Abstract
Currently, almost five million Americans and 23 million people worldwide are living with congestive heart failure (CHF), with 2 million new cases diagnosed each year. The etiology is mostly ischemic, idiopathic, or viral, and more than $36 billion is spent each year on the care of congestive heart failure patients. Treatment of advanced CHF takes three forms: medical therapy, surgical therapy and cardiac replacement. Medical therapy, including inotropes and vasodilators, relieves symptoms by reducing cardiac work and increasing myocardial contractility. This has helped improve quality of life, but mortality remains unaffected. Surgical therapy, including revascularization, ventricular restoration and valve replacement/repair, relieves symptoms and improves function, but in most cases does not stop the underlying disease process from progressing. When conventional medical or surgical therapies are exhausted, cardiac assist or replacement, including ventricular assist device (VAD), heart transplant or a total artificial heart (TAH) may become the only therapeutic options. This article will not discuss the use of extracorporeal membrane oxygenation for cardiac support that is described in an additional article in this issue of Organogenesis.
Introduction
Currently, almost five million Americans and 23 million people worldwide are living with congestive heart failure (CHF), with two million new cases diagnosed each year. The etiology is mostly ischemic, idiopathic or viral, and more than $36 billion is spent each year on the care of congestive heart failure patients.
Treatment of advanced CHF takes three forms: medical therapy, surgical therapy and cardiac replacement. Medical therapy, including inotropes and vasodilators, relieves symptoms by reducing cardiac work and increasing myocardial contractility. This has helped improve quality of life, but mortality remains unaffected. Surgical therapy, including revascularization, ventricular restoration and valve replacement/repair, relieves symptoms and improves function, but in most cases does not stop the underlying disease process from progressing. When conventional medical or surgical therapies are exhausted, cardiac assist or replacement including a ventricular assist device (VAD), heart transplant or a total artificial heart (TAH) may become the only therapeutic options. This article will not discuss the use of extracorporeal membrane oxygenation for cardiac support that is described in an additional article in this issue of Organogenesis.
Background
The impetus for Dr. John Gibbon to develop a heart-lung machine was due to his witnessing of a patient's death due to pulmonary embolism and failed pulmonary embolectomy in 1930. By 1939, Gibbon reported the survival of cats in experiments that involved gradual occlusion of the pulmonary artery while gas exchange and perfusion were taken over by a rotating cylinder film oxygenator. In the late 1940s and early 1950s, Gibbon received engineering help from the IBM Corporation to develop a larger capacity oxygenator, and in May 1953, Dr. Gibbon used the Gibbon-IBM heart-lung machine successfully during the closure of an atrial septal defect in an 18 year-old girl. However, high mortality in the first few cases led Gibbon to abandon the use of his heart-lung machine. After these unsuccessful efforts, C. Walton Lillehei and his colleagues at the University of Minnesota began working in the laboratory with controlled cross-circulation, and in April 1954, they began a series of operations for congenital heart disease using “controlled cross-circulation” with the mother or father as the oxygenator. However, because of the controversy created by Lillehei's procedure, researchers continued efforts to develop a machine that would allow open-heart surgery. By 1955, Dr. John Kirklin at the Mayo Clinic had refined the Mayo-Gibbon machine and the heart-lung bypass techniques that allowed open-heart surgery; likewise, DeWall and Lillehei had developed their machine that also allowed for safe open-heart operations.Citation1 By 1960, Kirklin and Lillehei in Minneapolis and DeBakey and Cooley in Houston had improved their machines and techniques to the point where open heart surgery was becoming “routine” in Minnesota and Texas.
Because of the growing use of cardiopulmonary bypass (CPB) and the increase in open heart surgery, the need arose to develop means of supporting patients with postcardiotomy shock. The intraaortic balloon pump was the first mechanical cardiac assist device and is now the most widely used. The selection of post-CPB support devices has been expanded to include a variety of ventricular assist devices (VAD) to serve as a “bridge to transplantation” or in lieu of transplantation for those who are candidates for destination therapy. The development of an implantable “artificial heart” is one of the ultimate goals of research in this field and is discussed in a later section.
