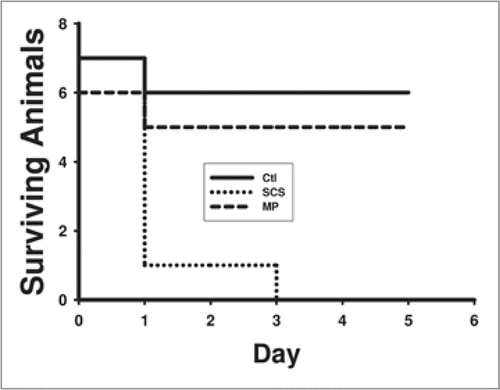
Table 1 Composition of preservation solutions
Acknowledgements
We would like to thank Alexandra Lim for compiling and consolidating the information for the table of preservation solutions.
References
- Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation 1988; 45:673 - 676
- Southard JH, Belzer FO. Control of canine kidney cortex slice volume and ion distribution at hypothermia by impermeable anions. Cryobiology 1980; 17:540 - 548
- Collins GM, Bravo-Shugarman M, Novom S, Terasaki PI. Kidney preservation for transplantation I. Twelve-hour storage in rabbits. Transplant Proc 1969; 1:801 - 807
- Collins GM, Bravo-Shugarman M, Terasaki PI. Kidney preservation for transportation. Initial perfusion and 30 hours' ice storage. Lancet 1969; 2:1219 - 1222
- Mees N, Southard JH, Belzer FO. Inhibition of ischemic induced cellular swelling in kidney cortex tissue by lactobionate anions. J Trauma 1982; 22:118 - 120
- Belzer FO, Sollinger HW, Glass NR, Miller DT, Hoffmann RM, Southard JH. Beneficial effects of adenosine and phosphate in kidney preservation. Transplantation 1983; 36:633 - 635
- McAnulty JF, Southard JH, Belzer FO. Improved maintenance of adenosine triphosphate in five-day perfused kidneys with adenine and ribose. Transplant Proc 1987; 19:1376 - 1379
- Southard J, Lutz M, Ametani M, Belzer F. Stimulation of ATP synthesis in hypothermically perfused dog kidneys by adenosine and PO4. Cryobiology 1984; 21:13 - 19
- Southard JH, Ametani MS, Lutz MF, Belzer FO. Effects of hypothermic perfusion of kidneys on tissue and mitochondrial phospholipids. Cryobiology 1984; 21:20 - 24
- Southard JH, Senzig KA, Hoffmann RM, Belzer FO. Toxicity of oxygen to mitochondrial respiratory activity in hypothermically perfused canine kidneys. Transplantation 1980; 29:459 - 461
- Southard JH, van Gulik TM, Ametani MS, Vreugdenhil PK, Lindell SL, Pienaar BL, Belzer FO. Important components of the UW solution. Transplantation 1990; 49:251 - 257
- Vreugdenhil P, Evans W, Belzer F, Southard J. Glutathione depletion in cold-stored organs. Transplant Proc 1990; 22:455 - 457
- Bretschneider HJ. Survival Time and Recuperative Time of the Heart in Normothermia and Hypothermia. Verh Dtsch Ges Kreislaufforsch 1964; 30:11 - 34
- Isemer FE, Ludwig A, Schunck O, Bretschneider HJ, Peiper HJ. Kidney procurement with the HTK solution of Bretschneider. Transplant Proc 1988; 20:885 - 886
- Groenewoud AF, Isemer FE, Stadler J, Heideche CD, Florack G, Hoelscher M. A comparison of early function between kidney grafts protected with HTK solution versus Euro-Collins solution. Transplant Proc 1989; 21:1243 - 1244
- Gubernatis G, Pichlmayr R, Lamesch P, Grosse H, Bornscheuer A, Meyer HJ, et al. HTK-solution (Bretschneider) for human liver transplantation. First clinical experiences. Langenbecks Arch Chir 1990; 375:66 - 70
- Lindell S, Gandolph D, Southard J, Belzer F. Comparison of PBS HTK, and UW solutions for kidney preservation. Transplant Proc 1991; 23:2399 - 2401
- Menasche P, Pradier F, Grousset C, Peynet J, Mouas C, Bloch G, Piwnica A. Improved recovery of heart transplants with a specific kit of preservation solutions. J Thorac Cardiovasc Surg 1993; 105:353 - 363
