Abstract
Isolation of adipose-derived stem cells (ASCs) typically involves 8+ hours of intense effort, requiring specialized equipment and reagents. Here, we present an improved technique for isolating viable populations of mesenchymal stem cells from lipoaspirate saline fractions within 30 minutes. Importantly, the cells exhibit remarkable similarities to those obtained using the traditional isolation protocols, in terms of their multipotent differentiation potential and immunophenotype. Reducing the acquisition time of ASCs is critical for advancing regenerative medicine therapeutics, and our approach provides rapid and simple techniques for enhanced isolation and expansion of patient-derived mesenchymal stem cells.
Brief Report
The original, pioneering work on the isolation of adipose-derived stem cells (ASCs) from liposuction waste typically involves 8–10 hours of continuous intense effort,Citation1,Citation2 making it a labor-intensive endeavor and increasing the risk of culture contaminations due to excessive handling. Based on recent reports,Citation3 we originally postulated that the blood/saline portion of lipoaspirate waste would prove to be a rich source of ASCs due to their association with the perivascular space. In addition, because of the violent nature of liposuction and the ultrasonic procedure used to obtain our samples, we expected to find significant number of ASCs in the blood and saline fractions of the lipoaspirate waste.
We present an enhanced method for isolating and quickly expanding a robust population of mesenchymal stem cells (MSCs) derived from lipoaspirate in less than 30 minutes. This isolation process yields an abundant population of ASCs (∼100,000 cells per 100 ml of blood/saline collected from sonicated lipoaspirate) with differentiation potential, characteristic cell surface markers, and proliferative lifespan indistinguishable from MSCs extracted from bone marrow (BMSCs) or conventionally processed ASCs.Citation1,Citation2
As outlined in , a viable population of adherent ASCs is easily obtained from the blood and saline phase that carries over with liposuction surgical refuse. This layer is located below the floating, more buoyant adipose tissue and is processed though a simple 5-step process. First, the blood/saline phase is isolated and cells pelleted (10 minutes). Second, the resulting pellet is gently re-suspended in NH4Cl for red blood cell lysis (2–5 minutes). Third, the cells are pelleted again (10 minutes). Fourth, the cell pellet is gently re-suspended in DMEM with 40–50% fetal bovine serum (FBS), followed by plating the cells (2–5 minutes) and incubation overnight. Finally, the non-adherent cells and debris are washed away with phosphate-buffered saline (PBS), and the 30 minute ASC cultures are grown. This isolation method not only requires less than half an hour to complete but uses only standard tissue culture materials and equipment without the need for collagenase digestion, Percoll gradients, or extensive washing. Use of this isolation technique allows for the straightforward establishment of both patient-specific ASCs for regenerative medicine and disease-specific cell strains for scientific discovery.
Beyond sharing a mesenchymal morphology with other MSCs, the ASCs isolated in this streamlined manner were shown to share multipotent differentiation potential and an immunophenotype with the traditionally isolated ASCs (shown in for differentiation, for flow cytometry, performed as reported previouslyCitation1,Citation2,Citation4), characteristics which are also indistinguishable from BMSCs (not shown). Rapidly isolated ASCs have a CD14−, CD29+, CD31−, CD34low/+, CD45−, CD73+ and CD105+ immunophenotype (), consistent with classically isolated ASCs () and BMSCs (not shown). In agreement with previous reports on ASCs and BMSCs,Citation1,Citation2,Citation4–Citation7 rapidly isolated ASCs also have undetectable levels of telomerase, assessed either by TRAP assay or RT-PCR for the catalytic subunit of telomerase, hTERT (not shown). Because rapidly isolated ASCs are nearly identical to both BMSCs and ASCs isolated in the traditional long protocol in terms of important MSC characteristics, ASCs isolated using our accelerated protocol warrant the same adult stem cell classification.
We also show that the simple addition of epidermal growth factor (EGF) to the regular ASC growth media elicits a marked enhancement to the proliferation rate for ASCs with no adverse effects on proliferative lifespan or differentiation potential ( and data not shown). ASC cultures supplemented with EGF show a sharp increase in proliferation rate and an increased senescence population doubling (PD) timing compared to unsupplemented ASCs (). With EGF added to normal growth media, doubling times, as measured over a 120 day span, were calculated at 28 hours for EGF supplemented cells, compared to 65 hours for unsupplemented cells. Importantly, EGF-ASCs still undergo robust differentiation and appear otherwise unchanged compared to ASCs under standard conditions, apart from the enhanced proliferation rate and increased senescence timing, which is consistent with a recent report suggesting ASCs have functional EGF receptors.Citation8
We have detailed a simple, rapid, and effective method for isolating adipose-derived stem cells from the blood/saline fraction of lipoaspirates. These ASCs exhibit somatic progenitor (i.e., stem cell traits) and phenotypic properties indistinguishable from ASCs isolated using the labor-intensive traditional protocol and the gold-standard BMSCs, while also showing that EGF supplementation increases proliferation rates of ASC cultures. Thus, the methods described here mutually facilitate the autologous stem cell extraction process and the ability to generate a large number of cells, both of which have been longstanding hurdles for basic regenerative medicine research and therapeutics.
