Abstract
For centuries, reconstructive surgeons have restored form and function with autografts. These techniques are highly effective, but they are associated invariably with donor site morbidity. To avoid this, surgeons have long dreamed of using cadaveric sources for reconstructive material. However, allografts have two major limitations: rejection and limited donor tissue. In order to limit rejection, the allograft must be rendered more tolerable to the host or the host must be rendered more tolerant of the allograft. Both strategies have been used with considerable success in recent years. As understanding of the human immune response increases, clinical immunosuppressive regimens will undoubtedly become less morbid, and the indications for allotransplantation will broaden. This will place an even greater burden on the already small donor pool. One way to relieve this burden would be through the development of strategies for the long-term preservation of donated tissues and organs. Cryopreservation has been used clinically for decades, and recent advances in the field have allowed the preservation of an ever widening array of tissues and organs. As cold storage has been shown to reduce the antigenicity of parts, cryopreservation may actually serve to improve the survival rate of transplanted parts, as well as increase their availability. As the era of autotransplantation gives way to the age of allotransplantation, cryopreservation will play an increasingly important role.
Introduction
The reconstruction of defects with autografts (tissue that is taken from one site and grafted to another site on the same person) has been performed for centuries. The first recorded use of autografts was by the early Ayurvedic practitioners in India, at least as early as the 6th century BCE.Citation1 Grafting techniques were reported sporadically over the intervening centuries, but consistent success was not achieved until the seminal experimental work of Boronio and Reverdin in the 19th century.Citation2,Citation3 Since that time, autografting has been the cornerstone of all reconstructive surgery. Over the years, a greater understanding of anatomy and physiology, as well as technical advances, have led to the development of advanced autografting techniques such as composite grafts, free microvascular flaps, and pre-fabricated flaps. These techniques have allowed today's reconstructive surgeons to restore form and function to patients with complex post-traumatic, post-ablative and congenital defects. However, these techniques have one fundamental drawback: they all create a donor site defect. Donor morbidity can be very minor, as in the case of a small skin graft, but can be quite severe in the case of more complex reconstructions. In addition, some defects, such as the loss of a hand or a severe facial disfigurement, cannot be adequately addressed with autologous reconstructive techniques.
Allotransplantation
Surgeons have long dreamed of using cadaveric donors as a source of reconstructive material. SchöneCitation4 in 1912 and LexerCitation5 in 1914 presented a series of experiments where allogeneic (from a cadaveric donor) and xenogeneic (from a non-human donor) skin grafts were transplanted to human recipients. They demonstrated that these grafts do not survive more than three weeks after transplantation. Additional evidence was provided the following decade by Padgett, who reported rejection of all skin allografts in a series of 40 patients. However, he observed that skin grafts exchanged between identical twins survived indefinitely.Citation6 The improved battlefield care of injuries in the Second World War meant that greater numbers of patients presented to secondary hospitals with severe burns or composite defects. Gibson, a plastic surgeon at the Glascow Royal Infirmary, gained extensive experience with skin allografts while treating pilots with severe burn injuries. He was the first to observe “second set rejection,” or the accelerated rejection of allogeneic tissue due to the presence of humeral antibodies from prior exposure to tissue from the same donor. After the war, Gibson joined Medawar and others in experimental work which better delineated the mechanisms of rejection. These studies laid the foundation for modern immunology and transplant biology.Citation7
Since their inception, the usefulness of allografts has been limited by two factors: rejection and limited donor tissues. Rejection of allogeneic tissue occurs through cellular and humoral immunologic responses. These responses are generated when the host immune system detects the expression of major histocompatibility complex (MHC) antigens on the surface of donor cells. This sets into motion a complex rejection amplification cascade resulting in the production of antibodies, antibody binding to allogeneic cell membrane, and cell destruction.Citation8 Researchers and clinicians have used two basic strategies to limit rejection following the transplantation of allogeneic tissue across an MHC barrier. Namely, the allograft must be rendered more tolerable to the host or the host must be rendered more tolerant of the allograft.
