Abstract
The main obstacle to achieving favorable outcome of soft-tissue augmentation after autologous fat transplantation is unpredictable long-term results due to the high rate of absorption in the grafted site. At the present time, adipose aspirates can only be used for immediate autologous fat grafting during the same procedure in which liposuction is performed; therefore adipose aspirates obtained from the procedure are usually discarded. It has been a strong desire of both surgeons and patients to be able to preserve the adipose aspirates, if an optimal technique were available, for potential future applications. For the last several years, cryopreservation of adipose tissue has been studied extensively in the author’s laboratory. Several findings from this exciting translational research will lead to develop a reliable method for long-term preservation of adipose tissue in the future. In addition, successful long-term preservation of adipose tissue may open a new era in adipose tissue related tissue regeneration.
Autologous fat transplantation (AFT) in cosmetic and reconstructive plastic surgery has revolutionized surgical treatment for soft-tissue augmentation of the face, hands or other parts of the body for the last decade. As liposuction has gained popularity recently, a source of graft materials for AFT should be reconsidered. Adipose aspirates obtained from liposuction have many properties desirable for soft-tissue augmentation because they are abundant, readily available, inexpensive, host compatible and can be harvested easily and repeatedly.Citation1 Up to now, the main obstacle to achieving favorable outcome of soft-tissue augmentation after AFT is its unpredictable long-term results due to the high rate of absorption (up to 70% of the original injected volume) in the grafted site.Citation2 This high rate of absorption often necessitates overcorrection or repeated procedures in the desired area, causing patient discomfort, less than optimal appearance, high cost and morbidity or trauma of the donor sites.Citation1,Citation2
At the present time, adipose aspirates can only be used for immediate autologous fat grafting during the same procedure in which liposuction is performed; therefore adipose aspirates obtained from the procedure are usually discarded. It has been a strong desire of both plastic surgeons and patients to preserve the adipose aspirates, if an optimal technique were available, for potential future applications. With the level of perfection plastic surgeons have come to expect of surgical results, the option to preserve and bank previously harvested fatty tissues for possible future use or repeated application is in great demand.
In this review, the author will summarize previous studies in cryopreservation of adipose tissue, the development of a cryopreservation protocol for adipose tissue, and the findings from several recent studies. In addition, future perspectives on this existing field of translational research will be discussed.
Previous Studies in Cryopreservation of Adipose Tissue
Until recently, only a few empirical experimental studies have examined the role of frozen storage for short-term preservation of adipose aspirates obtained by liposuction at a relatively high temperature (+1°C to −18°C). In one study, human adipose tissue obtained by liposuction was stored in a domestic refrigerator at −18°C for 2 weeks. After thawing, the fat was injected into nude mice. In the control group, the fat was injected immediately after being harvested. Injected fat survived in both the study and control groups. No significant differences were found between the groups in fat graft weight, volume or histology.Citation3 In another study, commercially available refrigeration or freezing equipment was used to store suction-harvested autologous subcutaneous fat of Sprague-Dawley rats. The aspirated fat was stored at −16°C (frozen) or 1°C (refrigerated) for a period of 1 or 2 weeks and was then implanted subcutaneously in the rats. A histological comparison clearly demonstrated a decrease in viable adipocytes and an increase in signs of inflammation and fat necrosis in those animals that received preserved fat versus immediate fat implantation.Citation4 These changes become more severe with increased length of storage and the use of refrigeration over freezing.
