Abstract
The property of vasoactivity is important for both resistance vessels and larger arteries. Evaluation of smooth muscle cell phenotype is often done in place of functional testing in engineered tissues, assuming a direct correlation between cell phenotype and tissue contractile force. In this study we look at a large panel of vasoactive agents to determine the functionality of our collagen-based tissue. The engineered vascular media elicited a measurable change in force in response to seven of the nine agents used. As part of this characterization, TGF-β1 and TNF-α were used to promote a more contractile and synthetic cell phenotype respectively. Both smooth muscle α-actin and vasoconstriction were evaluated in ring sections. Due to large differences in cell-compaction and cell distribution in the tissues, no correlation was found between α-actin expression and contractile strength. This highlights the need for functional testing of engineered tissue and the importance of cell-matrix interactions in vasoactivity.
Introduction
There are several characteristics that are critical to the ultimate success of tissue engineered blood vessel substitutes for coronary bypass graft in vivo. These include (1) having mechanical properties that are like those of a coronary artery both in terms or its strength and viscoelastic properties and (2) possessing a non-thrombogenic inner lining. If such a tissue-engineered substitute is to truly mimic a normal large artery, however, it also must be able to exhibit vasoactivity. This property is not only important for the resistance vessels, but also for larger arteries where the local hemodynamic environment, including wall shear stress, can be regulated through vasoactivity.
There is a complex system of controls that regulate vasoactivity in order to meet the local and systemic needs of the body. This includes signaling by means of endothelial cell secretions (e.g., endothelin-1 and nitric oxide), circulating hormones (e.g., norepinephrine), neurotransmitters [e.g., adenosine triphosphate (ATP)], local paracrine secretions (e.g., bradykinin) and vasoactive metabolites (e.g., K+), as well as responses due to changes in temperature and pressure. In response to these signals the smooth muscle cells in the vascular media will contract or dilate to change the diameter of the vessel in order to regulate the blood flow.Citation1 Many of these agonists can produce constriction or dilation at different concentrations by binding to different receptors. For example, bradykinin can elicit vasoconstriction or vasodilation in a dose-dependent manner by binding to the B2 versus the B1 receptor.Citation2–Citation5
Vasoactivity has long been considered a desirable characteristic for a tissue-engineered blood vessel, yet few laboratories have demonstrated this function. Most that have shown functionality have chosen to look at a small number of vasoactive agents. Vasoactivity testing may be relatively uncommon in engineered tissues; however, there have been some notable studies. Cell sheet tissue-engineered vascular medias (TEVM) have been extensively tested through use of a comprehensive panel of agents,Citation6 as well as investigations of the endothelin system.Citation7 Despite issues with cell culture, vasoactivity has been seen in TEVM that used adult smooth muscle cellsCitation8–Citation10 as well as TEVM using umbilical cord derived,Citation6,Citation7,Citation11,Citation12 bone marrow progenitor,Citation13 and neonatal cells.Citation14,Citation15 One reason why few groups have demonstrated function is that obtaining the number of cells necessary to make a TEVM requires extensive passaging of cells. Smooth muscle cells in culture modulate towards a more synthetic phenotype, losing much of their contractile apparatus, reducing their capacity to respond to vasoconstrictors and vasodilators.Citation16,Citation17 Biochemical stimulation can be employed to promote a more contractile cell phenotype. One of the more popular choices for biochemical stimulation is TGFβ1, which is known to promote a more contractile smooth muscle cell phenotype and increase engineered blood vessel strength.Citation15,Citation18–Citation23 While much is known about how TGFβ and other biochemical stimulation affects cell phenotype, functional testing has rarely been done. Often it is assumed that there is a direct correlation between cell phenotype and the stress generated by the tissue in response to a given agent.