Avoiding Rejection I: The Graft
Since MHC antigens are expressed on the surface of cells, treatments which remove the cellular component from tissues of allogeneic origin can result in allografts which are relatively well tolerated by the recipient. Cadaveric bone is the most commonly used allogeneic material. It is prepared through low-dose irradiation, freezing or chemical sterilization.Citation9,Citation10 As very few, if any, donor cells survive this preparation the risk of rejection is essentially eliminated. The bone serves primarily as a scaffold for the ingrowth of recipient mesenchymal stem cells, which repopulate the donor bone by creeping substitution. The graft is usually well tolerated, but at a cost of elevated failure rates and long union times, as compared to autografts.Citation11
Skin is strongly antigenic, and skin allografts are subject to rejection, even in patients already receiving immunosuppression for an organ transplant.Citation12 Skin allografts are commonly used in severely burned patients for temporary coverage following burn excision. The immunocompromised state of patients after a major burn often delays rejection for several weeks. Skin can be rendered immunologically inert by removal of the epidermis and enzymatic treatment of the dermis to remove the cellular components. Processed, acellular dermis has been available commercially since 1996 (Alloderm®, LifeCell Corp., Branchburg, NJ). Dermal allografts have many applications, including abdominal wall and breast reconstruction.Citation13–Citation15 Using similar preparation techniques, acellular nerve allografts have been developed. The early clinical results with acellular nerve autografts has shown them to be comparable to autografts in the treatment of a peripheral nerve injury with a critical gap.Citation16
Allografts may find their most important application in the field of tissue engineering. One of the biggest hurdles to tissue engineering has been creating effective scaffolds, artificial structures capable of supporting three dimensional tissue formation. An ideal scaffold must be biocompatible, allow cell attachment and migration, deliver and retain cells and biochemical factors, and enable diffusion of vital cell nutrients and expressed products.Citation17 Acellular allografts can meet these requirements and in many cases serve as a more effective scaffold than any synthetic product. For example, the use of decellularized cancellous allograft bone in conjunction with recombinant human BMP-2 has largely replaced autograft in many orthopedic procedures.Citation18,Citation19 Nerve allografts have also been shown in animal models to be effective scaffolds and can be seeded with either Schwann cells or bone marrow stromal cells, resulting in improved nerve regeneration.Citation20,Citation21 Modifying the local environment with basic fibroblastic growth factor or nerve growth factor improved axonal repopulation.Citation22,Citation23 Most dramatically, tissue engineered heart valves have been constructed in vitro using decellularized allograft valves and human mesenchymal stem cells.Citation24 All of these tissue engineered allografts will have clinical applications in the near future. Despite these advances, however, the application of acellular allografts is limited to the reconstruction of small defects of a single tissue type. They are not suitable for large, composite defects.