A simple freezing technique has been recently used by a number of investigators to explore the possibility of long-term storage of fat grafts. In one study, fat grafts were frozen in liquid nitrogen and stored at −195.8°C for up to 8 days. The results demonstrated that those frozen fat grafts showed remarkable maintenance of mitochondrial metabolic activity.Citation5 However, a separate study by others indicated that after simple freezing, up to 92.7% of metabolic activity of fat grafts was lost, but the addition of a cryoprotective agent led to preservation of up to 54% of the baseline activity. The authors indicated that the widely used practice of simple freezing in a freezer leads to nonviable tissue and cell survival can be improved by addition of a cryoprotective agent.Citation6 In another study, fat grafts were simply frozen in liquid nitrogen and stored at −35°C for 6 months. Interestingly, the authors found that the viability and histology of fat grafts frozen in liquid nitrogen were similar to those of fresh grafts.Citation7 However, their finding has been questioned by other investigators who have found that to achieve optimal cryopreservation of adipose tissues, it would be necessary to add a cryoprotective agent before freezing and fat grafts mixed with a cryoprotective agent should also undergo controlled freezing and thawing during cryopreservation.Citation8,Citation9
In our laboratory, a preliminary study was conducted to evaluate the effect and role of temperature alone for storage of adipose tissue. In these studies, the viability of adipose aspirates was assessed by a glycerol-3-phosphate dehydrogenase (G3PDH) assay at both 4°C and −20°C. After 2 weeks of storage, the viability of adipose aspirates decreased about 80% at 4°C. However, the viability of adipose aspirates only decreased about 5% at −20°C. Furthermore, a series of experiments was performed initially with different types and concentrations of cryoprotective agents, and their different combinations to determine what would be the best cryoprotective agent that can be used especially for adipose tissues. A number of freezing and thawing protocols were also evaluated for adipose tissue during the preliminary studies.Citation10
Although a few studies showed encouraging preliminary results for cryopreservation of autologous fat grafts for possible future application, the technique described in the study can only be used for a short-term preservation (a few days or weeks) and may not be optimal due to uncontrolled cooling/warming process and the lack of use of a cryoprotective agent. With application of modern cryopreservation techniques, a better long-term preservation of adipose tissues can possibly be achieved. Such a method will likely allow fat grafts being stored for months or years (below −85°C) or for more than 10 years (at −196°C in liquid nitrogen).
Development of a Cryopreservation Protocol for Fat Grafts
Modern techniques in cryopreservation.
The modern technique of cryopreservation permits the long-term storage of living cells and tissues that may have many potential clinical applications such as blood transfusion, bone marrow transplantation, in vitro fertilization, vascular grafts, bone grafts and skin grafts.Citation11 The major steps of cryopreservation process can be summarized as follows: (1) add cryoprotective agents to cells/tissues before cooling, (2) cool the cells/tissues in a controlled rate toward a low temperature at which the cells/tissues are stored, (3) warm the cells/tissues, and (4) remove the cryoprotective agents from the cells/tissues after thawing.Citation12 The optimum cooling rate for cell survival should be slow enough to avoid intracellular ice formation but fast enough to minimize the cell damage.
Choices of cryoprotective agents.
Dimethyl sulfoxide (DMSO) is a permeable agent that can reduce cell injury due to the intracellular ice formation and “solution effects”.Citation11,Citation12 It has been widely used as an effective cryoprotective agent (CPA) in cryopreservation of living cells or tissues. The concentration of DMSO, when used alone as a cryoprotective agent, is usually 10%. Because this agent is tissue toxic at normal body temperature, it should be removed from the previously cryopreserved cells or tissues after thawing. In our previous study, we attempted to lower the concentration of DMSO used in cryopreservation of adipose tissues by adding another non-tissue toxic cryoprotective agent, such as trehalose. As a CPA, trehalose can dehydrate cells and thus reduce the amount of water present before freezing. It also can stabilize cellular membranes and proteins during freezing and drying. A combination of trehalose, a non-permeable cryoprotective agent, with DMSO, a permeable cryoprotective agent, may significantly enhance the protective effect of adipose tissue during cryopreservation through a possible synergistic mechanism but details of the mechanism remain uncertain.Citation13 Therefore the concentration of DMSO can theoretically be reduced when it is used in combination with trehalose. The combined use of both DMSO and trehalose as cryoprotective agents may be valuable to achieve optimal cryopreservation of adipose tissues or other type of tissues. From our preliminary studies, the optimal combination was determined to be 0.5 Molar (3.3%) DMSO (Sigma, St. Louis, MO) and 0.2 Molar (7.6%) trehalose (Sigma). This combination of agents was used in our subsequent studies.Citation14–Citation16
Establishment of a freezing and thawing protocol.
