Abstract
The Xenopus laevis cyclin dependent kinase inhibitor p27Xic1 has been shown to be involved in exit from the cell cycle and differentiation of cells into a quiescent state in the nervous system, muscle tissue, heart and retina. We show that p27Xic1 is expressed in the developing kidney in the nephrostomal regions. Using over-expression and morpholino oligonucleotide (MO) knock-down approaches we show normal levels of p27Xic1 regulate pronephros organ size by regulating cell cycle exit. Knock-down of p27Xic1 expression using a MO prevented myogenesis, as previously reported; an effect that subsequently inhibits pronephrogenesis. Furthermore, we show that normal levels of p27Xic1 are required for somite segmentation also through its cell cycle control function. Finally, we provide evidence to suggest correct paraxial mesoderm segmentation is not necessary for pronephric induction in the intermediate mesoderm. These results indicate novel developmental roles for p27Xic1, and reveal its differentiation function is not universally utilised in all developing tissues.
Introduction
In the amphibian Xenopus laevis, the first fully functional embryonic kidney to form is the pronephros. It is a paired organ, consisting of four distinct components; the glomus, coelomic cavity, nephrostomes and tubules (which are further characterized as proximal, intermediate, distal and connecting tubules), together these components form a non-integrated nephron.Citation1–Citation4 The pronephros develops from nephrogenic mesenchyme within the intermediate mesoderm lateral to the anterior somites.Citation5–Citation8 A signal from the somites establishes the intermediate mesoderm; therefore myogenesis is a necessary prerequisite to pronephrogenesis.Citation9–Citation11 The pronephros is a simple organ to study, showing both morphological and physiological similarities to more complex kidney forms, the meso- and metanephros, which make it an ideal model to study with reference to kidney development.Citation3
p21Cip1, p27Kip1 and p57Kip2 are mammalian members of the Cip/Kip family of Cdk inhibitors (CKIs) that play important roles in development, particularly in cell fate determination, in addition to their function of blocking cell cycle progression.Citation12 In Xenopus, three Cip/Kip family members have been described; p27Xic1, p16Xic2 and p17Xic3, the latter two are orthologues of p21Cip1 and p27Kip1 respectively.Citation13–Citation16 The expression of p16Xic2 and p17Xic3 is highly developmentally regulated, suggesting they might be involved in cell fate determination in a tissue-specific manner.Citation15 However, expression was not detected within the pronephros. The other homologue is p27Xic1, which shows structural and functional similarities to p21Cip1, p27Kip1 and p57Kip2.Citation16,Citation17 The expression of p27Xic1 in early and tail bud embryos has been described and shown to play a role in neurogenesis, myogenesis, gliogenesis and cardiogenesis where it regulates cell fate and differentiation, in a similar manner to mammalian homologues.Citation13,Citation14,Citation18–Citation20 Analysis of the p27Xic1 domains has revealed the region responsible for this regulatory effect overlaps with but is distinct from the Cdk-cyclin binding domain located in the N-terminus.Citation13,Citation14,Citation18 The phenotypic effect of p27Xic1 depletion in X. laevis, using morpholino oligonucleotides, on both muscle and neural development can be rescued by co-injecting the human homologue p21Cip1.Citation13,Citation14 If p27Xic1 functions in a similar manner to the mammalian p21Cip1, then it may be involved during development of the Xenopus kidney.
Here, we describe the characterization of p27Xic1 in X. laevis pronephric development. We report that p27Xic1 expression is detected within the developing pronephros at tadpole stages by in situ hybridization. We interfere with the normal expression of this gene by targeted overexpression, or knock-down of the endogenous protein with a specific antisense morpholino oligonucleotide (MO). Overexpression of p27Xic1 disrupted somitogenesis and reduced the size of the pronephric anlagen. MO knock-down also reduced the size of the pronephric anlagen and inhibited myogenesis. Pronephric phenotypes were lost upon injection of a mutant of p27Xic1 that had inactivated cyclin/cdk binding capabilities, suggesting p27Xic1 is not involved in regulating differentiation in the pronephros. These experiments identify previously unrecognized, functional roles for p27Xic1 in X. laevis pronephros development and somitogenesis and confirm the importance of this gene in diverse developmental processes.
Results
p27Xic1 expression in the pronephros.
p27Xic1 expression has been previously shown in the notochord, neural plate, differentiating muscle and in the eye and brain but its distribution has not been reported past stage 30 in whole embryos.Citation13,Citation14,Citation19,Citation21 To analyse p27Xic1 expression in pronephric development, we investigated expression in these stages and later using an improved whole mount in situ hybridization protocol.
p27Xic1 expression in early stage embryos followed similar domains to that previously reported (data not shown) but is also detected initially in the dorso-anterior part of the pronephric region and then in the region corresponding to the nephrostomes between stages 27 and 32 (). Prior to and past these stages no pronephric expression of p27Xic1 was detected by in situ hybridization. In conclusion, p27Xic1 expression is temporally and spatially appropriate for a role in pronephros development.
p27Xic1 and p21Cip1 mRNA overexpression inhibited pronephric development.
In order to examine the overexpression phenotype of p27Xic1 mRNA in vivo, we injected p27Xic1 mRNA and p21Cip1 mRNA into a single V2 blastomere of embryos at the 8-cell stage, βgal mRNA was co-injected to act as a lineage tracer. This targeted mRNA to the developing pronephric and somitic compartments. All microinjections described subsequently are carried out in this way unless otherwise stated. Little βgal staining was observed in pronephroi due to its immunostaining by antibody or in situ analysis. However, correct targeting could be determined by βgal staining in the somites. In each case the largest amount of mRNA (20–50 pg) that did not cause gross developmental defects was injected. Injected embryos were cultured to stage 41, correctly targeted embryos were sorted for βgal staining and then subjected to whole mount antibody staining with 3G8, which detects nephrostomes and proximal pronephric tubules, and 4A6, which detects intermediate and distal pronephric tubules.Citation3 The effects on pronephric structure were scored by comparing the lineage labelled injected side against the control, uninjected side, of the same embryo and Chi squared analysis was carried out to assess the statistical significance. All experiments involving embryo microinjection were repeated multiple times and each time statistical analysis was carried out. Due to slight differences in embryo batches, results from individual repeat experiments were not pooled, a representative experiment is quoted in each case. Control embryos injected with βgal mRNA alone had a negligible effect on proximal tubule (2% reduced, n = 58) or intermediate and distal tubule (0% affected, n = 58) morphology (). In embryos injected with p27Xic1 mRNA, the proximal tubules were reduced on the injected side in 91% of embryos (n = 23) (). Inspection of the intermediate and distal tubules revealed that p27Xic1 mRNA also reduced their size to a similar degree (83%, n = 23) (). Chi-squared analysis confirmed that the pronephros of p27Xic1 mRNA injected embryos was significantly reduced in size when compared to βgal mRNA injected embryos (p < 0.05).
p21Cip1 has functional and sequence homology with p27Xic1 and has been used to rescue the effect of the p27Xic1 MO.Citation13 Injecting p21Cip1 mRNA produced a similar phenotype to that of p27Xic1 mRNA (), the pronephric tubules were statistically significantly reduced in 82% of embryos (n = 52, p < 0.05).