History and Overview
In 1963, DeBakey implanted the first ventricular assist device in a patient suffering a cardiac arrest following aortic valve replacement. The patient subsequently died on post-op day 4. In 1966, DeBakey and Liotta implanted the paracorporeal Liotta-DeBakey LVAD (MicroMed, Houston, TX) in a patient suffering from postcardiotomy shock. The patient was supported for 10 days and ultimately survived to discharge.Citation2 Soon thereafter, Cooley reported the first successful bridge to transplantation using a pneumatically driven, implantable artificial heart.Citation3 Although total artificial heart (TAH) research predates VAD research, the latter developed and advanced more quickly. Clinically, the VAD was first intended to perform temporarily until either the heart function recovered or a donor heart became available for transplantation. Intensive research led to the first clinically usable systems in the late 1980s and culminated in FDA approval of a left ventricular assist device (LVAD) as a bridge to transplantation in 1994, with two more devices receiving approval in 1998.
Patients considered for mechanical circulatory support are those who can no longer sustain adequate systemic oxygen delivery to maintain normal end-organ function despite maximal medical therapy. The current criteria for VAD implantation is NYHA class IV heart failure with failing hemodynamics, persistent pulmonary edema, neurologic or renal failure due to low perfusion, fluid and electrolyte imbalance related to low cardiac output and severe arrhythmias despite medical therapy.Citation4 Patients in chronic decompensated heart failure generally fall into two categories: those who are eligible for cardiac transplantation and those who are not. Mechanical circulatory support can therefore be instituted as either a bridge to transplantation or destination therapy. Patients who are listed for transplant with a long anticipated waiting time due to limited donor availability, blood type, patient size or immunologic sensitization (with the need for prospective cross-matching) may benefit from elective LVAD placement to maintain or restore normal end-organ function. The normalization of hemodynamics and end-organ function with mechanical support in pre-transplant patients has been shown to significantly decrease post-transplant mortality rates.Citation5–Citation7
In 2001, the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) study group evaluated patients with congestive heart failure who were ineligible for transplant. Patients were randomized to receive either optimal medical therapy or LVAD insertion. The study showed survival at 2 years for 23% for those implanted with an LVAD, compared with 8% for those receiving medical therapy alone.Citation8 The results of this study validated the feasibility of using mechanical support as an approach to the treatment of end-stage heart failure.
The operative risk of device implantation must be weighed against the potential lifestyle and survival benefit of mechanical support. The revised Columbia screening scale published in 2003 offers a method of stratifying the risk for LVAD therapy based on several clinical factors: mechanical ventilation, postcardiotomy, prior LVAD insertion, CVp >16 mmHg and prothrombin time >16 seconds.Citation9 Each factor is given a weight with a cumulative score of >5, predicting an operative mortality of 46%, versus a mortality rate of 12%, for a score <5. The timing of intervention is also an important determinant of clinical outcomes. Optimization of the patient's clinical status is important but should not delay institution of support in critically ill patients with severe ventricular failure and ongoing end-organ malperfusion. VAD implantation is contraindicated in patients with irreversible end-organ damage, particularly renal, hepatic or respiratory failure, as these patients have consistently demonstrated poor clinical outcomes. Severe and unrecoverable neurologic injury also represents a contraindication to device implantation.
Types of Circulatory Support Devices
Several modalities exist for providing mechanical circulatory support. Although each device has its own unique characteristics, most of the available pumps can be classified into the following types: counterpulsation pumps, centrifugal pumps, volume-displacement pumps and axial-flow pumps.
Kantrowitz and colleagues reported the first clinical application of an intra-aortic balloon pump (IABP) in 1968 for the treatment of postinfarction cardiogenic shock.Citation10 Counterpulsation, which is synchronized to either ECG or arterial waveforms, provides balloon inflation within the descending thoracic aorta during diastole, with deflation at the onset of systole. The result is a reduction in myocardial work through afterload reduction and improvement in myocardial oxygen supply through augmentation of diastolic blood pressure and coronary perfusion.Citation11
Centrifugal pumps have traditionally been used as short-term support as a means to provide either intraoperative cardiopulmonary bypass or either right, left or biventricular mechanical circulatory support. These pumps generate a vortex either through impellers (Sarns™ centrifugal pump, 3M, Ann Arbor, MI and the St. Jude Lifestream centrifugal pump St. Jude Medical, Chelmsford, MA) or through nested cones (Bio-Medicus Bio-Pump®, Medtronic Bio-Medicus, Inc., Eden Prairie, MN) to drive nonpulsatile blood flow through a circuit. Blood is drawn in through the apex of the cone by the vortex and expelled through a port that is oriented tangential to the housing.Citation12 Unlike positive displacement pumps, these pumps will not generate high forward drive pressure in the face of occlusion and are less prone to pump air, because air in the pump leads to a loss of suction at the vortex. They are widely available, easy to use and relatively low cost. However, they require systemic anticoagulation, are not durable long-term and tend to create high levels of hemolysis.