The freezing and thawing protocol described herein represents the best possible method developed for cryopreservation of adipose tissue and was used in our subsequent studies.Citation14–Citation16 Fresh adipose aspirates after preparation were put into a vial and mixed with the combined DMSO (in 0.5 Molar) and trehalose (in 0.2 Molar) solution in a one to one ratio. After adding cryoprotective agents, the vial was placed in room temperature for 10 minutes and then placed into a methanol bath (Kinetics, Stone Ridge, NY). The freezing system was programmed to cool at 1∼2°C per minute from 22°C to −30°C without artificial ice formation. The vial was then transferred to liquid nitrogen (−196°C) after it reached −30°C for long-term preservation.
Before thawing, the vial containing cryopreserved adipose aspirates was taken from the liquid nitrogen tank and placed at room temperature for 2 minutes to let the liquid nitrogen vapor out of the vial. The vial was then dropped into a stirred 37°C water bath until the preserved adipose aspirates were thoroughly thawed.
Findings from Our Recent Studies
In the following studies, samples of human fat grafts were obtained from adult female patients during liposuction and collected from the middle layer after centrifugation. In the in vitro study, a combination of DMSO and trehalose as cryoprotective agents with the optimal concentration (0.5 Molar of DMSO and 0.2 Molar of trehalose) was used throughout the study. In addition, maximal recovery of adipose tissue was achieved after cryopreservation using slow cooling (1∼2°C/min to −30°C, followed by immersing to −196°C for storage) and fast warming (in a 37°C water bath, averaging 35°C per minute). Fresh fat grafts (group 1), cryopreserved fat grafts without adding cryoprotective agents (group 2), or cryopreserved fat grafts with added cryoprotective agents (group 3) were evaluated by viable adipocyte counts with trypan blue vital stain), G3PDH assay, and routine histology. Significantly higher viable adipocyte counts, determined by intact cell membrane under microscopy, were found in group 3 compared with group 2 (p < 0.0011). Group 3 had only a marginally lower viable adipocyte count than group 1 (p = 0.083). There was a significant increase of G3PDH activity in group 3 compared with group 2 (p < 0.01). However, there was no statistical difference of G3PDH activity in group 3 compared with group 1, indicating good cellular function of fat grafts maintained after optimal cryopreservation performed in the above study. Histologically, various degrees of adipose tissue shrinkage were found in both groups 2 and 3. However, more tissue shrinkage was evident in group 2 compared with group 3 ().Citation14,Citation16
In the in vivo study, an athymic nude mouse was selected because of its limited ability to reject foreign antigenic grafts. This animal model allows evaluation of the injected human fat grafts, thus effectively imitating the clinical situation.Citation17 Fresh fat grafts (group 1), cryopreserved fat grafts without adding cryoprotective agents (group 2), or cryopreserved fat grafts with adding cryoprotective agents (group 3) from the same patient were injected into the posterior scalp of a nude mouse. The maintained fat grafts were harvested in each animal at 4 months and their weight, volume and histology were evaluated. Various degrees of absorption of injected fat grafts were seen in all three groups. However, group 3 had significantly greater weight and volume of the injected fat grafts than group 2 (both p < 0.0001) but had significantly less weight and volume than group 1 (both p < 0.01). Histologically, a large amount of tissue fibrosis was seen in group 2, and reasonably well maintained fatty tissue with only a small amount of tissue fibrosis was seen in group 3 compared with group 1 ().Citation15,Citation16
Future Perspectives