Methods
Traditional and rapid ASC isolation.
ASC isolation was carried out as previously described with slight variations.Citation2 Briefly, oil from the top of the lipoaspirate was immediately removed from freshly harvested fat, followed by the isolation of the saline/blood fraction (see ). For the traditional isolation, 200 ml aliquots of fat were washed 3–4 times with equal volume PBS and incubated in 50% volume 150 µg/mL collagenase (Sigma-Aldrich) with 1% ABAM (Invitrogen) for 60 min at 37°C shaking at 250 rpm. Collagenase was inactivated with 10% FBS, followed by redistribution of the mixture into 50 ml conicals and centrifugation at 1,000 xg for 10 min to separate the oil and remaining fat lobules from the stromal vascular fraction (SVF). The pelleted SVF was treated with 160 mM NH4Cl at room temperature for 10 min to lyse red blood cells, and applied to a Percoll gradient (Sigma-Aldrich) to purify the mononucleated cells.
The rapid isolation protocol involves multiple steps at room temperature including centrifugation of the saline/blood fraction at 400 xg for 10 min, followed by red blood cell lysis with 160 mM NH4Cl for 5 min, and a final centrifugation for 10 min at 400 xg.
For both traditional and rapid protocols, the final pelleted fraction of mononuclear cells was then resuspended in 25 ml DMEM:F12 media (Invitrogen) supplemented with 40% FBS (Invitrogen), ABAM, and 10 ng/mL EGF (BD Biosciences) incubated overnight to select for adherent cells. The remaining floating cells were aspirated off the following day, and the plate was washed with PBS to remove any remaining debris. ASCs were maintained on DMEM low glucose supplemented with 10% FBS, 1% ABAM, with or without 10 ng/ml EGF at 5% CO2 at 37°C. For quantification of growth, cells were maintained on 100 mm dishes and split at confluence at a ratio of 1:4 until a senescent growth and phenotypic state was achieved.
Osteocyte differentiation.
Cells are plated and grown until approximately 75% confluent, followed by osteogenic differentiation in DMEM with high glucose, 10% FBS, 0.01 µm 1.25-dihydroxyvitamin D3 (Sigma-Aldrich), 50 µm ascobate-2-phosphate (Sigma-Aldrich), 10 mM β-glycerophosphate (Sigma-Aldrich), and 1% ABAM. The cells were cultured for 2 weeks and then washed and fixed in 4% paraformaldhyde. The cells were then stained using Alizarin Red S (Sigma-Aldrich), which specifically stains calcium deposits, and whole field light microscopy images were then captured at 10x.
Adipocyte differentiation.
100% confluent cells were treated with adipogenic media containing DMEM low glucose, 10% FBS, 0.5 mM isobutyl-methylxanthine (Sigma-Aldrich), 1 µM dexamethasone (Sigma-Aldrich), 10 µM insulin, 200 µM indomethacin (Sigma-Aldrich), and 1% ABAM. The plates were maintained for 2 weeks until lipid droplet formation was observed, followed by fixation in 4% paraformaldehyde. Fixed cells were stained with Oil Red O (Sigma-Aldrich), which specifically stains lipid droplets, and whole field light microscopy images were captured at 10x.
Chondrocyte differentiation.
2 × 105 cells were pelleted in a 2 ml V bottomed tube and placed in the incubator at 37°C 10% CO2 in chondrogenic media, which contained DMEM low glucose, 1% FBS, 6.25 µg/ml insulin, 10 ng/ml recombinant TGFβ3 (R&D Systems), 50 nM ascorbate-2-phosphate, and 1% ABAM. After 1 week, the pellets were transferred to a 10 cm dish and cultured in the same media formulation for another 2 wks. The pellets were fixed in 4% paraformaldehyde and stained with Safranin O (Sigma-Aldrich), which specifically stains GAG proteins present in chondrocyte extracellular matrix. The micromass pellets were imaged with a 1 mm scale bar to indicate gross anatomy and size.
Flow cytometry.