Avoiding Rejection II: The Recipient
The second strategy for limiting rejection involves modification of the recipient immune system to tolerate the allotransplant. In organ and composite tissue transplantation, this usually takes the form of immunosuppressive drugs, which are used for induction, maintenance and treatment of rejection.Citation25 The aim of induction therapy is to silence the immune system so that it is unable to mount a response that leads to acute rejection. Currently, polyclonal antithymocyte globulins and monoclonal antibodies such as anti-CD3 (OKT-3) and anti-interleukin-2 receptor antibody are the most commonly used agents.Citation25–Citation27 The agents most commonly used for maintenance therapy include calcineurin inhibitors, such as cyclosporine and tacrolimus, which inhibit activation of T cells through suppression of interleukin-2 production by T-cells.Citation28 These immunosuppressive agents have significantly improved the life of transplant recipients; however, they are associated with severe and potentially life-threatening side-effects, including nephro- and neurotoxicity, hypertension, hyperglycemia and opportunistic infections.Citation25 The most serious complication of chronic immunosuppression is the risk of malignancy, which may be an effect of prolonged calcineurin inhibitors and antimetabolite therapy.Citation29 However, new pharmacologic strategies are being developed, such as selective T-cell depletion with monoclonal antibodies, which have more tolerable side-effect profiles.Citation30
The oldest strategy for the induction of tolerance is through chimerism, defined as the coexistence of two genetically distinct cell populations in the same organism.Citation31 Chimerism can be either full, when the recipient immune system is destroyed by myeloablation and replaced fully by donor hematopoietic cells, or mixed. Mixed chimerism describes a state wherein hematopoietic populations of both the recipient and donor coexist in the recipient and is achieved through nonmyeloablative host conditioning.Citation32 Long-term survival of limb allografts associated with the presence of stable donor-specific chimerism, without chronic immunosuppression, has been achieved in the rat model. This was achieved through T-cell depletion and short-term administration of calcineurin inhibitors.Citation33–Citation35 Recently, clinical trials of simultaneous kidney and donor bone marrow transplantation without long-term immunosuppression have resulted in successful graft acceptance.Citation36 As strategies for achieving tolerance become less morbid, the risk-benefit ratio of allotransplantation will undergo a fundamental change, with a dramatic broadening of the indications for transplantation.
The importance of MHC matching in the development of tolerance has been demonstrated in animal models and in the clinical setting. Bourget et al. demonstrated that tolerance to solid organ transplantation can be achieved by MHC matching and a short-term, 12-day course of cyclosporine.Citation37 In a series of experiments in the pig model, Lee et al. showed that tolerance to composite tissue allotransplants (CTAs) can be achieved using short-term cyclosporine and MHC-matched donors. Rejection was observed in most animals, however, when skin was incorporated in the CTA, and the authors theorized that topical cyclosporine may be a necessary component of a clinical application of this regimen.Citation38,Citation39 The induction of tolerance between a donor and a MHC-matched recipient without long-term immunosuppression is feasible for both solid organ transplantation and CTA, but the clinical application is limited by the difficulty of finding a matched donor.
A Limited Donor Pool
As the clinical methods of achieving immune tolerance become less morbid, the indications for organ transplantation and, especially, composite tissue allotransplantation, will likely broaden dramatically. Today's indications for free composite flaps, such as complex post-traumatic or post ablative defects, may be tomorrow's indication for composite tissue allotransplantation. Consider a patient with a large mandibular defect following resection of an oral cancer. It would be unconscionable for a surgeon to submit this patient to the harvest of a fibular flap, if he could be treated with a partial-face allotransplant with minimal morbidity and without the need for long-term immunosuppression (). These expanded indications will place additional strain on the already-limited donor pool. One way to improve the availability of donor composite tissues would be to devise strategies for long-term preservation of these tissues.