Based on our recent studies, the fat grafts previously cryopreserved with our preferred method described above after thawing had near normal appearance as fresh ones and may readily be used for future fat grafting if indicated (). Our cryopreservation method developed specifically for adipose tissues appears to provide good long-term preservation of fat grafts but so far, the overall quality of the cryopreserved fat grafts is poorer than fresh ones. This might be true not only for adipose tissue but also for other types of tissues after an “optimal” cryopreservation.Citation11–Citation13 Refinements may still be needed to improve the techniques used for cryopreservation of adipose tissue to achieve the best possible viability and histology of the fat grafts after cryopreservation. Clearly, further studies need to be done to develop a reliable and clinically feasible cryopreservation method that can be used for successful long-term preservation of adipose tissue. The favorable results from our recent studies warrant further investigations on cryopreservation of human adipose tissue for possible future autologous fat grafting. Furthermore, it becomes obvious that simple “freezing” fat grafts with liquid nitrogen should not be considered as a “standard” method for long-term preservation of fat grafts because of poorly maintained viability of fat grafts and higher degree of absorption after they are transplanted. Without adding CPAs to adipose tissue before cryopreservation, the results were clearly poor based on our in vitro study, and this was again confirmed by the findings from our in vivo study.Citation7,Citation8 Undoubtedly, the development of a practical but optimal cryopreservation technique will benefit many patients who desire soft-tissue augmentation with their own fat for either cosmetic or reconstructive reasons.
Adipose aspirates, collected from conventional liposuction of humans, can be processed in vitro to obtain a fibroblast-like population of cells, called processed lipoaspirate (PLA) cells.Citation18,Citation19 Several studies demonstrate that PLA cells can differentiate in vitro into adipogenic, chondrogenic, myogenic and osteogenic cells in the presence of lineage-specific induction factors.Citation20 PLA cells can also differentiate in vitro into non-mesodermal lineage cells, such as early progenitors of neurons and/or glia, again in the presence of lineage-specific induction factors.Citation21 Furthermore, adipose tissue may represent an important source of adult human stem cells because of abundant sources, similarities with bone marrow in term of embryonic origin, and easy availability through liposuction.Citation22 The current proposed strategy for the use of PLA cells for cell-based tissue engineering and transplantation would process adipose aspirates right away for PLA cells after they were collected from conventional liposuction and then store these cells by means of one of the tissue banking techniques, i.e., cryopreservation. One of our recent studies demonstrates that human adipose aspirates after cryopreservation with our preferred technique may still be a reliable source of adult human PLA cells because they could be processed later in good quantity.Citation23
In conclusion, adipose aspirates as “raw material” may be preserved effectively by an optimal cryopreservation method to meet future needs of patients either for repeated fat transplantation or for cell-based therapy ().Citation18,Citation19 Successful long-term preservation of adipose aspirates may open a new era in plastic and reconstructive surgery, in both the area of autologous fat transplantation and adipose tissue related tissue regeneration.Citation24
Figures and Tables
Figure 1 Histology of fresh or cryopreserved fat grafts (H&e staining, original magnification ×200, Bar = 100 µm). (A-Top) Group 1 (Fresh control) showing essentially normal histology of adipose tissue; (B-Middle) Group 2 (Cryopreservation without CPA) showing very prominent shrinkage of adipose tissues with evidence of broken adipocytes compared with Group 1; (C-Bottom) Group 3 (Cryopreservation with 0.5 M DMSO and 0.2 M trehalose) showing near normal histology of adipose tissue compared with Group 1. (reprinted with permission from Pu LLQ, et al. Long-term preservation of adipose aspirates after conventional liposuction. Aesthetic Surg J 2004; 24:6).