- Ambiru S, Uryuhara K, Talpe S, Dehoux JP, Jacobbi L, Murphy CJ, et al. Improved survival of orthotopic liver allograft in swine by addition of trophic factors to University of Wisconsin solution. Transplantation 2004; 77:302 - 319
- McAnulty JF, Reid TW, Waller KR, Murphy CJ. Successful six-day kidney preservation using trophic factor supplemented media and simple cold storage. Am J Transplant 2002; 2:712 - 718
- Schmiedt CW, Schwab MC, Dubielzig RR, Murphy CJ, McAnulty JF. Trophic factor supplemented UW solution reduces intimal hyperplasia in the rat aortic transplant model. Cryobiology 2007; 54:204 - 211
- Kwon YS, Foley JD, Murphy CJ, McAnulty JF. The effect of trophic factor supplementation on cold ischemia-induced early apoptotic changes. Transplantation 2007; 83:91 - 94
- Bahde R, Palmes D, Gemsa O, Minin E, Stratmann U, de Groot H, et al. Attenuated cold storage injury of rat livers using a modified HTK solution. J Surg Res 2008; 146:49 - 56
- Radovits T, Lin LN, Zotkina J, Koch A, Rauen U, Kohler G, et al. Endothelial dysfunction after long-term cold storage in HTK organ preservation solutions: effects of iron chelators and N-alpha-acetyl-L-histidine. J Heart Lung Transplant 2008; 27:208 - 216
- Rauen U, Wu K, Witzke O, de Groot H. Custodiol-N A new mechanism-based organ preservation soluiton. Cryobiology 2008; 57:331
- Carrel A, Lindbergh CA. The culture of whole organs. Science 1935; 81:621 - 623
- Lindbergh CA, Perry VP, Malinin TI, Mouer GH. An apparatus for the pulsating perfusion of whole organs. Cryobiology 1966; 3:252 - 260
- Lapchinsky AG. Recent results of experimental transplantation of preserved limbs and kidneys and possible use of this technique in clinical practice. Ann N Y Acad Sci 1960; 87:539 - 571
- Manax WG, Bloch JH, Longerbeam JK, Lillehei RC. Successful 24 hour in vitro preservation of canine kidneys by the combined use of hyperbaric oxygenation and hypothermia. Surgery 1964; 56:275 - 282
- Hoffman A, Burger C, Persky L. Extracorporeal renal storage. Invest Urol 1965; 2:567 - 573
- Belzer F, Ashby B, Dunphy J. 24-hour and 72-hour preservation of canine kidneys. Lancet 1967; 2:536 - 538
- Belzer FO, Ashby BS, Gulyassy PF, Powell M. Successful seventeen-hour preservation and transplantation of human-cadaver kidney. N Engl J Med 1968; 278:608 - 610
- Rooney SJ, Levine AJ, Parkes K, Revell M, Shimada I, Bonser RS. Differential time scale of fluid and solute permeability following hypothermic lung preservation. J Heart Lung Transplant 2000; 19:179 - 184
- Belzer FO, Park HY, Vetto RM. Factors influencing renal blood flow during isolated perfusion. Surg Forum 1964; 15:222 - 224
- Hoffmann RM, Southard JH, Lutz M, Mackety A, Belzer FO. Synthetic perfusate for kidney preservation. Its use in 72-hour preservation of dog kidneys. Arch Surg 1983; 118:919 - 921
- Adams JA, Mangino MJ, Bassuk J, Kurlansky P, Sackner MA. Regional blood flow during periodic acceleration. Crit Care Med 2001; 29:1983 - 1988
- Dekker RJ, van Thienen JV, Rohlena J, de Jager SC, Elderkamp YW, Seppen J, et al. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol 2005; 167:609 - 618
- Hutcheson IR, Griffith TM. Release of endothelium-derived relaxing factor is modulated both by frequency and amplitude of pulsatile flow. Am J Physiol 1991; 261:257 - 262