Cryopreservation
The successful cryopreservation of human embryos was reported by Wilmut and Whittingham in 1972,Citation40 and since that time there has been steady progress in the field. To date, most of the clinical applications of these technologies have been in fertility medicine. Sperm and embryos can now be reliably preserved. The cryopreservation of oocytes has been a clinical reality for the past two decades, and has been offered to prepubertal or adolescent cancer patients undergoing treatments resulting in the loss of fertility. However, the live birth rate per injected cryopreserved oocyte is approximately 2%. This is much lower than that with IVF using fresh oocytes.Citation41 The cryopreservation of ovarian cortical strips is a newer technique which has already yielded its first live births and is being offered increasingly to patients undergoing cancer treatments.Citation42
One of the fundamental tenets of cryobiology, demonstrated through several decades of study, is that intracellular crystal formation must be avoided during freezing for the cryopreservation of viable cells to be successful. A slow, controlled cooling rate allows the efflux of intracellular water prior to ice formation. At the same time, the cryoprotective agent (CPA) is added, which prevents cells from shrinking too quickly and thwarts the formation of intracellular ice.Citation40 Over the past decade, improved programmed cooling devices have allowed more precise control of intracellular crystal formation. In addition, refinements in the composition and application of cryoprotectant solutions have allowed more reliable survival of single cell lines and tissues in animal models. For the first time, whole organs have been successfully cryopreserved and transplanted.Citation43,Citation44
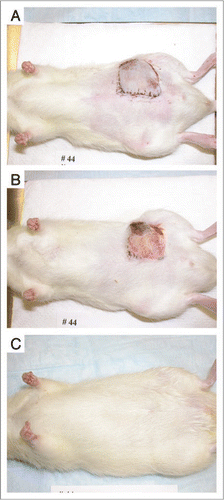
The cryopreservation of composite tissues poses technical challenges beyond those seen in the preservation of single tissue types or organs. The parameters of cryopreservation, such as freezing and thawing rates, concentration of CPA, or type of CPA which are ideal for one component tissue of a composite flap may not be suitable for another component. The successful cryopreservation of microvascular composite flaps consisting of skin, fat and blood vessels, has been achieved in the rat model ().Citation45 However, the parameters need to be refined before the cryopreservation of composite tissues can be reliably performed. These technologies, albeit in their infancy, have the potential to revolutionize organ and composite tissue transplantation. One may envision “tissue banks” where organs, limbs and other composite parts could be stored from the time they are harvested to when they are needed. This would allow much more precise MHC-matching than what is currently practiced.
The experience with blood vessels, tracheal grafts and solid organs demonstrates that cryopreservation can reduce the antigenicity of parts.Citation43,Citation46,Citation47 This suggests that cryopreservation may actually improve the survival of allotransplanted tissues as well as increase their availability. Due to the morbidity of long-term immunosuppression, the indications for composite tissue allotransplantation are very narrow. The worldwide experience with hand transplantation is fewer than 50 patients, and only a handful of partial face transplants have been performed.Citation48 These cases have been accompanied by intense scrutiny and debate over the ethical issues. These debates are appropriate and healthy, but will likely be rendered moot by future advances in transplant biology. As the immunosuppressive regimens become less morbid, today's indications for free tissue transfer will be tomorrow's indications for composite tissue allotransplantation. Methods of preserving and extending the “shelf life” of donated tissues will be critically important. As the age of the autotransplant gives way to the age of allotransplant, cryopreservation will play a central role.
Figures and Tables
Figure 1 (A) A 48 year old patient following free fibular osteocutaneous composite reconstruction of a defect involving mandible, chin, lower lip and oral mucosa. (B) The typical donor site defect. Today's indications for free tissue transfer will likely be tomorrow's indications for composite tissue allotransplantation.
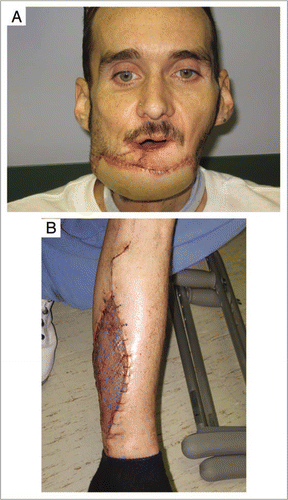
Figure 2 (A) Experimental animal on postoperative day one following microsurgical transplantation of a cryopreserved and thawed composite flap. (B) Appearance of transplant on postoperative day seven, with a small area of peripheral necrosis. (C) Appearance of transplant on postoperative day 60, with full survival of the transplant and normal hair growth.