Volume-displacement (positive-displacement) pumps consist of a chamber or sac that fills and empties cyclically. An external driveline provides electrical power to a motor within the device. The motor drives a pusher plate up and down repeatedly, expanding and compressing the volume-displacement chamber. Inflow and outflow valves maintain the direction of blood flow. These pumps produce a pulsatile flow and mimic the pumping action of the heart. They tend to generate noise during their filling and emptying cycles.
Axial flow (rotary) pumps contain a rotating impeller with helical blades that curve around a central shaft. An external driveline provides electrical power to a motor that drives the rotation of the impeller by electromagnetic induction. The spinning impeller draws blood from the inflow cannula to the outflow cannula, and blood flow is essentially non-pulsatile. These pumps are typically quiet and use less power than the pulsatile devices. Due to a decreased number of moving parts and contact bearings, they offer the distinct advantage of enhanced durability.
In the current era, continuous-flow blood pumps (centrifugal pumps and axial flow pumps) represent a significant advance in mechanical circulatory support, particularly due to the enhanced mechanical longevity when compared with pulsatile systems. Questions remain as to the long-term physiological effect of continuous non-pulsatile blood flow. Despite this theoretical concern, clinical experience to date has been encouraging,Citation3,Citation16–Citation21 and recent evidence suggests that normal end-organ function can be maintained with continuous non-pulsatile blood flow with no adverse effects or increased morbidity or mortality.Citation22,Citation23
Short-Term Support Devices
Short-term support devices have the advantage of relative ease of implementation and provide either a bridge to recovery or a bridge to a more long-term ventricular support device. They are used primarily in clinical situations requiring support for several hours, days or up to two weeks.
IABP counterpulsation is used today in a variety of clinical settings, including cardiogenic shock associated with myocardial infarction, postcardiotomy shock, mechanical complications of infarction, such as acute mitral regurgitation and VSD, postinfarction angina and for the treatment of refractory ventricular arrhythmias in the setting of ongoing ischemia. IABP has also been used preoperatively as an adjunct to high-risk percutaneous interventions or coronary artery bypass grafting. Current models include Autocat 2 WAVE® (Arrow, Reading, PA) and the Datascope C5100® (Maquet, Mahwah, NJ).
Bio-Medicus, St. Judes and Sarns are three of the most commonly used centrifugal pumps (). These pumps utilize a spindle/bearing design to directly turn the spinning rotor. This “direct drive” method has been implicated in the high incidence of hemolysis and thrombus formation. A new generation of centrifugal pumps are now available that eliminate the central drive shaft/bearing. Each of these new generation pumps can generate in excess of 9 L/min of blood flow with very low priming volumes. Two of the most common examples of these pumps are the Jostra Rotaflow® (Maquet, Wayne, NJ) and the CentriMag® (Levitronix, Waltham, MA). The Rotaflow® (32 ml prime) is magnetically stabilized on a mono-pivot with a sapphire bearing. The Centrimag utilizes a magnetically levitated impeller to avoid the central drive shaft. Currently there are two sizes available, adult (32 ml prime) and pediatric (14 ml prime), which can be utilized with the same pump console, depending on the patient size. Early clinical experience has shown these newer generation pumps to be more durable and less prone to thrombus and hemolysis generation.