- Moers C, Smits JM, Maathuis MH, Treckmann J, van Gelder F, Napieralski BP, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med 2009; 360:7 - 19
- Kamada N, Calne RY, Wight DG, Lines JG. Orthotopic rat liver transplantation after long-term preservation by continuous perfusion with fluorocarbon emulsion. Transplantation 1980; 30:43 - 48
- Tamaki T, Kamada N, Wight DG, Pegg DE. Successful 48-hour preservation of the rat liver by continuous hypothermic perfusion with haemaccel-isotonic citrate solution. Transplantation 1987; 43:468 - 471
- Bessems M, Doorschodt B, van VA, van GT. Improved rat liver preservation by hypothermic continuous machine perfusion using polysol, a new, enriched preservation solution. Liver Transpl 2005; 11:539 - 546
- Jain S, Xu H, Duncan H, Jones J, Zhang J, Clemens M, Lee C. Ex-vivo study of flow dynamics and endothelial cells structure during extended hypothermic machine perfusion preservation of livers. Cryobiology 2004; 48:322 - 332
- 't Hart N, van dPA, Leuvenink H, van GH, Wiersema BJ, Verkerke G, et al. Hypothermic machine perfusion of the liver and the critical balance between perfusion pressures and endothelial injury. Transplant Proc 2005; 37:332 - 334
- Xu H, Lee C, Clemens M, Zhang J. Pronlonged hypothermic machine perfusion preserves hepatocellular function but potentiates endothelial cell dysfunction in rat livers. Transplantation 2004; 77:1676 - 1682
- Pienaar B, Lindell S, Van GT, Southard J, Belzer F. Seventy-two-hour preservation of the canine liver by machine perfusion. Transplantation 1990; 49:258 - 260
- Guarrera J, Estevez J, Boykin J, Boyce R, Rashid J, Sun S, Arrington B. Hypothermic machine perfusion of liver grafts for transplantation: technical development in human discard and miniature swine models. Transplant Proc 2005; 37:323 - 325
- Friend P, Imber C, St PS, Lopez I, Butler A, Rees M. Normothermic perfusion of the isolated liver. Transplant Proc 2001; 33:3436 - 3438
- Imber CJ, St Peter SD, de Cenarruzabeitia IL, Lemonde H, Rees M, Butler A, et al. Optimisation of bile production during normothermic preservation of porcine livers. Am J Transplant 2002; 2:593 - 599
- Butler AJ, Rees MA, Wight DG, Casey ND, Alexander G, White DJ, Friend PJ. Successful extracorporeal porcine liver perfusion for 72 hr. Transplantation 2002; 73:1212 - 1218
- St Peter SD, Imber CJ, Lopez de Cenarruzabeitia I, Hughes D, Friend PJ. Extended preservation of non-heart-beating donor livers with normothermic machine perfusion. Br J Surg 2002; 89:609 - 616
- Imber CJ, St Peter Sd, Lopez de Cenarruzabeitia I, Pigott D, James T, Taylor R, et al. Advantages of normothermic perfusion over cold storage in liver preservation. Transplantation 2002; 73:701 - 709
- Tolboom H, Pouw R, Uygun K, Tanimura Y, Izamis ML, Berthiaume F, et al. A model for normothermic preservation of the rat liver. Tissue Eng 2007; 13:2143 - 2151
- Isselhard W, Berger M, Denecke H, Witte J, Fischer JH, Molzberger H. Metabolism of canine kidneys in anaerobic ischemia and in aerobic ischemia by persufflation with gaseous oxygen. Pflugers Arch 1972; 337:87 - 106