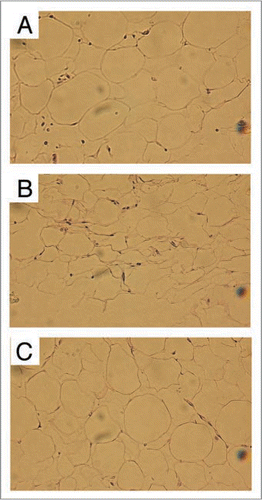
Figure 2 Histological examinations of encapsulated “fat grafts” after 4 months (H&E staining, original magnification ×400, Bars: 100 µm) show a well-retained fatty tissue structure in Group 1 (A, Top), large amount of tissue fibrosis with almost no maintained fatty tissue structure in Group 2 (B, Middle), and a reasonably well-retained fatty tissue structure associated with only minimal amount of tissue fibrosis in Group 3 (C, bottom) (Reprinted with permission from Pu LLQ, et al. The fate of cryopreserved adipose aspirates after in vivo transplantation. Aesthetic Surg J 2006; 26:9).
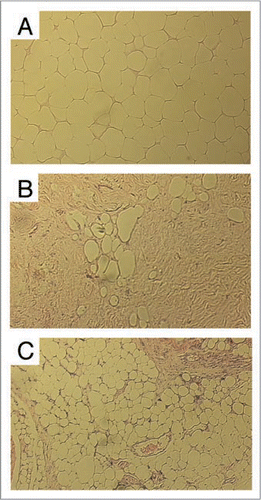
Figure 3 Gross appearance of fresh (left) and previously cryopreserved fat grafts after thawing with our preferred technique (right). The cryopreserved fat grafts have normal appearance and may possibly be used for future fat grafting if indicated. (From Pu LLQ. Cryopreservation of adipose tissue for fat grafting: Problems and potential solutions. In eds. Coleman SR, Mazzola RF. Fat injection: From Filling to Regeneration. St. Louis: Quality Medical Publishing 2009; 84).
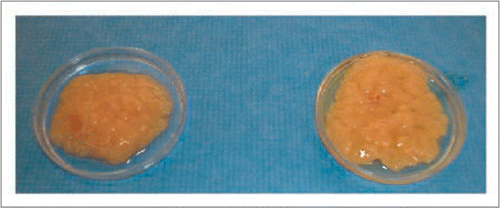
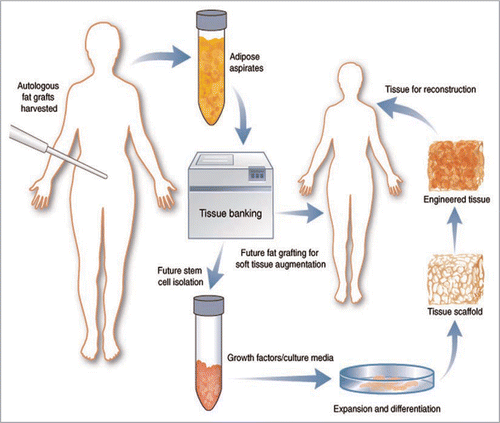
Figure 4 A schematic diagram shows possible future autologous fat transplantation or cell-based therapy for engineered tissue reconstruction after successful cryopreservation of the patient's own adipose tissue collected from conventional liposuction. (From Pu LLQ. Cryopreservation of adipose tissue for fat grafting: Problems and potential solutions. in eds. Coleman SR, Mazzola RF. Fat injection: From Filling to Regeneration. St. Louis: Quality Medical Publishing 2009; 85).
Acknowledgements
The author would like to express his sincere appreciation to Ms. Amy Dykstra for her secretarial support during the preparation of the manuscript.