The p27Xic1 pronephric phenotype is dependent on the N-terminus of the protein.
p27Xic1 possesses three characteristic domains (Fig. S1A). The N-terminal half contains a Cyclin/Cdk binding domain, highly conserved between p21Cip1, p27Kip1 and p57Kip2. The C-terminal half possesses a PCNA-binding domain, also found in p21Cip1, and a QT-domain, a potential cdc2 phosphorylation site that occurs in p27Kip1.Citation22,Citation23 Similar to full length p27Xic1, p27Xic1 N (amino acids 1–96) inhibits Cyclin/Cdk kinase activity and stops cell division before MBT. p27Xic1 C (amino acids 97–210) can inhibit PCNA driven replication but cannot stop cell division before MBT.Citation19 A third construct, p27Xic1 #2 (amino acids 35–96), possesses Cdk2 inhibitory activity but has been shown to lack the Müller cell inductive activity observed with p27Xic1 N in the Xenopus retinaCitation19 and lack the ability to promote primary neurogenesis.Citation13 In order to investigate the effect these constructs had on pronephric development, we injected p27Xic1 N mRNA, p27Xic1 C mRNA and p27Xic1 #2 mRNA into a V2 blastomere as previously described. Inspection of p27Xic1 N mRNA injected embryos, revealed a significant reduction in proximal tubule (58% reduced, n = 33, p < 0.05) and intermediate and distal tubule (40% reduced, n = 33, p < 0.05) morphology similar to full length p27Xic1 mRNA when compared against the control, un-injected side (). Therefore, p27Xic1 N, like the full length protein, perturbed pronephric development. In contrast, analysis of pronephric tubule morphology after p27Xic1 #2 mRNA (n = 34) and p27Xic1 C mRNA (n = 32) injections showed 0% effect on the pronephric development, similar to control embryos injected with βgal mRNA ( and F). In the absence of western analysis comparing the amounts of protein translated from the different constructs, it is formally possible that the differences in phenotype observed are due to differences in protein amounts as a consequence of different stability or translation efficiency.
In order to separate the effects of overexpressing p27Xic1 on cell cycle from potential later roles in differentiation, we injected a mutant of p27Xic1 that had inactivated cyclin and cdk binding domains, we term this mutant p27Xic1 CK− (Fig. S1B illustrates sites of mutagenesis, this mutant was a kind gift from Mehregan Movassagh, Cambridge University). Injection of p27Xic1 CK− mRNA failed to show a significant pronephric phenotype at stage 41, as observed by whole mount 3G8/ 4A6 immunostaining (8% reduced, n = 28, p > 0.05) ().
p27Xic1 depletion using p27Xic1 MO reduced the size of the pronephric tubules and glomus.
To investigate whether depletion of p27Xic1 affects pronephric development, we utilized a previously characterized anti-sense morpholino oligonucleotide (MO) designed to be complementary to p27Xic1, and shown to specifically knock down p27Xic1 translation in vivo in our hands by western blot analysisCitation13,Citation20 (for MO sequence see Fig. S1C). Indeed, we have shown p27Xic1 MO knocked down p27Xic1 translation completely in vitro ( and S3). To determine if p27Xic1 depletion affected development of the pronephros, we targeted the p27Xic1 MO with βgal mRNA to the pronephric region as previously described. Embryos were allowed to develop to stage 32, when they were fixed and whole mount in situ hybridized for nephrin expression (a glomus marker), or were allowed to develop to stage 41 where whole mount antibody staining using 3G8 and 4A6 was performed. Injection of a Control MO had no effect on glomus development, reducing nephrin expression in a statistically insignificant 3% of embryos (n = 61, ), confirming that the process of injecting a random sequence MO does not adversely affect development. MO knock-down of p27Xic1 inhibited glomus development, 86% had reduced nephrin expression (n = 22, ). Overexpressing p27Xic1 reduced nephrin expression in 66% of embryos scored (n = 48, ). Additionally, injection of a Control MO had 0% effect on the morphology of the pronephric tubules (n = 24) as observed by 3G8 and 4A6 antibody staining (). However, 3G8 immunostaining was reduced after p27Xic1 MO injection in 93% of embryos (n = 27) (). Analysis of the intermediate and distal tubule morphologies revealed p27Xic1 MO reduced their size in 81% of embryos (n = 27).
Co-injecting p21Cip1 with p27Xic1 MO rescued development of the pronephros.
Since p21Cip1 has functional homology with p27Xic1, but is not translationally inhibited by the p27Xic1 MO (Fig. S3),Citation13 we attempted to rescue the p27Xic1 MO phenotype by coinjecting p21Cip1 mRNA with the p27Xic1 MO into a V2 blastomere of an 8-cell stage embryo. Embryos were co-injected with 20 ng p27Xic1 MO, 20 pg p21Cip1 mRNA, and βgal mRNA to act as a lineage tracer, and cultured to stage 41. Correctly targeted embryos were selected and whole mount antibody stained using 3G8 and 4A6. Alongside these co-injections, single injections of 20 ng p27Xic1 MO and 20 pg p21Cip1 mRNA were also performed to act as controls. Both p27Xic1 MO (78% reduced, n = 27) and p21Cip1 mRNA (91% reduced, n = 34) inhibited pronephros development. However when 20 ng p27Xic1 MO was coinjected together with 20 pg p21Cip1 mRNA pronephrogenesis is almost completely rescued, only a very small proportion of embryos had a reduced pronephros (9%, n = 23, p > 0.05, ). We have also attempted this rescue experiment with 10 pg p21Cip1 mRNA co-injected with 20 ng p27Xic1 MO. At these concentrations, we observed a reduction in pronephros size (58%, n = 26, data not shown).
The anti-apoptotic protein BclXL had no effect on pronephric phenotypes observed when p27Xic1 is overexpressed or depleted using a MO.