In this study, the importance of functional testing is explored by characterizing the response of collagen-based TEVMs to a panel of vasoconstrictors and vasodilators that consist of drugs from both the intrinsic and extrinsic pathways. As part of this characterization, the correlation between cell phenotype and contractile stress was investigated. TGFβ1 and TNFα were chosen as biochemical cell phenotype modifiers because they are known to promote a more contractile or synthetic cell phenotype, respectively.Citation24,Citation25
Results
Vasoactivity characterization.
TEVM were constructed by encapsulating rat aortic smooth muscle cells in a type I collagen hydrogel. The smooth muscle cells used for these experiments were isolated using a facilitated migration technique and were passaged when close to 80% confluent. Relatively high passages between P6 and P9 were used for these studies. The tubular TEVM were cultured statically for 3 weeks at which time the ring segments were cut and placed into a physiologic organ-bath for functional testing. To determine which receptors were present and functional, a panel of seven vasoconstrictors and two vasodilators were chosen. Isometric contractile and relaxation tensions were measured after the addition of increasing concentrations of the agents. Representative responses to the vasoconstrictors are shown in and responses to vasodilators are shown in . All but two of the agents produced a measurable response. Only sodium nitroprusside (SNP), a nitric oxide donor and BHT-920, an α2-adrenergic receptor agonist resulted in no measurable vasoactive response. The presence of a contractile response was confirmed by administering endothelin-1 after BHT-920. Phenylephrine, an α1-adrenergic receptor agonist, resulted in a biphasic response. Lower doses resulted in vasodilation while higher concentrations resulted in vasoconstriction. A summary of the results is shown in .
Biochemical stimulation and cell phenotype.
To determine if there was any correlation between cell phenotype and vasoconstriction, TEVMs were cultured with 2 ng/ml TGFβ1, 10 ng/ml TNFα or both. TGFβ1 is known to improve TEVM strength and promote a more contractile cell phenotype, while TNFα is known to promote a more synthetic cell phenotype. After 3 weeks in culture the TEVMs were cut into ring segments for histologic analysis and functional testing. The addition of TGFβ1 resulted in a significant increase in smooth muscle α-actin while the addition of TNFα alone or in combination with TGFβ1 resulted in a significant decrease in a-actin staining as shown in . Hoechst staining showed differences in cell number and distribution in the cross-section. Initially the cells were homogeneously distributed throughout the hydrogel, but after 3 weeks of culture the smooth muscle cells had proliferated and formed a cell-dense ‘capsule’ on the outer surface of the hydrogel that is exposed to culture medium. Staining for smooth muscle α-actin was found to be much more pronounced in the outer cell-dense portion of the TEVM; cells within the collagen hydrogel exhibited little to no α-actin staining.
Vasoactivity of biochemically stimulated TEVM.
Vaso-activity testing was performed on ring sections. Only two of the more potent agonists, endothelin-1 (10−7 M) and bradykinin (10−4 M), were used in this study. Both agonists were followed by the non-specific antagonist papaverine (10−4 M). The maximum contractile force was determined and was converted to a nominal contractile stress in order to account for differences in the amount of tissue tested. Significant differences were seen in the contractile stress as shown in ; however, there is no correlation between the cell phenotype and the contractile force. All of the TEVMs subjected to biochemical treatment exhibited significantly diminished contractile stresses as compared to the untreated controls. Due to the differences seen in the cellularity of the TEVMs, cell number was determined using DNA quantification. The force generated per cell of the ring segments was then calculated in order to determine if the number of cells was influencing the contractility results. The results are shown in . When the contractile force per cell is considered, the results are consistent with what was seen with contractile stress. All of the contractile stresses generated by the treated TEVMs are less than those generated by the untreated controls. The contraction in response to endothelin-1 of the TNFα-treated TEVMs are now significantly higher than that of the TGFβ1-treated TEVMs, further highlighting the impact of the TEVM composition on the contractile and relaxation response.
TEVM composition.