- Sachweh D, Isselhard W, Dennecke H, Stelter WJ, Berger M, Lauschke H, et al. Short time kidney preservation by hypothermic oxygen persufflation. Bull Soc Int Chir 1972; 31:258 - 263
- Rolles K, Foreman J, Pegg DE. A pilot clinical study of retrograde oxygen persufflation in renal preservation. Transplantation 1989; 48:339 - 342
- Minor T, Isselhard W, Kunz G, Saad S. Involvement of oxygen in harvesting injury of the liver. An experimental study including substrate free organ persufflation to evaluate a specific therapeutic approach. Res Exp Med (Berl) 1991; 191:167 - 175
- Minor T, Klauke H, Vollmar B, Isselhard W, Menger M. Biophysical aspects of liver aeration by vascular persufflation with gaseous oxygen. Transplantation 1997; 63:1843 - 1846
- Minor T, Klauke H, Vollmar B, Menger M, Isselhard W. Rat liver transplantation after long-term preservation by venous systemic oxygen persufflation. Transplant Proc 1997; 29:410 - 411
- Minor T, Isselhard W, Yamamoto Y, Obara M, Saad S. The effects of allopurinol and SOD on lipid peroxidation and energy metabolism in the liver after ischemia in an aerobic/anaerobic persufflation. Surg Today 1993; 23:728 - 732
- Kootstra G, Daemen JH, Oomen AP. Categories of non-heart-beating donors. Transplant Proc 1995; 27:2893 - 2894
- Kootstra G, Wijnen R, van Hooff JP, van der Linden CJ. Twenty percent more kidneys through a non-heart beating program. Transplant Proc 1991; 23:910 - 911
- Daemen JH, de Wit RJ, Bronkhorst MW, Yin M, Heineman E, Kootstra G. Non-heart-beating donor program contributes 40% of kidneys for transplantation. Transplant Proc 1996; 28:105 - 106
- Daemen J, Oomen A, Kelders W, Kootstra G. The potential pool of non-heart-beating kidney donors. Clin Transplant 1997; 11:149 - 154
- Stratta RJ, Moore PS, Farney AC, Rogers J, Hartmann EL, Reeves-Daniel A, et al. Influence of pulsatile perfusion preservation on outcomes in kidney transplantation from expanded criteria donors. J Am Coll Surg 2007; 204:873 - 882
- Lindell S, Compagnon P, Mangino M, Southard J. UW solution for hypothermic machine perfusion of warm ischemic kidneys. Transplantation 2005; 79:1358 - 1361
- Brasile L, Stubenitsky B, Haisch CE, Kon M, Kootstra G. Potential of repairing ischemically damaged kidneys ex vivo. Transplant Proc 2005; 37:375 - 376
- Brasile L, Stubenitsky BM, Booster MH, Lindell S, Araneda D, Buck C, et al. Overcoming severe renal ischemia: the role of ex vivo warm perfusion. Transplantation 2002; 73:897 - 901
- Brasile L, Stubenitsky BM, Haisch CE, Kon M, Kootstra G. Repair of damaged organs in vitro. Am J Transplant 2005; 5:300 - 306
- Lee CY, Jain S, Duncan HM, Zhang JX, Jones J, Southard JH, Clemens MG. Survival transplantation of preserved non-heart-beating donor rat livers: preservation by hypothermic machine perfusion. Transplantation 2003; 76:11432 - 11436
- Stegemann J, Minor T. Energy charge restoration, mitochondrial protection and reversal of preservation induced liver injury by hypothermic oxygenation prior to reperfusion. Cryobiology 2009; 58:331 - 336
- Bessems M, Doorschodt B, van VA, van GT. Machine perfusion preservation of the non-heart-beating donor rat livers using polysol, a new preservation solution. Transplant Proc 2005; 37:326 - 328