Programmed cell death (PCD) is a key developmental process required by multi-cellular organisms to eliminate unwanted cells. Recently, molecular connections between cell cycle exit and PCD have been established.Citation24 We have previously shown injection of p27Xic1 mRNA and the p27Xic1 MO, at the concentrations used here, did not increase levels of apoptosis during early development.Citation13,Citation14,Citation19,Citation20 To confirm this result, and ensure phenotypic pronephric reductions after p27Xic1 mis-expression were not a result of ectopic apoptosis, we inhibited apoptosis by co-injecting the apoptotic inhibitor BclXL with p27Xic1 mRNA or p27Xic1 MO. Injecting BclXL mRNA with βgal mRNA into one cell of a two cell stage embryo significantly reduced apoptosis in 36% of embryos assayed by TUNEL analysis at stage 22 (n = 55, p < 0.05, Fig. S4), indicating the correct biological activity of this mRNA. We injected p27Xic1 mRNA and p27Xic1 MO with or without BclXL mRNA into one cell of a two cell stage embryo and left the embryos to develop to stage 22. Expression of Lim-1, an early marker for the pronephros anlagen, was then detected by in situ hybridization (). The pronephros anlagen was reduced in 92% of embryos injected with the p27Xic1 MO (n = 24) () and 74% of embryos injected with p27Xic1 mRNA (n = 38) (). The same phenotypes with similar degrees of severity were observed when BclXL was co-injected with the p27Xic1 MO (97% reduced, n = 41) () and p27Xic1 mRNA (84% reduced, n = 37) (). In conclusion, PCD is not the mechanism by which pronephrogenesis is inhibited following injection of p27Xic1 mRNA and p27Xic1 MO.
Overexpression of p27Xic1 reduced cell division, but MO depletion of p27Xic1 had no effect.
To test whether an effect on cell cycle could explain the pronephric phenotype, we injected p27Xic1 mRNA and p27Xic1 MO into one cell of a two cell stage embryo and allowed development to proceed to stage 20, where Lim-1 in situ hybridizations and anti-phosphohistone H3 (pH3, a marker of dividing cells) immunostains were performed on the same embryos. Overexpressing p27Xic1 reduced Lim-1 expression in 75% of scored embryos (n = 24), similarly 84% of embryos injected with the p27Xic1 MO had reduced Lim-1 expression (n = 31) ( and C). Injection of p27Xic1 mRNA reduced the total number of pH3 immunostained cells on the injected side by 63% on average (n = 11) (). However, MO depletion of p27Xic1 had no statistically significant effect on pH3 immunostaining. Injection of the Control MO had no significant effect on either Lim-1 expression (2% reduced, n = 55) or pH3 immunostaining (4% average reduction, n = 10) (). Injection of the cyclin/cdk mutant p27Xic1 CK− mRNA showed that on average out of 10 embryos numerically scored for total pH3 immunostained cells, there was a statistically insignificant increase of 3.44% on the injected side over the un-injected side (p > 0.05) ( and E), confirming the lack of cell cycle inhibitory function in this mutant.
These results suggest that overexpressing p27Xic1 caused premature cell cycle exit, reducing the number of cells contributing to the anlagen, and therefore decreasing pronephros size. In contrast, reduction in size of the pronephros following p27Xic1 MO knock-down could not be attributed to a cell cycle effect.
Loss of p27Xic1 inhibits the development of myotomal muscle without affecting early mesoderm formation.
To determine if p27Xic1 depletion induced gross developmental defects which could inhibit pronephrogenesis, we first injected the equatorial region of both cells of a 2-cell stage embryo with the p27Xic1 MO, along with βgal mRNA to act as a lineage tracer. Embryos were left to develop to stage 10/11, when they were fixed, stained for the lineage label and whole mount in situ hybridized for a marker of the early mesoderm, Xbrachyury (Xbra).Citation25 Injection of the Control MO had 0% effect on Xbra expression (n = 73, ). Injection of the p27Xic1 MO also caused no effect (5% reduction n = 105, ). Although, V2 blastomere injections of the Control MO had no effect on MyoD or Lim-1 expression (n = 36) ( and S5Aa), injections of p27Xic1 MO caused severe reduction in expression of both the muscle marker MyoD, and an early pronephric anlagen marker, Lim-1 (79%, n = 33) ( and S5Ab). We suggest normal levels of p27Xic1 are required for myogenesis, the first phase of somite development, as loss of p27Xic1 expression inhibited MyoD expression. As early mesoderm development is not perturbed by p27Xic1 depletion, we suggest this effect on myogenesis is direct.
Overexpression of p27Xic1 results in segmentation defects in somites.
To test the effects overexpressing p27Xic1 had on myogenesis and somitogenesis, we injected p27Xic1 mRNA into a V2 blastomere of embryos at the 8-cell stage. The embryos were left to develop to stage 24, then fixed and subjected to double in situ hybridization for MyoD and Lim-1 expression. Overexpressing p27Xic1 caused the expected reduction in Lim-1 expression in 78% of embryos (n = 27) (). 76% of these affected embryos also had fused, un-segmented somites, indicated by MyoD and MHC in situ analysis (MHC data not shown), without reducing MyoD and MHC expression. This effect could be attributed to cell cycle function since no significant effect on the pronephros anlagen formation or myogenesis, as seen by Lim-1/MyoD double in situ hybridizations, was observed (4.7% reduced Lim-1, 0% disrupted MyoD, n = 43) after p27Xic1 CK− overexpression (). Thus we conclude that the reduced pronephros phenotype and the non-segmented somite phenotype we observed when we overexpressed p27Xic1 were due to premature cell cycle exit through inhibition of cyclinA2/cdk2 activity.
Inhibiting cell division using hydroxyurea and aphidicolin prevented formation of the pronephros and disrupted segmentation of the somites.
To confirm that the reduction in the size of the pronephros we observed when we overexpressed p27Xic1 mRNA could be achieved by premature cell cycle exit, we interfered with normal cell division by incubating embryos from stage 10.5 in the cell division inhibitors Hydroxyurea and Aphidicolin (HUA).Citation27 HUA treatment inhibited cell division, as observed by an average 82% reduction in the number of positively stained pH3 cells on the left side of the embryos treated with HUA compared to the controls (Fig. S5, n = 10). At stage 41, 3G8/4A6 antibody staining was reduced in all embryos treated with HUA, with 4A6 staining completely absent (n = 14) (). At these later stages embryos incubated in HUA had severely perturbed and delayed development. HUA treatment, like p27Xic1 mRNA overexpression, inhibited pronephros anlagen formation and disrupted somite segmentation in all the embryos treated (n = 17) ( and C; S7A and B). Incubation of embryos in HUA from stage 17 had no effect on muscle segmentation or pronephros anlagen formation (Fig. S7C and D).
p35.1 overexpression disrupted somitic segmentation, but had no lasting effect on mature pronephros development.
p35.1 is a member of the p35 family, a group of proteins that bind to and activate cdk5. Philpott et al. showed p35.1 mRNA overexpression disrupted somite segmentation in a similar manner to our p27Xic1 mRNA overexpression phenotype.Citation28 Reduced myogenesis is known to inhibit pronephrogenesis.Citation9–Citation11 To observe if disrupted somite segmentation affects pronephros development we injected p35.1 mRNA. Embryos injected into a V2 blastomere were then cultured to stage 24, where Lim-1/MyoD double in situ hybridizations were performed, and stage 41, where whole mount 3G8/4A6 immunostaining was carried out. At stage 24 we observed a similar muscle phenotype to that of overexpression of p27Xic1 mRNA, 69% of embryos had disrupted somite segmentation (n = 23). However, in 75% of embryos with disrupted somite segmentation Lim-1 expression covered a broader domain, but was not reduced ( and B). The remaining embryos showed no Lim-1 expression defects. At stage 41, p35.1 mRNA overexpression had no statistically significant effect on 3G8/4A6 staining (p > 0.05, n = 24, ). Thus the early broader domain of the pronephros anlagen induced by p35.1 mRNA injection was sufficient to form a mature pronephros in later stage embryos. In conclusion, we suggest correct somite segmentation is not essential for pronephros development.