References
- Bhishagratna KKL. The sushrute samhite 1963; (English translations based on original sanskrit text). Varanasi, Chowkhamba, Sanskrit series
- Gibson T. Early free grafting: The restitution of parts completely separated from the body. Br J Plast Surg 1965; 18:1 - 11
- Reverdin JL. Greffe epidermique. Bull Soc Imperiale Chir Paris 1869; 493
- Schöne G. Die heteroplastische und homöoplastische Transplantation. Eigene Untersuchungen und vergleichende Studien 1912; Berlin
- Lexer E. Die freien Transplantationen 1919; Stuttgart Ferdinand Enke
- Padgett EC. Is iso-skin grafting practicable?. South Med J 1932; 25:895
- Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature 1952; 172:603
- Auffray C, Stomiger JL. Molecular genetics of the human major histocompatibility complex. Adv Hum Genet 1985; 15:197
- Scarborough NL. Current procedures for banking allograft human bone. Orthopedics 1992; 15:1161
- Hardin CK. Banked bone. Otolaryngol Clin North Am 1994; 27:911
- Stevenson S, Horowitz M. The response to bone allografts. J Bone Joint Surg 1992; 74:939
- Silvers WK, Bartlett ST, Chen HD. Major histocompatibility complex restriction and transplantation immunity. A possible solution to the allograft proplem. Transplantation 1984; 37:28
- Patton JH Jr, Berry S, Kralovich KA. Use of human acellular dermal matrix in complex and contaminated abdominal wall reconstructions. Am J Surg 2007; 193:360 - 363
- Espinosa-de-los-Monteros A, de la Torre JI, Marrero I, Andrades P, Davis MR, Vasconez LO. Utilization of human cadaveric acellular dermis for abdominal hernia reconstruction. Ann Plast Surg 2007; 58:264 - 267
- Spear SL, Parikh PM, Reisin E, Menon NG. Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg 2008; 32:418 - 425
- Karabekmez FE, Duymaz A, Moran SL. Early clinical outcomes with the use of decellularized nerve allograft for repair of sensory defects within the hand. Hand (NY) 2009; [Epub ahead of print]
- Liu X, Ma PX. Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng 2004; 32:477 - 486
- Subach BR, Haid RW, Rodts GE, Kaiser MG. Bone morphogenetic protein in spinal fusion: overview and clinical update. Neurosurg Focus 2001; 10:3
- Jones AL, Bucholz RW, Bosse MJ, Mirza SK, Lyon TR, Webb LX, et al. BMP-2 Evaluation in Surgery for Tibial Trauma-Allgraft (BESTT-ALL) Study Group. Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J Bone Joint Surg Am 2006; 88:1431 - 1441
- Wang D, Liu XL, Zhu JK, Jiang L, Hu J, Zhang Y, et al. Bridging small-gap peripheral nerve defects using acellular nerve allograft implanted with autologous bone marrow stromal cells in primates. Brain Res 2008; 1188:44 - 53
- Aszmann OC, Korak KJ, Luegmair M, Frey M. Bridging critical nerve defects through an acellular homograft seeded with autologous schwann cells obtained from a regeneration neuroma of the proximal stump. J Reconstr Microsurg 2008; 24:151 - 158
- Ide C, Tohyama K, Tajima K, Endoh K, Sano K, Tamura M, et al. Long acellular nerve transplants for allogeneic grafting and the effects of basic fibroblast growth factor on the growth of regenerating axons in dogs: a preliminary report. Exp Neurol 1998; 154:99 - 112
- Yu H, Peng J, Sun H, Xu F, Zhang L, Zhao B, et al. Effect of controlled release nerve growth factor on repairing peripheral nerve defect by acellular nerve graft. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2008; 22:1373 - 1377
- Bin F, Yinglong L, Nin X, Kai F, Laifeng S, Xiaodong Z. Construction of tissue-engineered homograft bioprosthetic heart valves in vitro. ASAIO J 2006; 52:303 - 309