One reason for the lack of correlation between the cell phenotype and the contractile force is the significant difference in the TEVM hydrogel compaction as shown in . Masson's trichrome staining was performed on 7 µm thick sections (see ). There were dramatic differences not only in the wall thickness, but also in the collagen density. The addition of TGFβ1 results in significant hydrogel compaction, especially in the longitudinal direction resulting in a TEVM with a thick, dense collagenous inner region. The addition of TNFα resulted in a much more diffuse collagen layer. There is still some radial compaction, but little to no longitudinal compaction. TEVMs treated with TNFα have the thinnest walls, but the least dense collagen layer. The overall wall thickness of the samples is affected by both differences in the thickness of the collagen hydrogel and the thickness of the cell layers. These striking differences in hydrogel compaction and cellularity, taken along with the cell phenotype and vasoactivity studies, highlight the importance of the cell-matrix interactions involved in the generation of a contractile force in tissue.
Discussion
This study describes tissue function, not smooth muscle function because it is functionally important for a TEVM. It is possible for smooth muscle cell contraction to occur without generating enough force to cause a measurable tissue contraction. This work has shown that the collagen-based TEVMs are capable of generating a measurable response to seven different vasoconstrictors and vasodilators. These drugs were chosen to represent multiple classes of intrinsic and extrinsic stimulation. A response was recorded for seven of the nine drugs used; no response was measured for SNP or BHT-920. The number of drugs tested in this study is surpassed only by the study of cell sheet TEVMs by L'Heureux et al.Citation6 The list of agents studied is significant, but not all-encompassing. Even if a response was found to all of the agents, it would not have proven that the cells were fully functional. The most significant contractions were found in response to endothelin-1, a potent vasodilator. Constriction was also seen in response to ATP, serotonin, KCl and bradykinin. Bradykinin is often thought of as a vasodilator, but is known to cause constriction in pulmonary tissues. This result of constriction in a TEVM is consistent with results from other laboratories utilizing different tissue engineering strategiesCitation6,Citation26 as well as some veins.Citation27 This reaction may be due to changes induced during smooth muscle cell isolation and culture or due to the different mechanical environments. Lack of response to SNP is not consistent with what other groups have found.Citation6,Citation12,Citation26 The response to isoproterenol shows that the cells in the collagen-based TEVM are capable of dilation, but that the nitric oxide pathway is non-functional.
The addition of TGFβ1 and TNFα increased and decreased smooth muscle α-actin, respectively; however, the expression of and changes in a-actin expression appear to be localized to cells in the cell dense region on the outer surface of the collagen hydrogel. While the addition of TGFβ1 promoted a more contractile cell phenotype, the contractile stress generated by the tissue was significantly less than the force generated by the untreated control and similar to that of the tissue treated with TNFα. Many studies have shown a correlation between α-actin expression and the force generated by a single smooth muscle cell or fibroblast.Citation23,Citation28–Citation30 We believe that the cells in our experiments are generating a greater force, but we are measuring tissue function, not smooth muscle function. The more contractile cells are found on the outside of the tissue surrounding a collagenous tissue. In order for there to be a measurable force generated by the tissue, the cells need to compress this inner layer. While TGFβ1 promoted a more contractile cell phenotype, it also resulted in significant hydrogel compaction, of which a majority was in the longitudinal direction. This compaction led to a very thick dense tissue, which then required the generation of a larger force for compression.