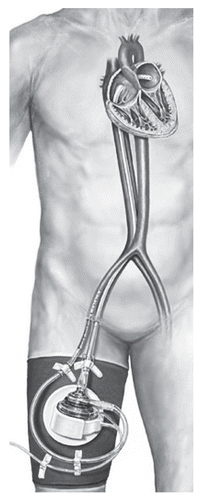
The TandemHeart™ (CardiacAssist Inc., Pittsburgh, PA) percutaneous VAD uses a centrifugal pump that pumps blood from the left atrium to one or both femoral arteries (). A transseptal cannula allows direct unloading of the left heart at blood flow rates sufficient to support patients until cardiac recovery occurs or until a long-term device can be implanted.Citation13
Abiomed BVS 5000i (Abiomed Inc., Danvers, MA) is an FDA-approved device for acute postcardiotomy failure (). It is a dual-chambered, pneumatically driven extracorporeal pump designed for short-term cardiac support. Blood is drained into the upper atrial chamber under gravity, then the lower ventricle is ejected, according to the filling conditions, with pneumatically driven ejection. The device is capable of generating pulsatile flow of up to 6 L/min. The ease of implantation, operation, weaning, cost effectiveness and widespread availability have made it one of the most commonly used devices in the setting of acute cardiac failure. Disadvantages include the need for systemic anticoagulation and very limited patient mobility during support.
The Impella Recover device (Impella CardioSystems AG, Aachen, Germany) is a miniaturized, catheter-based axial flow pump designed for short-term, left ventricular support (). It is able to generate flows of up to 5 L/min, with the advantage of ease of implantation and minimal requirement for anticoagulation. The device can be placed percutaneously using ECHO guidance and is designed for up to seven days use.Citation14,Citation15
First-Generation VADs
First-generation VADs deliver pulsatile blood flow and are mainly positive-displacement pumps (see ).
HeartMate XVE (Thoratec Inc., Pleasanton, CA) is an electrically vented unit with a portable control console and batteries. The pumping chamber uses 25 mm porcine valves in the inflow and outflow conduits, generates pulsatile flow with a stroke volume of 83 mL and a maximal output of 10 L/min. Due to the size and physical characteristics of the HeartMate XVE LVAD, patients undergoing implantation must have a body surface area of at least 1.5 m2. A unique feature of the device is the titanium and textured polyurethane internal surfaces, which encourage the deposition of circulating cellular elements and the formation of a pseudointima. This allows the device to have a relatively low incidence of thromboembolic events without the need for systemic anticoagulation with warfarin.Citation24 The FDA has approved the HeartMate XVE LVAD as a bridge to transplantation, and it was the first device approved for destination therapy.
Thoratec (Pleasanton, CA) also offers both an intracorporeal (Thoratec® IVAD) and a paracorporeal (Thoratec® PVAD) VAD system. They both allow the option of right, left or biventricular support and are approved for use as a bridge to transplantation and for support in the setting of postcardiotomy shock (). They provide pulsatile flow with a 65 ml blood chamber and unidirectional flow, achieved with tilting disk mechanical valves. The paracorporeal placement of the PVAD allows use in patients with BSAs of <1.5 m2 and has been used successfully in patients <0.8 m2. Both the intracorporeal and paracorporeal devices require systemic anticoagulation with warfarin.
Novacor Left Ventricular Assist System (LVAS) (World Heart Inc., Oakland, CA) is a device that was first successfully used as a bridge to transplantation in 1984 and is FDA approved for this indication. It generates pulsatile blood flow of up to 9 L/min. Unidirectional flow is achieved with bioprosthetic valves in both the inflow and outflow conduits and requires systemic anticoagulation with warfarin. World Heart discontinued the distribution of the Novacor LVAS in 2008 and now focus their research efforts on newer-generation VADs.
Arrow LionHeart LVAD 2000 (Arrow International Inc., Reading, PA) is a totally implantable pulsatile VAD designed with the goal of destination therapy for patients in end-stage heart failure. Implantable components of the device include the actual titanium blood pump with inflow and outflow assemblies, motor controller, a compliance chamber and a transcutaneous energy transmission system.Citation2 There are no percutaneous lines or connections, and recharging of the batteries is accomplished through a transcutaneous system with a wand overlying the recharging coil implanted under the skin. The pump has a stoke volume of 64 mL and generates pulsatile blood flow of up to 8 L/min. Arrow discontinued the production of the LionHeart LVAD in 2005.