Discussion
The expression of p27Xic1 has been previously shown in the differentiating muscle and notochord at neurula stages and at lower levels in the neural plate and heart.Citation13,Citation19,Citation20 We show by in situ hybridization and RT-PCR for the first time, p27Xic1 is expressed within the pronephros during development. Strong expression in the dorso-anterior region of the pronephros between stages 27 and 32, suggest p27Xic1 could have a role in pronephric development. To ascertain whether p27Xic1 is required for pronephros development, overexpression and depletion studies were performed.
Overexpressing p27Xic1 inhibited formation of all pronephric compartments. Injection of different domains of p27Xic1 mapped the N-terminus (amino acids 1–96) as the effector region inhibiting pronephrogenesis. Moreover, depleting endogenous p27Xic1 translation using a MO also inhibited development of distinct pronephric regions. We have demonstrated that overexpression and MO knock down of p27Xic1 indirectly inhibited formation of the pronephros, by different mechanisms. We have shown ectopic apoptosis was not a mechanism by which pronephros development was inhibited, and previous studies have shown that p27Xic1 mis-expression does not increase levels of apoptosis.Citation13,Citation14,Citation19,Citation20
p27Xic1 overexpression inhibited pronephros development as premature cell cycle exit reduced the number of cells in the anlagen.
Overexpressing p27Xic1 inhibited cell division and injection of the cyclin and cdk binding mutant, p27Xic1 CK−, had no effect on cell division or pronephros development. Thus injection of p27Xic1 mRNA impeded pronephrogenesis by inhibiting cdk2 kinase activity. Recent research has identified CKIs such as p27Xic1 and the mammalian Cip/Kip family as multifunctional proteins involved not only in cell cycle exit but also cell differentiation and migration.Citation29 p27Xic1 has been shown to be involved in muscle, neural and heart differentiation, independent of its cell cycle exit function.Citation13,Citation14,Citation20 p21-/- mutant mice have hypomyelinated cerebella due to failure of oligodendrocyte differentiation, importantly these mice had no aberrant cell cycle exit phenotypes.Citation30 p27Kip1 has been shown to be involved in neuronal differentiation and migration.Citation31 The N-terminus of p27Kip1 contains both the conserved cell cycle exit function and an uncharacterized differentiation function with the ability to stabilize Neurogenin-2. Nguyen et al. used a mouse homozygous for a cell cycle mutant allele of p27Kip1, p27CK−, to show the increase they observed in neuronal markers was independent of cell cycle exit. We overexpressed a similar cyclin/cdk mutant, p27Xic1 CK− which failed to show a pronephric phenotype. This suggests overexpression of p27Xic1, with concomitant cell cycle arrest, results in failure of the pronephric anlagen to proliferate. The function of p27Xic1 in myotome differentiation is separable from its ability to slow the cell cycle,Citation14 so this differentiation function of p27Xic1 in the myotome will affect pronephric induction as myogenesis is a necessary prerequisite to pronephrogenesis.Citation9–Citation11 Since p27Xic1 CK− mRNA retains the differentiation function, but did not alter pronephrogenesis, we suggest that in contrast to other organs, pronephros development is only affected by cell cycle effects. Thus the effects of CKIs on differentiation may vary between tissues, for example p21Cip1 overexpression inhibits keratinocyte differentiationCitation32 but promotes myelomonocytic leukemia cell differentiation.Citation33
Knock-down of p27Xic1 expression using a MO inhibited pronephros development by preventing myogenesis.
One of the earliest signals to the intermediate mesoderm to form pronephros originates from the anterior somites.Citation9–Citation11 Thus inhibition of myogenesis by the p27Xic1 MO may be sufficient to inhibit pronephros formation. Therefore, we suggest the p27Xic1 MO pronephric phenotype arises as an indirect effect, reduced myotome formation failing to induce the pronephric anlagen. Since MO knockdown of p27Xic1 expression did not inhibit expression of the early mesodermal marker Xbrachyury, our experiments suggest that the effect of p27Xic1 depletion on myogenesis was direct, and not a consequence of perturbed early mesoderm development.
MyoD has been shown to be stabilized by p57Kip2 in growing myoblasts through repression of Cyclin E/Cdk2 phosphorylation of MyoD, and by direct binding to and stabilization of MyoD.Citation34,Citation35 It is possible p27Xic1 interacts directly with MyoD, which would promote MyoD expression due to the positive feedback loop it has on its own expression.Citation36 This would explain why MyoD expression is lost in p27Xic1 depleted embryos. Moreover, MyoD has been shown to stimulate expression of p21Cip1 in C2C12 cells and further control cell proliferation by acting as a negative intercessor of MRF4, which accelerates cell proliferation.Citation37 Whether MyoD induces expression of cell cycle inhibitors to ensure cell cycle exit alone, or in addition to ensuring its stability in the cell, is not known.
p27Xic1 is necessary for co-ordinating cell cycle exit to aid segmentation of the somites.
The clock and wavelength model of segmentationCitation38 requires synchronous cell division to aid allocation of somites within the presomitic mesoderm. In the myotome, p27Xic1 expression overlaps with MyoD expression from stage 15.Citation14 Thus expression of p27Xic1 is temporally and spatially appropriate to have a role in directing cell cycle exit, and therefore somitogenesis. During gastrulation, mesodermal cells in the primitive streak divide synchronously in mice and chick embryos,Citation39–Citation41 this synchrony is maintained in somitogenic cells. Chick embryos incubated for 24 hours in S-phase inhibitors such as hydroxyurea display fused somites as a consequence of undirected cell cycle exit.Citation42 p27Xic1 is a cell cycle inhibitor, thus the non-segmentation pattern observed when p27Xic1 is overexpressed is not surprising. Nevertheless, this result establishes a previously unrecognized role for p27Xic1 in somite segmentation in X. laevis.
Muscle formation is required for pronephros development, but correct segmental organization is not.