Both traditional and rapid ASCs were trypsinized and counted in order to obtain 5 × 105 cells for primary antibody or 2 × 105 cells for secondary antibody only and no antibody controls. These cells were centrifuged at 100 xg for 1 min at 4°C and resuspended in 75 µl of FACS buffer (PBS, 2% FBS) and incubated on ice for 10 minutes. The primary antibody was added (CD14, CD29, CD34, CD45, CD73, CD105, InVitrogen; CD34, Millipore) and incubated with rotation at 4°C for 30 min, followed by centrifugation and 3 washes with FACS buffer. The secondary antibody (Alexa 488 goat anti-mouse; Invitrogen) was then added at a dilution of 1:400 and incubated in the dark at 4°C with rotation for 30 min. The washes were then repeated, and the resulting pellet was resuspended in 500 µl PBS and forced through a 35 µm mesh filter to remove any cell clumps. Immunolabeled cell suspensions were kept on ice until cytometric analysis, which was performed using a Coulter Epics XL-MCL.
Figures and Tables
Figure 1 Adipose-derived stem cell isolation techniques flow chart. The wide variance in the time, materials, and effort for obtaining ASCs via the Standard Isolation and our Rapid Isolation techniques is shown. A highly viable population of around 250,000 ASCs can be derived from 250 ml of blood/saline fraction of the liposuction waste in as little as 30 minutes, as compared to 8–10 hours to obtain ASCs using the traditional isolation method. SVF, stromal vascular fraction; ABAM, antibiotic/antimycotic; FBS, fetal bovine serum.
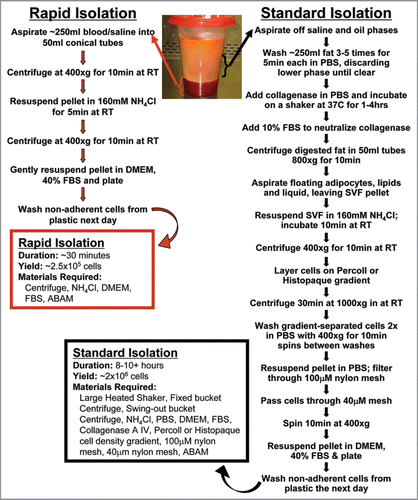
Figure 2 Characterization of ASC differentiation. The differentiation potential of ASCs isolated in the streamlined, rapid protocol were compared to ASCs isolated using the standard protocol (traditional ASCs). Cells were induced to differentiate into adipocytes ((A) for rapid ASCs and (G) for traditional ASCs; (D) rapid ASCs grown for 2 weeks without adipogenic media as control; all stained with oil red O and hematoxlin), osteocytes ((B) for rapid ASCs and (H) for traditional ASCs; (E) rapid ASCs grown 2 weeks without osteocyte induction media as control; all stained with Alizarin red S and hematoxylin), and chondrocytes [(C) for rapid ASCs and (I) for traditional ASCs, both in a micromass (solid micromass pellet, insert, (C)]; (F) unpelleted rapid ASCs grown 4 weeks in induction media as control; all samples stained for Safranin O).
![Figure 2 Characterization of ASC differentiation. The differentiation potential of ASCs isolated in the streamlined, rapid protocol were compared to ASCs isolated using the standard protocol (traditional ASCs). Cells were induced to differentiate into adipocytes ((A) for rapid ASCs and (G) for traditional ASCs; (D) rapid ASCs grown for 2 weeks without adipogenic media as control; all stained with oil red O and hematoxlin), osteocytes ((B) for rapid ASCs and (H) for traditional ASCs; (E) rapid ASCs grown 2 weeks without osteocyte induction media as control; all stained with Alizarin red S and hematoxylin), and chondrocytes [(C) for rapid ASCs and (I) for traditional ASCs, both in a micromass (solid micromass pellet, insert, (C)]; (F) unpelleted rapid ASCs grown 4 weeks in induction media as control; all samples stained for Safranin O).](/cms/asset/9ee2896e-21bb-4eb5-a725-95d2e6159d54/kogg_a_10910019_f0002.gif)
Table 1 Immunophenotype of ASCs isolated using the rapid and standard protocols
Table 2 EGF supplemented ASC media enhances growth
Acknowledgements
We would like to thank Dr. Matthew Beckman for providing bone marrow mesenchymal stem cells. We are also grateful to Dr. Stephen Chen and his surgical team for facilitating accrual of lipoaspirates and surgical coordination. Flow Cytometry was supported in part by the Massey Cancer Center core grant, NIH Grant P30 CA16059. Microscopy was performed at the VCU—Dept. of Neurobiology & Anatomy Microscopy Facility, supported, in part, with funding from NIH-NINDS Center core grant (5P30NS047463).
References
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002; 13:4279 - 4295
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001; 7:211 - 228
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008; 3:301 - 313
- Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells 2005; 23:412 - 423
- Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem 2006; 99:1285 - 1297
- Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006; 24:1294 - 1301
- Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki Y, Aiba-Kojima E. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol 2006; 208:64 - 76
- Baer PC, Schubert R, Bereiter-Hahn J, Plösser M, Geiger H. Expression of a functional epidermal growth factor receptor on human adipose-derived mesenchymal stem cells and its signaling mechanism. Eur J Cell Biol 2009; 88:273 - 283