References
- Coleman SR. Structural fat grafts: The ideal filler?. Clin Plast Surg 2001; 28:111 - 119
- Sommer B, Sattler. Current concepts of fat graft survival: Histology of aspirated adipose tissue and review of the literature. Dermatol Surg 2000; 26:1159 - 1166
- Shoshani O, Ullmann Y, Shupak A, Ramon Y, Gilhar A, Kehat I, et al. The role of frozen storage in preserving adipose tissue obtained by suction-assisted lipectomy for repeated fat injection procedures. Dermatol Surg 2001; 27:645 - 647
- Lidagoster MI, Cinelli PB, Levee EM, Sian CS. Comparison of autologous fat transfer in fresh refrigerated, and frozen specimens: an animal model. Ann Plast Surg 2000; 44:512 - 515
- MacRae JW, Tholpady SS, Ogle RC, Morgan RF. Ex vivo fat graft preservation: effects and implications of cryopreservation. Ann Plast Surg 2004; 52:281 - 283
- Wolter TP, Heimburg DV, Stoffels I, Groeger A, Pallua N. Cryopreservation of mature human adipocytes: In vitro measurement of viability. Ann Plast Surg 2005; 55:408 - 413
- Atik B, Ozturk G, Erdogan E, Tan O. Comparison of techniques for long-term storage of fat grafts: An experimental study. Plast Reconstr Surg 2006; 118:1533 - 1537
- Moscatello DK, Dougherty M, Narins RS, Lawrence N. Cryopreservation of human fat for soft tissue augmentation: Viability requires use of cryoprotectant and controlled freezing and storage. Dermatol Surg 2005; 31:1506 - 1510
- Pu LLQ. Re: Comparison of techniques for long-term storage of fat grafts. Plast Reconstr Surg 2007; 120:813
- Pu LLQ. Coleman SR, Mazzola RF. Cryopreservation of adipose tissue for fat grafting: Problems and solutions. Fat injection: From Filling to Regeneration 2009; St. Louis MO Quality Medical Publishing, Inc. 73 - 87
- Pegg DE. The history and principles of cryopreservation. Semin Reprod Med 2002; 20:5 - 13
- Gao DY, Critser JK. Mechanisms of cryoinjury in living cells. ILAR J 2000; 41:187 - 196
- Erdag G, Eroglu A, Morgan JR, Toner M. Cryopreservation of fetal skin is improved by extracellular trehalose. Cryobiology 2002; 44:218 - 228
- Pu LLQ, Cui X, Fink BF, Cibull ML, Gao D. Long-term preservation of adipose aspirates after conventional lipoplasty. Aesthetic Surg J 2004; 24:536 - 541
- Pu LLQ, Cui XD, Li JH, Fink BF, Gao DY. The fate of cryopreserved adipose aspirates after in vivo transplantation. Aesthetic Surg J 2006; 26:653 - 661
- Cui XD, Gao DY, Fink BF, Vascones HC, Pu LLQ. Cryopreservation of human adipose tissues. Cryobiology 2007; 55:269 - 278
- Ullmann Y, Hyams M, Ramon Y, Beach D, Peled IJ, Lindenbaum ES. Enhancing the survival of aspirated human fat injected into nude mice. Plast Reconstr Surg 1998; 101:1940 - 1944
- Ashjian PH, DeUgarte DA, Katz AJ, Hedrick MH. Lipoplasty: From body contouring to tissue engineering. Aesthetic Surg J 2002; 22:121 - 127
- DeUgarte DA, Ashjian PH, Elbarbary A, Hedrick MH. Future of fat as a raw material for tissue regeneration. Ann Plast Surg 2003; 50:215 - 219
- Zuk PA, Zhu M, Mizuno H, Hung J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng 2001; 7:211 - 218
- Ashjian PH, Elbarbary AS, Edmonds B, DeUgarte D, Zhu M, Zuk PA, et al. In vitro differentiation of human processed lipoaspirate cells into early neural progenitors. Plast Reconstr Surg 2003; 111:1922 - 1931
- DeUgarte DA, Morizono K, Elbarbary A, Alfonsa Z, Zuk PA, Zhu M, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 2003; 174:101 - 109
- Pu LLQ, Cui XD, Fink BF, Gao DY, Vasconez HC. Adipose aspirates as a source for human processed lipoaspirate cells after optimal cryopreservation. Plast Reconstr Surg 2006; 117:1845 - 1850
- Coleman SR. Structural fat grafting: More than a permanent filler. Plast Reconstr Surg 2006; 118:108 - 120