The p27Xic1 MO reduced the somite size, as observed by reduced MyoD expression, consequently inhibiting pronephrogenesis. However, when p27Xic1 mRNA was overexpressed somite formed, but segmentation was disrupted. To see if disrupted muscle development inhibited pronephrogenesis we overexpressed p35.1, a gene known to disrupt somite segmentation but which is not expressed in the pronephros.Citation28 These embryos displayed a slightly enlarged pronephros anlagen which subsequently recovered to form a normal mature pronephros at stage 41. Thus disrupted somite segmentation alone is insufficient to affect development of a mature pronephros. Overexpression of p27Xic1 did reduce mature pronephros development as well as disrupting somite segmentation, thus normal levels of p27Xic1 are essential for pronephrogenesis. We suggest this additional effect is due to premature cell cycle exit reducing the number of cells that can contribute to the pronephros anlagen. Thus in the pronephros, p27Xic1 does not promote differentiation like has been observed in primary neurogenesis and cardiogenesis.Citation13,Citation20
Materials and Methods
Whole-mount in situ hybridization.
Whole-mount in situ hybridization was carried out as described elsewhere.Citation43 The embryos were fixed in MEMFA (0.5 M MOPS, pH 7.4, 100 mM EGTA, 1 mM MgSO4, 4% formaldehyde) and linearized plasmid from p27Xic1 (XhoI/pBS), MyoD (HindIII/T7), MHC (NcoI/SP6) and Lim-1 (XhoI/T7) was used to generate digoxigenin-11-UTP-labelled (Boehringer Mannheim) antisense RNA probes from the polymerases indicated. Probes were visualized using anti-DIG-alkaline phosphatase secondary and NBT/BCIP for the color reaction according to the manufacturer's recommendations (Boehringer Mannheim).
Embryo culture.
Embryos were obtained by in vitro fertilization of hormonally stimulated Xenopus laevis and staged according to Nieuwkoop and Faber.Citation1 Standard embryological procedures were used as described by Jones and Woodland.Citation44 Embryos were dejellied in 2% cysteine hydrochloride pH 8 and cultured in 1/10th BarthX.
mRNA synthesis, morpholino antisense oligonucleotides and micro-injection.
Capped RNAs were synthesized in vitro from p27Xic1,Citation16 p27Xic1 N, p27Xic1 C, p27Xic1 #2,Citation19 p21Cip1,Citation45 p35.1,Citation28 and p27Xic1 CK− using the SP6 Message Machine Kit (Ambion). Pod-1 mRNACitation46 was synthesized using T7 Message Machine Kit (Ambion). Typically 50 pg p27Xic1, p27Xic1 CK− and p27Xic1 N, 20 pg p21Cip1, 80 pg p27Xic1 #2 and p27Xic1 C, 2 ng p35.1 and 400 pg βgal mRNA was injected. The p27Xic1 antisense morpholino oligonucleotide used was: 5′-GCA GGG CGA TGT GGA AAG CAG CCA T-3′ and Pod-1 MO is: 5′-CGG TGG ACA TGA TCT GTT ATG CTG C-3′ (Gene Tools LLC). The control morpholino is a random sequence of the same length. Typically 20 ng of p27Xic1 MO and Control MO was injected. Embryos were dejellied and injected with mRNA alone or in combination with MO (as specified in the text) into one blastomere of a 2-cell stage embryo or a V2 blastomere of an 8-cell stage embryo to target the future pronephros,47,48 under 5% Ficoll in BarthX.
Immunohistochemistry.
Whole mount immunohistochemistry was performed using standard methods on MEMFA fixed embryos. The primary antibodies used were monoclonal antibody 3G8, which detects the proximal tubules, and 4A6, which detects the intermediate and distal tubules.Citation3 The secondary antibody was alkaline phosphatase-conjugated goat anti-mouse (Sigma). BCIP/NBT (Boehringer) or Fast Red TR/Napthol AS/MX (Sigma) was used for the color reaction, according to the manufacturer's recommendations. The primary antibody used to detect phosphohistone H3 was rabbit polyclonal IgG anti-phospho- Histone H3 (Ser10) (Upstate). For these experiments the secondary antibody was alkaline phosphatase-conjugated goat anti-rabbit (Sigma).
RT-PCR.
Total RNA from whole embryos and pronephroi were isolated and used for RT-PCR as described by Barnett et al.Citation49 Primers used in this study are as follows. p27Xic1 primers: 5′-CAT CGA GCT CAG CAC TCA CA-3′ and 5′-GAC AGT CGG ACG CCT GGA TT-3′ (this work). ODC primers: 5′-GGA GCT GCA AGT TGG AGA-3′ and 5′-TCA GTT GCC AGT GTG TGG TC-3′.Citation50
In vitro translations of p27Xic1 construct mRNA.
mRNA alone, or with MO, was translated in vitro in the Rabbit Reticulocyte Lysate System (Promega) with 35S-Met according to the manufacturer's protocol. Reactions (5 µl) were denatured at 95°C in 2xSDS loading buffer51 and run on a 12% (w/v) SDS-PAGE resolving gel using a vertical minigel apparatus for 2 hour at 100 V. The gel was exposed to Kodak X-ray film overnight at room temperature, before being developed.
Figures and Tables
Figure 1 p27Xic1 is expressed at late tail bud stages in the pronephros by in situ analysis. Whole mount in situ hybridisation was carried out with a DIG-labelled anti-sense RNA probe for p27Xic1. Prior to and including stage 25, p27Xic1 expression in the presumptive pronephros is not detected by in situ hybridisation. At stage 27 p27Xic1 expression is clearly evident in the dorso-anterior region of the presumptive pronephros (white arrow). Pronephric staining is confirmed in transverse sections at stage 27 through the anterior pronephros anlagen (white arrow). At stage 30 a similar pattern of p27Xic1 expression to that seen at stage 27 is observed in the dorsal anterior pronephros anlagen. By stage 32 p27Xic1 expression is concentrated in the nephrostomes and proximal tubules. No pronephric expression of p27Xic1 is detected by in situ hybridisation at stage 38. (som, somites; nt, neural tube; ba, branchial arches; pr, pronephros; lh, lymph heart; ma, migrating muscle anlagen).
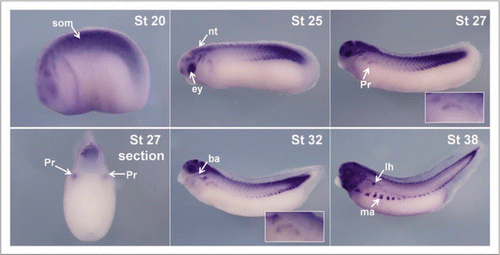
Figure 2 Overexpression of p27Xic1 identifies a pronephric phenotype. X. laevis embryos were injected at the 8 cell stage into a V2 blastomere to target the somites and presumptive pronephric region. mRNA of the construct indicated was co-injected with βgal mRNA to act as a lineage tracer (stained blue, black arrow). Embryos were cultured to stage 41 and whole mount antibody stained with 3G8, to detect proximal pronephric tubules (white arrow, stained purple), and 4A6, to detect intermediate and distal pronephric tubules (white arrowheads, stained red). Control βgal mRNA injected embryos had normal pronephros development (A). p27Xic1 mRNA and p21Cip1 mRNA injections inhibited proximal, intermediate and distal tubule development on the injected side (B and C). p27Xic1 N mRNA reduced the size of the pronephros, as indicated by reduced 3G8 and 4A6 staining (D). p27Xic1 #2 mRNA, p27Xic1 C mRNA and p27Xic1 CK− mRNA injections had no effect on pronephros development (E–G). These results are displayed in a histogram (H). *denotes injected side.