- Gorantla VS, Barker JH, Jones JW Jr. Immunosuppressive agents in transplantation: mechanism of action and current anti-rejection strategies. Microsurgery 2000; 20:420
- Yu X, Carpenter P, Anasetti C. Advances in transplantation tolerance. Lancet 2001; 357:1959
- Fehr T, Sykes M. Tolerance induction in clinical transplantation. Transpl Immunol 2004; 13:117
- Gerber DA, Bonham CA, Thomson AW. Immunosuppressive agents: recent developments in molecular action and clinical application. Transplant Proc 1998; 30:1573
- Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation 2005; 80:254
- Schneeberger S, Landin L, Kaufmann C, Gorantla VS, Brandacher G, Cavadas P, et al. Alemtuzumab: key for minimization of maintenance immunosuppression in reconstructive transplantation?. Transplant Proc 2009; 41:499 - 502
- Prabhune KA, Gorantla VS, Maldonado C, Perez-Abadia G, Barker JH, Ildstad ST. Mixed allogeneic chimerism and tolerance to composite tissue allografts. Microsurgery 2000; 20:441 - 447
- Wekerle T, Sykes M. Mixed chimerism and transplantation tolerance. Annu Rev Med 2001; 52:353
- Siemionow M, Ortak T, Izycki D. Induction of tolerance in composite tissue allografts. Transplantation 2002; 74:1211
- Ozer K, Oke R, Gurunluoglu R. Induction of tolerance to hind limb allografts in rats receiving cyclosporine A and antilymphocyte serum: effect of duration of the treatment. Transplantation 2003; 75:31
- Siemionow M, Izycki D, Zielinski M. Donor-specific tolerance in fully major histocompatibility complex-mismatched limb allograft transplants under an anti-alphabeta t-cell receptor monoclonal antibody and cyclosporine: a protocol. Transplantation 2003; 76:1662
- Fudaba Y, Spitzer TR, Shaffer J. Myeloma responses and tolerance following combined kidney and non-myeloablative marrow transplantation: in vivo and in vitro analyses. Am J Transplant 2006; 6:2121
- Bourget JL, Mathes DW, Nielsen GP, Randolph MA, Tanabe YN, Ferrara VR. Tolerance to musculoskeletal allografts with transient lymphocyte chimerism in miniature swine. Transplantation 2001; 71:851 - 856
- Lee WP, Rubin JP, Bourget JL, Cober SR, Randolph MA, Nielsen GP. Tolerance to limb tissue allografts between swine matched for major histocompatibility complex antigens. Plast Reconstr Surg 2001; 107:1482 - 1490
- Mathes DW, Randolph MA, Solari MG, Nazzal JA, Nielsen GP, Arn JS. Split tolerance to a composite tissue allograft in a swine model. Transplantation 2003; 75:25 - 31
- Mazur P. Freezing of living cells: mechanisms and implications. Am J Physiol 1984; 3:3 - 10
- Gosden RG. Prospects for oocyte banking and in vitro maturation. J Natl Cancer Instr Monogr 2005; 34:60 - 63
- Maltaris T, Beckmann MW, Dittrich R. Fertility preservation for young female cancer patients. In Vivo 2009; 23:123 - 130
- Tanaka H, Maeda K, Okita Y. Transplantation of the cryopreserved tracheal allograft in growing rabbits. J Pediatric Surg 2003; 38:1707 - 1711
- Yin H, Wang X, Kim SS, Chen H, Tan SL, Gosden RG. Transplantation of intact rat gonads using vascular anastomosis: effects of cryopreservation, ischaemia and genotype. Hum Reprod 2003; 18:1165 - 1172
- Cui X, Gao DY, Fink BF, Vasconez HC, Rinker B. Cryopreservation of composite tissues and transplantation: preliminary studies. Cryobiology 2007; 55:295 - 304
- Komorowska-Timek E, Zhang F, Shi D-Y. Effect of cryopreservation of patency and histological changes of arterial isogenetic and allogeneic grafts in the rat model. Ann Plast Surg 2002; 49:404 - 409
- Gu S, Liu CJ, Qiao T. Abdominal aorta transplantation after programmed cryopreservation. World J Gstroenterol 2004; 15:555 - 559
- Lanzetta M, Dubernard J-M. Hand Transplantation 2007; Milan Springer