This work demonstrates the importance of functional testing of TEVMs. When vasoactivity testing is done, the choice of drugs is also important. This study has shown that while a TEVM may respond to some drugs, a larger panel is needed to determine if the tissue is fully functional. One example is the response to phenylephrine, an α1-adrenergic receptor agonist, but the lack of response to BHT-920 an α2-adrenergic receptor agonist. Some groups have chosen to study responses to norepinephrine and have shown a functional response. Norepinephrine is an α-adrenergic receptor agonist capable of binding to either of the two a receptors. Constriction in response to norepinephrine proves that there is a functional α-adrenergic receptor, but not necessarily that both α receptors are present and functional. Along with choice of drugs, cell-matrix interactions are important. Much of the work in the literature is focused on increasing the strength of a TEVM so that the tissue can withstand physiological forces. Many of these changes that promote tissue compaction, extracellular matrix production or cross-linking will also increase the compressive strength of the tissue. In these cases, functional testing will be required as correlations may not exist between cell phenotype and tissue contraction or relaxation. Additionally one needs to be careful when comparing the force results from an engineered tissue to native tissue. Differences in the compressive forces of the tissues could allow tissue responses to be similar while the actual forces generated by the cells may be different. When a TEVM is remodeled either in vitro or in vivo, the changes in the strength, stiffness or thickness of the tissue could result in changes in the functional responses over time.
How the functional testing is reported is also important for engineered tissues and native tissues alike. Methods of reporting and differences between them make comparisons between tissues difficult if not impossible. Much of the reporting is done in terms of force generated by the tissue. This force is highly dependent on the length of the tissue. If force is reported, the tissue length should be tightly controlled and also reported. Some groups have chosen to normalize the force to another drug such as ATP. This will help to normalize for tissue length, but only when the tissues and cells are similar. Different isolation and culture methods could result in different responses between different tissues. In this work we have chosen to show force traces, but for quantification have chosen to look at stress instead of force. We believe that this is physiologically relevant and will make comparisons between tissues possible. Ideally actual stress would be completed using wall thickness measurements during contraction. In this case we calculated nominal stress using measurements taken in the relaxed state. While there are subtle differences, this method is still superior to measured force.
Vasoactivity of an engineered tissue is an important feature and is influenced by many factors including cell phenotype and cell-matrix interactions. Studies of cell phenotype alone give insight, but do not demonstrate tissue vasoactivity. Contraction of engineered tissue close to that of native tissue also does not indicate that the cells are producing forces near their in vivo counterparts as compressive strength differences of the tissue have an effect. What is clear, however, is that vasoactivity is an important test for TEVMs and studies of vasoactivity cannot be substituted simply by an evaluation of cell phenotype.
Materials and Methods
Tissue engineered vascular media fabrication and culture.
Rat aortic smooth muscle cells were isolated as previously described.Citation21 Briefly, smooth muscle cells were isolated from the thoracic portion of the aorta of normal adult Sprague-Dawley rats using a facilitated migration method combining both enzymatic digestion and migration techniques. Smooth muscle cells between passages 6 and 10 were incorporated into a type 1 collagen hydrogel for final concentrations of 1 million cells/ml and 2 mg/ml collagen. To create the hydrogel, cells in culture medium were added to acid solubilized type I bovine collagen (MP Biologics) that was neutralized with NaOH. The hydrogel was supplemented with concentrated Dulbecco's Modified Eagle Medium (DMEM, Mediatech Cellgro). The collagen-cell solution was placed into the tubular mold and allowed to polymerize. The hydrogel was then carefully removed from the mold and placed into culture in medium consisting of DMEM, 10% bovine growth serum (Hyclone, Logan, UT), 1% L-glutamine and 1% penicillin-streptomycin (Gibco, Grand Island, NY). Culture medium was changed weekly. For growth factor studies, medium was supplemented with 2 ng/ml TGFβ1 (Sigma, St. Louis, MO), 10 ng/ml TNFα (Sigma) or both; controls with no added growth factors were maintained. Culture medium was changed every other day to assure that growth factor concentrations remained high. TEVMs were cultured for three weeks.
Contractility testing.