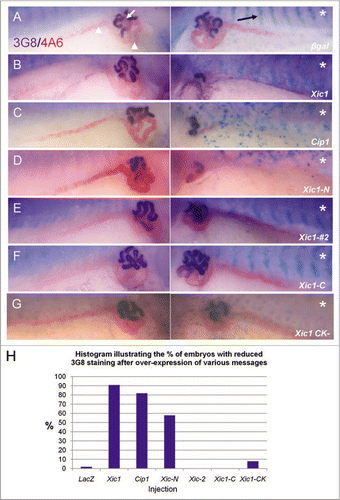
Figure 3 Inhibition of endogenous p27Xic1 mRNA translation using a specific MO identified a pronephros phenotype. X. laevis embryos were injected at the 8 cell stage into a V2 blastomere to target the somites and presumptive pronephric region. βgal mRNA was co-injected to act as a lineage tracer (blue staining in B–D and red staining in E–G). Embryos were cultured till stage 32, where they were whole mount in situ hybridised for nephrin expression (a marker of the glomus, A–C), or stage 41, where they were whole mount antibody stained with 3G8 and 4A6 to detect tubules (D–F). Control MO injected embryos had normal glomus development (A) and tubulogenesis (D). Injection of p27Xic1 MO inhibited formation of the glomus (B) and proximal, intermediate and distal tubules on the injected side (e). Overexpression of p27Xic1 also inhibited nephrin expression (C). p21Cip1 mRNA almost completely rescued development of the pronephros when co-injected with the p27Xic1 MO (F). *denotes injected side.
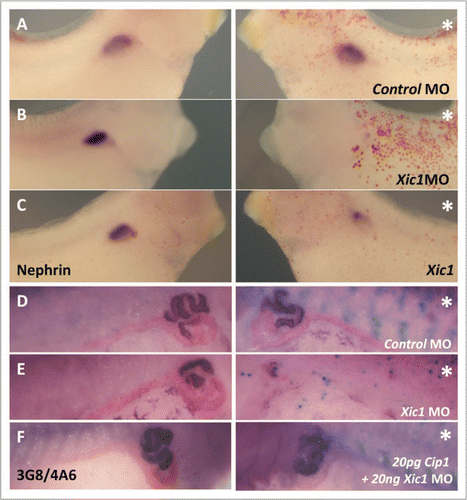
Figure 4 p27Xic1 overexpression and depletion, using a MO, inhibited pronephros anlagen formation with the same severity in the presence or absence of the apoptotic inhibitor BclXL. X. laevis embryos were injected at the 8 cell stage into a V2 blastomere to target the somites and presumptive pronephric region. βgal mRNA was co-injected to act as a lineage tracer (stained red, white arrow). embryos were cultured till stage 22 and whole mount in situ hybridised for expression of Lim-1, an early marker of the pronephros anlagen. Injection of the Control MO had no effect on Lim-1 expression (A). p27Xic1 mRNA, p27Xic1 MO, p27Xic1 mRNA/1 ng BclXL mRNA, and p27Xic1 MO/1 ng BclXL mRNA all reduced expression of Lim-1 on the injected side (B–E). Injection of p27Xic1 mRNA and p27Xic1 MO had effects on muscle development (see section 2.9); hence curling of the embryos towards the injected side was frequently observed, such as in the p27Xic1/BclXL injected embryo shown here (E). *denotes injected side.
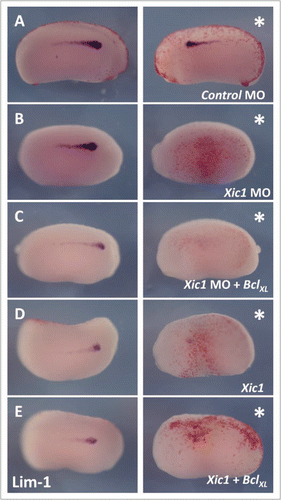
Figure 5 Overexpression of p27Xic1 mRNA reduced cell division, whereas depletion of endogenous p27Xic1 mRNA translation using a MO had no affect on cell division. X. laevis embryos were injected into one cell of a two cell stage embryo with βgal mRNA to act as a lineage tracer (red staining). Embryos were cultured till stage 22 and whole mount in situ hybridised for expression of Lim-1, and antibody stained for phosphohistone H3 (pH3, a marker of dividing cells). The Control MO had no effect on Lim-1 expression (white arrow) or pH3 immunostaining (white arrowhead) (A). p27Xic1 mRNA reduced both Lim-1 expression and pH3 immunostaining (B). p27Xic1 MO had no effect on pH3 immunostaining but reduced Lim-1 expression (C). p27Xic1 CK− mRNA had no effect on either Lim-1 expression or pH3 staining (D). *denotes injected side. To quantify the effect of these injections on cell division, positively pH3 immunostained cells on the injected and un-injected side of the embryos were numerically scored. The differences in the number of pH3 immunostained cells between the injected and un-injected sides were calculated as percentages and are presented as a bar chart (E). The Control MO, p27Xic1 MO and p27Xic1 CK− mRNA had no statistically significant effect on pH3 immunostaining. p27Xic1 mRNA statistically significantly reduced pH3 immunostaining on the injected side on average by 63% when compared to the un-injected side.
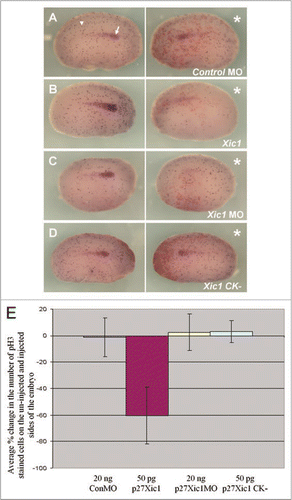
Figure 6 Mis-expression of p27Xic1 disrupted somite morphology and muscle differentiation, but not early mesoderm formation.to observe the effects p27Xic1 depletion had on early mesoderm formation, we injected both cells of a 2-cell stage embryo with the p27Xic1 MO, cultured the embryos to stage 10/11 and performed whole mount in situ hybridisation for Xbrachyury, a marker of the early mesoderm. Injection of neither the Control MO or the p27Xic1 MO had any effect on Xbrachyury expression (A and B).to observe the effects of p27Xic1 mis-expression on myogenesis, X. laevis embryos were injected at the 8 cell stage into a V2 blastomere to target the presumptive pronephric region. βgal mRNA was co-injected to act as a lineage tracer (red staining). embryos were cultured till stage 24 and whole mount in situ hybridised for expression of both Lim-1 and MyoD (a marker of differentiating muscle). The Control MO had no effect on either Lim-1 (as marked by the white arrowhead) or MyoD expression (as marked by the white arrow) (C). p27Xic1 MO reduced expression of both Lim-1 and MyoD (D). p27Xic1 mRNA reduced Lim-1 expression and disrupted MyoD expression (dashed white arrow), causing non-segmentation of the somites (E). p27Xic1 CK− had no effect either Lim-1 or MyoD expression (F). *denotes injected side.