Second-Generation VADs
Second-generation pumps are mostly rotary axial flow pumps with bearings immersed in blood or that can deliver diminished pulsatile or continuous blood flow. These devices are in different stages of development, testing and clinical use.
Micromed DeBakey VAD (MicroMed Technology Inc., Houston, Texas) is an axial flow pump measuring only 3.0 cm in diameter and weighing 95 grams. The impeller/inducer is capable of generating flows of up to 10 L/min. Components of the pump include a titanium inflow cannula, a flowmeter, a Dacron outflow graft and a percutaneous cable that connects to a portable control console and battery packs.Citation26 The rigid inflow cannula is inserted in the apex of the left ventricle and determines the position of the pump housing on the diaphragm during implantation. The device is currently in a redesign phase secondary to a high incidence of thromboembolic complications.
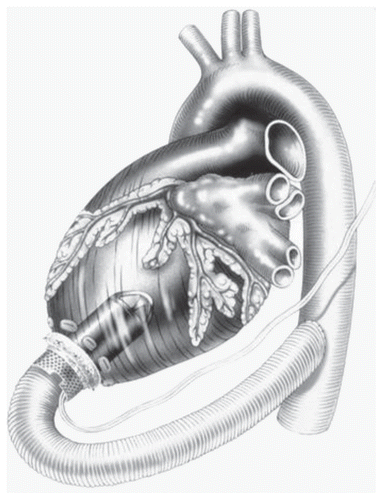
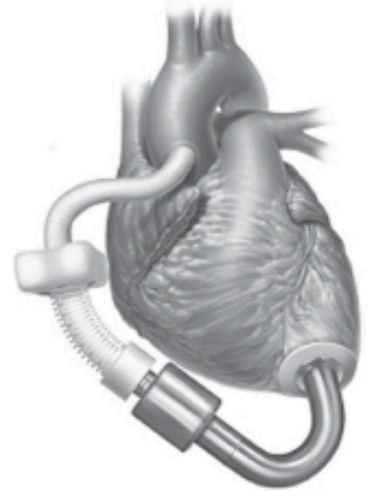
Jarvik 2000 (Jarvik Heart Inc., New York, NY) is an electromagnetically actuated pump constructed of titanium measuring 2.5 cm in diameter and weighing 90 grams. The impeller can generate maximum flows of 7 L/min. The actual pumping chamber is implanted within the left ventricular chamber through the apex and the outflow graft is anastomosed to the ascending or descending aorta (). The pump implantation can be done through a left thoracotomyCitation27 or a median sternotomy.
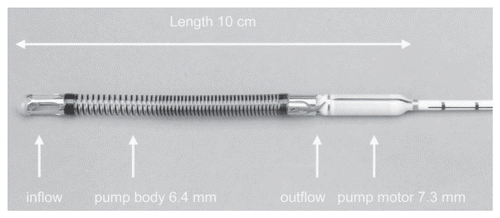
HeartMate II (Thoratec Inc., Pleasanton, CA) is an LVAD axial flow device that can generate flows of up to 10 L/min, weighs 176 grams and measures 40 mm in diameter (). Early clinical trials show very effective hemodynamic support with improved functional status and improved quality of life.Citation28–Citation30 The HeartMate II was FDA approved in January 2010 for use as destination therapy in adults with heart failure. Over 4000 HeartMate II devices have been implanted world-wide,Citation85 making it one of the most frequently used and successful VAD's in the current era.
Third-Generation VADs
Presently, over 20 new ventricular assist devices are either in development or in investigational use. These newer-generation devices try to address the shortcomings of current device technology, such as thromboembolic complications, device-related infection and limited durability. Many third-generation devices make use of magnetic levitation technology, in which a rotating impeller is magnetically suspended within a column of blood, obviating the need for contact-bearing moving parts. This provides the theoretical advantage of enhanced long-term durability. Continuous flow pumps are also smaller in size, allowing easier implantation with less surgical trauma and potentially decreased infectious complications. Advances in energy systems have also allowed for totally implantable devices without the need for percutaneous lines.