TEVMs were rinsed in phosphate-buffered saline (PBS, Sigma) and cut into 2 mm long ring segments. The rings were mounted on hooks in a physiological organ bath of 5 ml Krebs Henseleit solution with continuous of 95% O2, 5% CO2 at 37°C.Citation31–Citation34 All ring segments were preloaded to 0.15 g and subsequently stimulated with the non-specific agonist KCl (60 mM). The rings were subjected to the agonists endothelin-1, bradykinin, phenylephrine, 5,6,7,8-tetrahydro-6-(2-propenyl)-4H-thiazolo[4,5-d]azepine-2-amine dihydrochloride (BHT-920), ATP disodium salt or 5-hydroxytryptamine (5-HT). After contraction, the rings were subjected to the antagonists papaverine, isoproterenol or SNP. All drugs were purchased from Sigma. Changes in tension due to chemical stimulation were measured using a digital data acquisition system (Gould Instrument Systems, Valley View, OH). For quantitative studies the maximum change in force was recorded. Nominal stress was calculated by dividing the force by the cross-sectional area for the unloaded ring segment. To calculate the cross-sectional area, lengths were measured and pictures of the ring cross-section were taken. Wall-thickness was determined using Matrox Inspector 8 software.
Histology and immunohistochemistry.
Samples were rinsed in PBS and placed into 4% formalin at room temperature. After twenty four hours the samples were rinsed in PBS and transferred to 70% alcohol at 4°C until processing. The samples were paraffin-embedded; 7 µm sections were cut for histology and immunohistochemistry (IHC). Masson's trichrome staining was performed on deparaffinized sections. IHC was done using a FITC-conjugated smooth muscle a-actin antibody (Sigma); Hoechst 33258 dye (Sigma) was used to visualize the nuclei.
DNA quantification.
Segments of the tissue were taken, rinsed in PBS and lyophilized with a Freezone freeze dry system (Labconco) overnight. The dry weights were recorded. The tissue was then digested by incubating with 0.5 mg/ml Proteinase K in phosphate buffer with EDTA for 12–16 hours at 60°C or until no tissue was visible. The samples were loaded in triplicate and incubated for 15 minutes at room temperature with 0.1 µg/ml Hoechst 33258 dye in 10 mM Tris-HCl buffer with 1 mM EDTA and 0.2 M NaCl. The plate was read on a fluorometer using an excitation wavelength of 365 nm and emission wavelength of 458 nm. DNA quantity was determined by comparing to a standard curve of calf thymus DNA (Sigma). The results were normalized to dry weight.
Statistical analysis.
All graphs are shown as mean ± SEM. Statistical analysis was completed using a 95% confidence interval. Tests of two parameters were completed using a t-test. Tests of three or more parameters were completed using one way ANOVA with a post hoc t-test.
Abbreviations
| ATP | = | adenosine triphosphate |
| SNP | = | sodium nitroprusside |
| TEVM | = | tissue-engineered vascular media |
| TGFβ | = | transforming growth factor β |
| TNFβ | = | tumornecrosis factor α |
Figures and Tables
Figure 1 Representative responses to vasoconstrictors. Arrows represent when the agents were given and the numbers represent the dosage (mM). Representative responses to KCl (A), bradykinin (B), phenylephrine (C), serotonin (5-HT) (D), endothelin-1 (E), ATP (F) and BHT-920 (G) are shown. There was a biphasic response to phenylephrine. No contraction was elicited in response to BHT-920. A dose of endothelin-1 was given to show that the ring was capable of contraction; this is indicated by the double arrow.
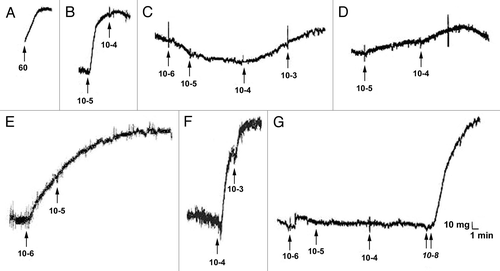
Figure 2 Representative response to vasodilators. Representative responses shown to sodium nitroprusside (A) and isoproterenol (B). No relaxation was found in response to SNP.