- Schon MR, Kollmar O, Wolf S, Schrem H, Matthes M, Akkoc N, et al. Liver transplantation after organ preservation with normothermic extracorporeal perfusion. Ann Surg 2001; 233:114 - 123
- Tolboom H, Pouw RE, Izamis ML, Milwid JM, Sharma N, Soto-Gutierrez A, et al. Recovery of warm ischemic rat liver grafts by normothermic extracorporeal perfusion. Transplantation 2009; 87:170 - 177
- Tolboom H, Milwid JM, Izamis ML, Uygun K, Berthiaume F, Yarmush ML. Sequential cold storage and normothermic perfusion of the ischemic rat liver. Transplant Proc 2008; 40:1306 - 1309
- Fischer JH, Czerniak A, Hauer U, Isselhard W. A new simple method for optimal storage of ischemically damaged kidneys. Transplantation 1978; 25:43 - 49
- Rolles K, Foreman J, Pegg DE. Preservation of ischemically injured canine kidneys by retrograde oxygen persufflation. Transplantation 1984; 38:102 - 106
- Minor T, Klauke H, Isselhard W. Resuscitation of cadaveric livers from non-heart-beating donors after warm ischemic insult: a novel technique tested in the rat. Experientia 1996; 52:661 - 664
- Saad S, Minor T, Nagelschmidt M, Fu Z, Kotting I, Paul A, et al. Revitalizing donor livers after cardiovascular arrest with venous oxygen persufflation. Langenbecks Arch Chir Suppl Kongressbd 1998; 115:705 - 708
- Lauschke H, Kotting M, Akbar S, Minor T. Use of taurine as antioxidant in resuscitating livers from non-heart-beating donors by gaseous oxygen persufflation. J Invest Surg 2003; 16:7 - 11
- Stegemann J, Hirner A, Rauen U, Minor T. Gaseous oxygen persufflation or oxygenated machine perfusion with Custodiol-N for long-term preservation of ischemic rat livers?. Cryobiology 2009; 58:45 - 51
- Sun Z, Klein A, Radaeva S, Hong F, El AO, Pan H, et al. In vitro interleukin-6 treatment prevents mortality associated with fatty liver transplants in rats. Gastroenterology 2003; 125:202 - 215
- Hata K, Tolba RH, Wei L, Doorschodt BM, Buttner R, Yamamoto Y, Minor T. Impact of polysol, a newly developed preservation solution, on cold storage of steatotic rat livers. Liver Transpl 2007; 13:114 - 121
- Minor T, Akbar S, Tolba R, Dombrowski F. Cold preservation of fatty liver grafts: prevention of functional and ultrastructural impairments by venous oxygen persufflation. J Hepatol 2000; 32:105 - 111
- Caraceni P, Bianchi C, Domenicali M, Maria PA, Maiolini E, Parenti CG, et al. Impairment of mitochondrial oxidative phosphorylation in rat fatty liver exposed to preservation-reperfusion injury. J Hepatol 2004; 41:82 - 88
- Fukumori T, Ohkohchi N, Tsukamoto S, Satomi S. Why is fatty liver unsuitable for transplantation? Deterioration of mitochondrial ATP synthesis and sinusoidal structure during cold preservation of a liver with steatosis. Transplant Proc 1997; 29:412 - 415
- Seifalian A, Chidambaram V, Rolles K, Davidson B. In vivo demonstration of impaired microcirculation in steatotic human liver grafts. Liver Transpl Surg 1998; 4:71 - 77
- Fukumori T, Ohkohchi N, Tsukamoto S, Satomi S. The mechanism of injury in a steatotic liver graft during cold preservation. Transplantation 1999; 67:195 - 200
- Bessems M, Doorschodt BM, Kolkert JL, Vetelainen RL, van Vliet AK, Vreeling H, et al. Preservation of steatotic livers: a comparison between cold storage and machine perfusion preservation. Liver Transpl 2007; 13:497 - 504