Incor® LVAD (Berlin Heart AG, Berlin, Germany) is a magnetically-actuated axial flow pump that weighs 200 grams and is 3 cm in diameter. An impeller that is held in place by a magnetic bearing creates flow, and the impeller is not in contact with any other parts. It is capable of producing flow of up to 7 L/min.Citation31
VentrAssist™ left ventricular assist system (Ventracor Inc., Foster City, CA) is a centrifugal pump that has a hydrodynamically suspended rotor as its only moving part. The design of the pump maximizes blood flow across all blood-contacting surfaces to avoid stasis and the potential for thrombus formation. The pump weighs about 300 grams and measures 2.5 inches in diameter.Citation32–Citation34
WorldHeart has a newer-generation LVAD in development and preclinical testing. The Levacor™ VAD (World Heart Inc., Oakland, CA) is a magnetically levitated centrifugal pump designed for either bridge-to-transplantation or destination therapy.
Thoratec is also in the process of preclinical evaluation of the HeartMate III (Thoratec Inc., Pleasanton, CA), which is a magnetically suspended centrifugal pump that uses transcutaneous energy transfer system technology to be totally implantable.Citation35,Citation36
Other devices in development and clinical trials that employ magnetic levitation technology include the Terumo Dura-Heart™ (Terumo Heart Inc., Ann Arbor, MI),Citation37 the CorAide blood pump (The Cleveland Clinic Foundation, Cleveland, OH)Citation38,Citation39 and the Heartware HVAD™ (HeartWare Ltd., Sydney, Australia).Citation40,Citation41
VAD complications.
Mediastinal bleeding following LVAD implantation is relatively common. Predisposing factors include hepatic congestion and dysfunction related to chronic heart failure, compromised nutritional status, the use of preoperative anticoagulation, extensive surgical dissection, reoperative procedures, prolonged CPB and coagulopathy secondary to interactions between circulating blood elements and the artificial device surfaces.Citation42 Due to the requirement of systemic anticoagulation for many devices, as well as the routine use of aspirin as an antiinflammatory agent, late bleeding and tamponade can occur as well.
Infection remains a significant source of morbidity and mortality in patients receiving mechanical circulatory support. In the REMATCH trial, the leading cause of death in patients receiving devices was sepsis, accounting for 20 of 52 deaths in this group.Citation8 A subsequent analysis focusing on infection during the REMATCH trial showed that freedom from sepsis in patients with an LVAD was 58% at 1 year and 48% at 2 years. The peak hazard for sepsis occurred within 30 days from implantation.Citation43 Infections in LVAD patients can affect different components of the device. These include infections at the driveline exit site, device pocket infections, device endocarditis and blood stream infection.Citation44 Bacteria that are able to form a biofilm are common pathogens, including staphylococcus, pseudomonas, enterococcus and candida.Citation45,Citation46 Other patient factors that may increase susceptibility to infection include generalized debilitation and malnutrition, diabetes, renal failure and immunologic derangements. Overall infection rates in patients receiving devices have been approximately 50%,Citation44,Citation47 and up to 25% of deaths in LVAD patients are due to systemic sepsis.Citation46,Citation48
Thromboembolic events can lead to devastating neurologic and end-organ injury and remain a significant concern in patients undergoing mechanical device placement. In the REMATCH trial, the rate of neurologic events was 4.35 times higher than in the medically treated group, with 47% of such events being transient.Citation8 All device patients are maintained on aspirin, mainly for its anti-inflammatory effect, and most devices presently require anticoagulation with heparin and warfarin. In addition, depending on the system used, many patients are maintained on anti-platelet therapy with dipyridamole and clopidogrel.