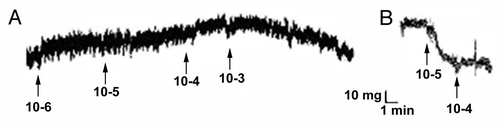
Figure 3 TEVM cell phenotype modulation. Comparison of untreated control (A), with TEVMs treated with TGFβ1 (B) demonstrated a significant increase in smooth muscle α-actin while the addition of TNFα alone (C) or in combination with TGFβ1 (D) resulted in a significant decrease in α-actin staining. Smooth muscle a-actin (green) and Hoechst nuclei dye (blue) are shown. The scale bar represents 50 µm and is applicable to all panels.
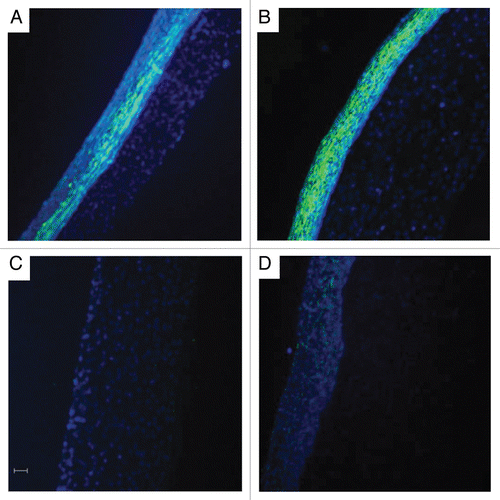
Figure 4 Contractile stress of the TEVMs. The addition of TGFβ1 and TNFα alone or combined resulted in significantly diminished contractile stress when TEVMs were stimulated with endothelin-1 and bradykinin. * signifies p value ≤0.05 as compared to the control using the same drug.
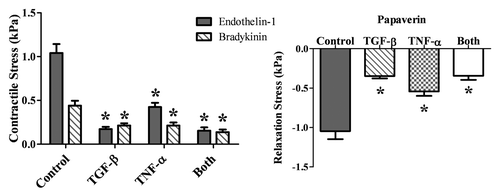
Figure 5 Cellularity and contractile force per cell. There are significant differences in the number of cells found in the different TEVMs. The TGFβ1-treated TEVMs contain the most cells while the TEVMs treated with the combination of TGFβ1 and TNFα have the fewest. All treated TEVMs elicit a significantly lower contractile force per cell than the untreated controls. The TNFα-treated TEVMs elicit a higher contractile force per cell than the TGFβ1-treated TEVMs.
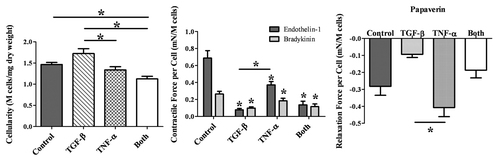
Figure 6 Differences in TEVM compaction. Representative macroscopic views of a control (A), TGFβ1-treated (B), TNFα-treated (C) and combined treatment (D) TEVM are shown. There are obvious differences in compaction visible to the naked eye (right) as well as significant differences in wall thickness. * signifies p-value ≤0.05 between the wall thicknesses as denoted.
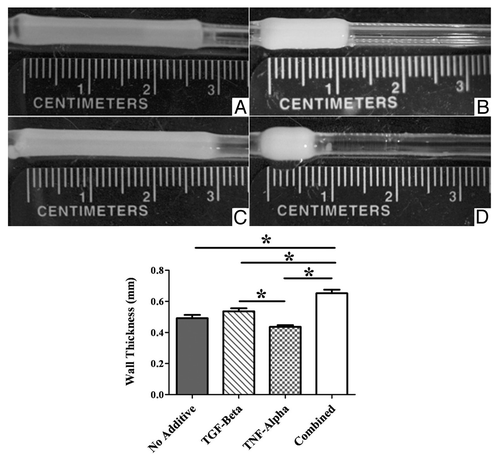
Figure 7 Masson's trichrome-stained TEVM sections. Representative sections of a control (A), TGFβ1-treated (B), TNFα-treated (C) and combined treatment (D) TEVM are shown demonstrating differences in wall thickness, collagen density and cellularity of the outer layer. Scale bar represents 100 µm and is applicable to all panels.