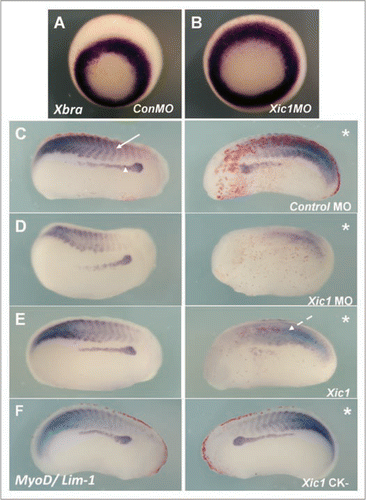
Figure 7 HUA treatment severely perturbed pronephric and muscle development. Stage 10.5 X. laevis embryos were incubated in either HUA and DMSO or DMSO only (control) and left to develop to stage 41 where whole mount 3G8/4A6 antibody staining was performed to detect the mature pronephros. All embryos treated with HUA had delayed development and reduced 3G8/4A6 staining (arrowheads). The control embryo showed normal development at stage 41 (A). Stage 10.5 X. laevis embryos were incubated in either HUA and DMSO or DMSO alone (control) and left to develop to stage 23 where whole mount in situ hybridisation for Lim-1/MyoD (B) and Lim-1/MHC (C) expression was performed. The HUA treated embryo is shown above the control untreated embryo in (B and C). HUA treatment clearly inhibited pronephros anlagen formation, as seen by reduced Lim-1 expression (white arrow), and disrupted the normally segmented MyoD expression (B) (white arrowhead) and MHC expression (C) (black arrowhead).
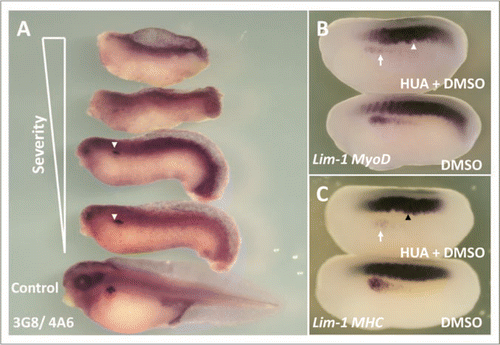
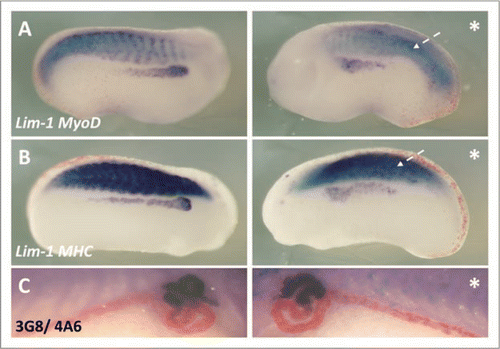
Figure 8 p35.1 disrupted somitogenesis and pronephros anlagen formation, but not mature pronephros development. X. laevis embryos were injected at the 8 cell stage into a V2 blastomere to target the presumptive pronephric region. βgal mRNA was co-injected to act as a lineage tracer (red staining). Embryos were cultured till stage 24, where whole mount in situ hybridised for expression of Lim-1/MyoD (A) and Lim-1/MHC (B) was carried out, and to stage 41, where whole mount 3G8/4A6 antibody staining was performed. p35.1 mRNA overexpression disrupted development of the myotome and pronephros anlagen (A and B). Both MyoD and MHC expression were abnormal on the injected side, with segmentation of the somites apparently lacking. Lim-1 expression was in a broader domain on the injected side. However at stage 41 p35.1 mRNA overexpression had no effect on the size of the mature pronephros (C). In this image the lineage tracer is not lined up in the typical chevron pattern associated with appropriate segmentation, indicating to us the early effects on somitogenesis have not recovered. *denotes injected side.
Additional material
Download Zip (403.3 KB)Acknowledgements
We would like to thank Karine Massé for advice on molecular techniques and unpublished TUNEL assays, Mehregan Movassagh for making and supplying the p27Xic1 CK− mutant, Ian Horan for helpful comments and Paul Jarrett for maintenance of frogs. This work was supported by BBSRC grant, BB/C00406X/1 and The Wellcome Trust.
References
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) 1994; 4th ed. New York Garland Publishing, Inc.