Right heart failure occurs in approximately 20% of patients undergoing LVAD placement.Citation49 A recent series from Columbia University examined the incidence of right heart failure in patients with chronic CHF undergoing LVAD implantation.Citation50 Right-sided circulatory failure was seen in 39%, with approximately 35% of those patients requiring implantation of an RVAD. Post-op right ventricular failure has a significant effect on clinical outcomes, leading to increased ICU stay, increased 30 day mortality following LVAD implantation and a lower bridge-to-transplantation rate.Citation50–Citation52
Right-sided circulatory failure can result from abnormalities in the right ventricle as well as in the pulmonary vascular bed. Preoperative factors include myocardial stunning, ischemia, infarction, air embolism and arrhythmias. Changes in ventricular interdependence and septal shifting secondary to mechanical unloading of the LV can also contribute to impaired right-sided function.Citation53 In addition, preexisting right ventricular dysfunction may be unmasked secondary to the augmented preload presented to the right side following LVAD implantation.Citation51 Improvements in medical management of RV failure have resulted in a decreased incidence of RVAD implantation.Citation54,Citation55 When RVAD support is necessary, improved clinical results have been demonstrated by early implantation within 24 hours.Citation74
Device failure and durability are critical factors in establishing mechanical support as a feasible option for providing long-term support and destination therapy. In the REMATCH trial, device failure was the second most common cause of death, after sepsis.Citation44 While 1-year freedom from device failure and replacement was 87%, this dropped off to 37% by the second year.Citation8,Citation56
Device failure can occur in any of the components of the system. These include the inflow and outflow conduits, the pumping chamber or the external components of the system, such as the driveline, power source or controller units. As clinical experience with individual devices accumulates, modes of failure that are amenable to design change become apparent, allowing for device modification and improvement. Design alterations during and since the conclusion of the REMATCH trial in the HeartMate LVAD include a bend relief to prevent outflow graft kinking, modification in the inflow assembly to facilitate valve replacement and smaller size and greater compliance of the percutaneous driveline.Citation56 Newer-generation axial and centrifugal flow pumps attempt to address the shortcomings of first-generation devices with smaller pumping chambers and drivelines, transcutaneous energy sources and the use of magnetic levitation technology to eliminate contact-bearing moving parts, offering the hope of enhanced durability.
Researchers have begun working toward eliminating or drastically reducing the more common causes of device failures, decreasing size and weight, increasing efficiency and advancing several new concepts. These new concepts include non-blood contacting systems, biological augmentation and small volume centrifugal and axial flow pumps characterized by continuous or diminished pulse flow and blood-lubricated, plasma-lubricated or magnetically levitated bearings.Citation57 By nature of their operation, rotary blood pumps eliminate the need for venting gas from the system, resulting in a smaller diameter percutaneous driveline. Smaller caliber drivelines are believed to reduce the risk of infection, which is the most frequently occurring clinical adverse event associated with VADs.
Total Artificial Heart
In 1958, Domingo Liotta of Argentina began work on a fully implantable heart, and he presented a prototype of his artificial heart at the American Society of Artificial Organs in 1961. Liotta continued his work with Dr. Denton Cooley, and the two began testing their TAH in calves in 1969. Eventually, after several modifications, the Liotta TAH was ready for clinical testing. This was a pneumatically powered, double-chambered pump with Dacron-lined right and left inflow cuffs and outflow grafts. Hingeless valves controlled the direction of blood flow through the pump. The TAH itself was connected to a large external power unit.Citation58 The first implantation of a TAH into a human was done by Cooley on April 4, 1969 in a 47 year-old man who could not be weaned from CPB following left ventricular aneurysmectomy. The TAH performed adequately for 64 hours, pumping at 6 L/min,Citation59 until transplantation; the donor heart also functioned well, but the patient died of pseudomonal pneumonia 32 hours after transplantation. Though the Liotta device performed as designed, it was never used clinically again. The next artificial human heart implant was not performed for another 12 years.