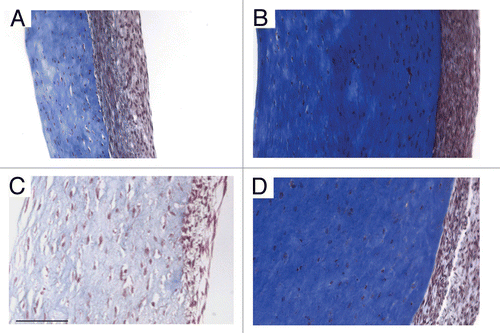
Table 1 Summary of responses to drugs
References
- Levick JR. An introduction to cardiovascular physiology 2003; London New York Arnold
- Hyman AL. The effects of bradykinin on the pulmonary veins. J Pharmacol Exp Ther 1968; 161:78 - 87
- Bateson EA, Schulz R, Olley PM. Response of fetal rabbit ductus arteriosus to bradykinin: role of nitric oxide, prostaglandins and bradykinin receptors. Pediatr Res 1999; 45:568 - 574
- Yu H, Carretero OA, Juncos LA, Garvin JL. Biphasic effect of bradykinin on rabbit afferent arterioles. Hypertension 1998; 32:287 - 292
- Drummond GR, Cocks TM. Endothelium-dependent relaxations mediated by inducible B1 and constitutive B2 kinin receptors in the bovine isolated coronary artery. Br J Pharmacol 1995; 116:2473 - 2481
- L'Heureux N, Stoclet JC, Auger FA, Lagaud GJ, Germain L, Andriantsitohaina R. A human tissue-engineered vascular media: a new model for pharmacological studies of contractile responses. FASEB J 2001; 15:515 - 524
- Laflamme K, Roberge CJ, Labonte J, Pouliot S, D'Orleans-Juste P, Auger FA, et al. Tissue-engineered human vascular media with a functional endothelin system. Circulation 2005; 111:459 - 464
- Dahl SL, Chen Z, Solan AK, Brockbank KG, Niklason LE, Song YC. Feasibility of vitrification as a storage method for tissue-engineered blood vessels. Tissue Eng 2006; 12:291 - 300
- Laflamme K, Roberge CJ, Grenier G, Remy-Zolghadri M, Pouliot S, Baker K, et al. Adventitia contribution in vascular tone: insights from adventitia-derived cells in a tissue-engineered human blood vessel. FASEB J 2006; 20:1245 - 1247
- Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, et al. Functional arteries grown in vitro. Science 1999; 284:489 - 493
- Laflamme K, Roberge CJ, Pouliot S, D'Orleans-Juste P, Auger FA, Germain L. Tissue-engineered human vascular media produced in vitro by the self-assembly approach present functional properties similar to those of their native blood vessels. Tissue Eng 2006; 12:2275 - 2281
- Swartz DD, Russell JA, Andreadis ST. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am J Physiol Heart Circ Physiol 2005; 288:1451 - 1460
- Liu JY, Swartz DD, Peng HF, Gugino SF, Russell JA, Andreadis ST. Functional tissue-engineered blood vessels from bone marrow progenitor cells. Cardiovasc Res 2007; 75:618 - 628
- Yao L, Liu J, Andreadis ST. Composite fibrin scaffolds increase mechanical strength and preserve contractility of tissue engineered blood vessels. Pharm Res 2008; 25:1212 - 1221
- Yao L, Swartz DD, Gugino SF, Russell JA, Andreadis ST. Fibrin-based tissue-engineered blood vessels: differential effects of biomaterial and culture parameters on mechanical strength and vascular reactivity. Tissue Eng 2005; 11:991 - 1003
- Campbell GR, Campbell JH. Campbell GR, Campbell JH. Phenotypic modulation of smooth muscle cells in primary culture. Vascular smooth muscle in culture 1987; Boca Raton, FL CRC Press Inc 23 - 56