- Saxén L. Organogesis of the kidney 1987; Cambridge Cambridge University Press
- Vize PD, Jones EA, Pfister R. Development of the Xenopus pronephric system. Dev Biol 1995; 171:531 - 540
- Reggiani L, Raciti D, Airik R, Kispert A, Brändli AW. The prepattern transcription factor Irx3 directs nephron segment identity. Genes Dev 2007; 21:2358 - 2370
- Brändli AW. Towards a molecular anatomy of the Xenopus pronephric kidney. Int J Dev Biol 1999; 43:381 - 395
- Jones EA. Xenopus: a prince among models for pronephric kidney development. J Am Soc Nephrol 2005; 16:313 - 321
- Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol 2006; 22:509 - 529
- Carroll T, Wallingford J, Seufert D, Vize PD. Molecular regulation of pronephric development. Curr Top Dev Biol 1999; 44:67 - 100
- Seufert DW, Brennan HC, DeGuire J, Jones EA, Vize PD. Developmental basis of pronephric defects in Xenopus body plan phenotypes. Dev Biol 1999; 215:233 - 242
- Mauch TJ, Yang G, Wright M, Smith D, Schoenwolf GC. Signals from trunk paraxial mesoderm induce pronephros formation in chick intermediate mesoderm. Dev Biol 2000; 220:62 - 75
- Mitchell T, Jones EA, Weeks DL, Sheets MD. Chordin affects pronephros development in Xenopus embryos by anteriorizing presomitic mesoderm. Dev Dyn 2007; 236:251 - 261
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 1999; 13:1501 - 1512
- Vernon AE, Devine C, Philpott A. The cdk inhibitor p27Xic1 is required for differentiation of primary neurones in Xenopus. Development 2003; 130:85 - 92
- Vernon AE, Philpott A. A single cdk inhibitor, p27Xic1, functions beyond cell cycle regulation to promote muscle differentiation in Xenopus. Development 2003; 130:71 - 83
- Daniels M, Dhokia V, Richard-Parpaillon L, Ohnuma S. Identification of Xenopus cyclin-dependent kinase inhibitors, p16Xic2 and p17Xic3. Gene 2004; 342:41 - 47
- Su JY, Rempel RE, Erikson E, Maller JL. Cloning and characterization of the Xenopus cyclin-dependent kinase inhibitor p27Xic1. Proc Natl Acad Sci USA 1995; 92:10187 - 10191
- Shou W, Dunphy WG. Cell cycle control by Xenopus p28Kix1, a developmentally regulated inhibitor of cyclin-dependent kinases. Mol Biol Cell 1996; 7:457 - 469
- Ohnuma S, Hopper S, Wang KC, Philpott A, Harris WA. Co-ordinating retinal histogenesis: early cell cycle exit enhances early cell fate determination in the Xenopus retina. Development 2002; 129:2435 - 2446
- Ohnuma S, Philpott A, Wang K, Holt CE, Harris WA. p27Xic1, a Cdk inhibitor, promotes the determination of glial cells in Xenopus retina. Cell 1999; 99:499 - 510
- Movassagh M, Philpott A. Cardiac differentiation in Xenopus requires the cyclin-dependent kinase inhibitor, p27Xic1. Cardiovasc Res 2008;
- Hardcastle Z, Papalopulu N. Distinct effects of XBF-1 in regulating the cell cycle inhibitor p27(XIC1) and imparting a neural fate. Development 2000; 127:1303 - 1314
- Chen J, Jackson PK, Kirschner MW, Dutta A. Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature 1995; 374:386 - 388
- Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, et al. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 1994; 78:59 - 66
- Greenwood J, Gautier J. From oogenesis through gastrulation: developmental regulation of apoptosis. Semin Cell Dev Biol 2005; 16:215 - 224
- Smith JC, Price BM, Green JB, Weigel D, Herrmann BG. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell 1991; 67:79 - 87
- Hartley RS, Sible JC, Lewellyn AL, Maller JL. A role for cyclin E/Cdk2 in the timing of the midblastula transition in Xenopus embryos. Dev Biol 1997; 188:312 - 321
- Harris WA, Hartenstein V. Neuronal determination without cell division in Xenopus embryos. Neuron 1991; 6:499 - 515
- Philpott A, Porro EB, Kirschner MW, Tsai LH. The role of cyclin-dependent kinase 5 and a novel regulatory subunit in regulating muscle differentiation and patterning. Genes Dev 1997; 11:1409 - 1421
- Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell 2008; 14:159 - 169
- Zezula J, Casaccia-Bonnefil P, Ezhevsky SA, Osterhout DJ, Levine JM, Dowdy SF, et al. p21Cip1 is required for the differentiation of oligodendrocytes independently of cell cycle withdrawal. EMBO Rep 2001; 2:27 - 34
- Nguyen L, Besson A, Heng JI, Schuurmans C, Teboul L, Parras C, et al. p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes Dev 2006; 20:1511 - 1524
- Di Cunto F, Topley G, Calautti E, Hsiao J, Ong L, Seth PK, et al. Inhibitory function of p21Cip1/WAF1 in differentiation of primary mouse keratinocytes independent of cell cycle control. Science 1998; 280:1069 - 1072
- Wang Z, Wang S, Fisher PB, Dent P, Grant S. Evidence of a functional role for the cyclin-dependent kinase inhibitor p21Cip1 in leukemic cell (U937) differentiation induced by low concentrations of 1-beta-D-arabinofuranosylcytosine. Differentiation 2000; 66:1 - 13
- Reynaud EG, Leibovitch MP, Tintignac LA, Pelpel K, Guillier M, Leibovitch SA. Stabilization of MyoD by direct binding to p57(Kip2). J Biol Chem 2000; 275:18767 - 18776
- Reynaud EG, Pelpel K, Guillier M, Leibovitch MP, Leibovitch SA. p57(Kip2) stabilizes the MyoD protein by inhibiting cyclin E-Cdk2 kinase activity in growing myoblasts. Mol Cell Biol 1999; 19:7621 - 7629
- Thayer MJ, Tapscott SJ, Davis RL, Wright WE, Lassar AB, Weintraub H. Positive autoregulation of the myogenic determination gene MyoD1. Cell 1989; 58:241 - 248
- Jin X, Kim JG, Oh MJ, Oh HY, Sohn YW, Pian X, et al. Opposite roles of MRF4 and MyoD in cell proliferation and myogenic differentiation. Biochem Biophys Res Commun 2007; 364:476 - 482
- Cooke J, Zeeman EC. A clock and wavefront model for control of the number of repeated structures during animal morphogenesis. J Theor Biol 1976; 58:455 - 476
- Ozato K. Cell cycle in the primitive streak and the notochord of early chick embryos. Embryologia (Nagoya) 1969; 10:297 - 311
- Snow MHL. Gastrulation in mouse—growth and regionalization of epiblast. J Embryol Exp Morphol 1977; 42:293 - 303
- Stern CD. Re-examination of mitotic-activity in the early chick-embryo. Anat Embryol 1979; 156:319 - 329
- Primmett DR, Norris WE, Carlson GJ, Keynes RJ, Stern CD. Periodic segmental anomalies induced by heat shock in the chick embryo are associated with the cell cycle. Development 1989; 105:119 - 130
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol 1991; 36:685 - 695
- Jones EA, Woodland HR. Development of the ectoderm in Xenopus: tissue specification and the role of cell association and division. Cell 1986; 44:345 - 355
- Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993; 75:805 - 816
- Haldin CE, Masse KL, Bhamra S, Simrick S, Kyuno JI, Jones EA. The lmx1b gene is pivotal in glomus development in Xenopus laevis. Dev Biol 2008;
- Dale L, Slack JM. Fate map for the 32-cell stage of Xenopus laevis. Development 1987; 99:527 - 551
- Moody SA, Kline MJ. Segregation of fate during cleavage of frog (Xenopus laevis) blastomeres. Anat Embryol 1990; 182:347 - 362
- Barnett MW, Old RW, Jones EA. Neural induction and patterning by fibroblast growth factor, notochord and somite tissue in Xenopus. Dev Growth Differ 1998; 40:47 - 57
- Bassez T, Paris J, Omilli F, Dorel C, Osborne HB. Post-transcriptional regulation of ornithine decarboxylase in Xenopus laevis oocytes. Development 1990; 110:955 - 962
- Harlow E, Lane D. Antibodies: A laboratory manual 1988; Cold Spring Harbour, New York Cold Spring Harbour Laboratory Press