- Owens GK. Schwartz SM, Mecham RP. Molecular identity of smooth muscle cells: overview. The vascular smooth muscle cell: molecular and biological responses to the extracellular matrix 1995; San Diego, CA Academic Press 163 - 167
- Mann BK, Schmedlen RH, West JL. Tethered-TGFbeta increases extracellular matrix production of vascular smooth muscle cells. Biomaterials 2001; 22:439 - 444
- Neidert MR, Lee ES, Oegema TR, Tranquillo RT. Enhanced fibrin remodeling in vitro with TGFbeta1, insulin and plasmin for improved tissue-equivalents. Biomaterials 2002; 23:3717 - 3731
- O'Callaghan CJ, Williams B. Mechanical strain-induced extracellular matrix production by human vascular smooth muscle cells: role of TGFbeta1. Hypertension 2000; 36:319 - 324
- Stegemann JP, Nerem RM. Phenotype modulation in vascular tissue engineering using biochemical and mechanical stimulation. Ann Biomed Eng 2003; 31:391 - 402
- Grassl ED, Oegema TR, Tranquillo RT. A fibrin-based arterial media equivalent. J Biomed Mater Res A 2003; 66:550 - 561
- Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 2001; 12:2730 - 2741
- Massague J, Wotton D. Transcriptional control by the TGFbeta/Smad signaling system. EMBO J 2000; 19:1745 - 1754
- Liu X, Kelm RJ Jr, Strauch AR. Transforming growth factor beta1-mediated activation of the smooth muscle alpha-actin gene in human pulmonary myofibroblasts is inhibited by tumor necrosis factor-alpha via mitogen-activated protein kinase kinase 1-dependent induction of the Egr-1 transcriptional repressor. Mol Biol Cell 2009; 20:2174 - 2185
- Bi D, Nishimura J, Niiro N, Hirano K, Kanaide H. Contractile properties of the cultured vascular smooth muscle cells: the crucial role played by RhoA in the regulation of contractility. Circ Res 2005; 96:890 - 897
- Song YC, Khirabadi BS, Lightfoot F, Brockbank KG, Taylor MJ. Vitreous cryopreservation maintains the function of vascular grafts. Nat Biotechnol 2000; 18:296 - 299
- Tomasek J, Rayan GM. Correlation of alpha-smooth muscle actin expression and contraction in Dupuytren's disease fibroblasts. J Hand Surg Am 1995; 20:450 - 455
- Moussallem MD, Olenych SG, Scott SL, Keller TC 3rd, Schlenoff JB. Smooth muscle cell phenotype modulation and contraction on native and cross-linked polyelectrolyte multilayers. Biomacromolecules 2009; 10:3062 - 3068
- Chen J, Li H, SundarRaj N, Wang JH. Alpha-smooth muscle actin expression enhances cell traction force. Cell Motil Cytoskeleton 2007; 64:248 - 257
- Huynh T, Abraham G, Murray J, Brockbank K, Hagen PO, Sullivan S. Remodeling of an acellular collagen graft into a physiologically responsive neovessel. Nat Biotechnol 1999; 17:1083 - 1086
- Song YC, Hunt CJ, Pegg DE. Cryopreservation of the common carotid artery of the rabbit. Cryobiology 1994; 31:317 - 329
- Song YC, Pegg DE, Hunt CJ. Cryopreservation of the common carotid artery of the rabbit: optimization of dimethyl sulfoxide concentration and cooling rate. Cryobiology 1995; 32:405 - 421
- Miller VM, Bergman RT, Gloviczki P, Brockbank KG. Cryopreserved venous allografts: effects of immunosuppression and antiplatelet therapy on patency and function. J Vasc Surg 1993; 18:216 - 226