In the summer of 1981, the Akutsu-III TAH was implanted into a patient after coronary bypass surgery complicated by postcardiotomy failure and ventricular fibrillation. This was a pneumatically driven pump, similar to the Liotta, but with refinements in material and valve design as well as an electrical monitoring system to follow rate, systolic durations and driveline vacuum pressures. The patient was sustained for approximately 6 hours until a donor heart became available for transplantation. However, the problem of infection and multiorgan failure, experienced with the Liotta TAH, led to the patient's death 7 days post-op.Citation58,Citation60
In the late 1970s, Dr. Willem Kolff and his team at the University of Utah developed the Jarvik-7 TAH (Jarvik Heart Inc., New York, NY), and in 1982 Dr. William DeVries and colleagues successfully implanted the Jarvik-7 total artificial heart into a dying man.Citation61,Citation62 The Jarvik-7 was a pneumatically powered, biventricular pulsatile device that replaced the heart.Citation61 In the initial clinical experience with the Jarvik-7, a total of five patients were permanently supported for periods ranging form 10 to 620 days. The Jarvik-7 was able to adequately support circulation, but its large drive console and frequent medical complications limited patient activity, and long-term outcomes were poor. Patients supported for longer periods suffered several complications, including thromboembolism, stroke, infection and multi-organ failure.Citation61,Citation63
In 1985 the Jarvik-7 (renamed the Symbion) entered clinical trials as a bridge to transplantation, and in 1986, Copeland reported the first successful use of this device for this indication.Citation64 Between 1985 and 1991, approximately 170 patients were supported with the Symbion TAH as a bridge to transplant. The FDA withdrew the device's investigational device exemption for clinical use in January 1991 because of inadequate compliance with FDA regulations,Citation65 but in January 1993, the investigational device exemption was restored to what was now called the CardioWest TAH (Syncardia Systems Inc., Tuscon, AZ), which differed little from the original Jarvik-7 ().
In the mid 1980s, artificial hearts were powered by dishwasher-sized pneumatic power sources (), and two sizeable catheters had to cross the body wall to carry the pneumatic pulses to the implanted heart, greatly increasing the risk of infection. To speed development of a new generation of technologies, the National Heart, Lung and Blood Institute opened a competition for implantable, electrically powered artificial hearts.
On October 15, 2004, the CardioWest became the first TAH to receive FDA approval. It is a pulsatile, pneumatically driven prosthetic pair of ventricles made of polyurethane, with Medtronic-Hall mechanical valves between chambers to maintain unidirectional flow. Each prosthetic ventricle has an air sac and a blood sac, and between the sacs is a four-layered polyurethane diaphragm. As air flows into the air sac, the diaphragm expands and provides a compression force against the blood sac and mimics cardiac systole. When the air sac is deflated, the diaphragm relaxes, and the blood sac fills with blood for diastole. The compressed air is provided by an external console that flows to the device via two intrathoracic ports, one port to each ventricle. The external console allows for regulation of rate, systolic duration and driving pressures for each ventricle. The maximum stroke volume is 70 mL with a cardiac output >9 L/min.Citation66 The CardioWest TAH has been used successfully in the US, Canada and France.Citation66
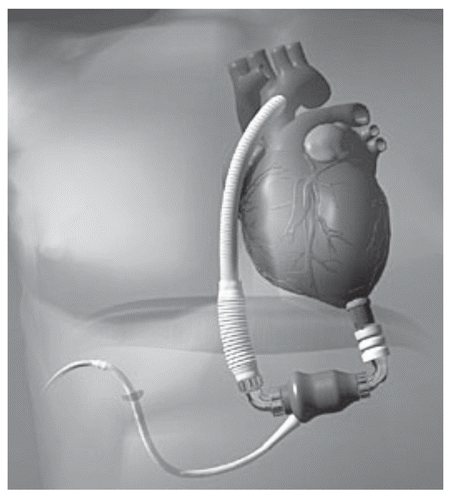
In 2001, as part of an FDA-sponsored phase I clinical trial, surgeons in Louisville, KY performed the first implantation of the AbioCor TAH (ABIOMED Inc., Danvers, MA) in a 59 year-old man suffering from end-stage CHF.Citation67 It is a self-contained, electrohydraulic TAH that is fully implantable and communicates to external hardware without penetrating the skin. The device utilizes a transcutaneous energy transfer system and a radiofrequency communication system that allows it to be powered and controlled by signals transmitted across intact skin.Citation68 A unique feature is a right-left flow-balancing mechanism that eliminates the need for an external vent or internal compliance chamber.Citation69 The thoracic unit (pump) weighs about two pounds and consists of two artificial ventricles, four valves and an innovative motor-driven hydraulic pumping system (). A miniaturized electronics package implanted in the patient's abdomen monitors and controls the pump rate, right-left balance and motor speed. In Phase I feasibility trials, 14 patients in five centers received the AbioCor as destination therapy. In these patients, thromboembolism was a significant adverse event, and there were two pump failures, but device-related infections were relatively rare. Cardiac output from the device may reach 8 L/min with a stroke volume of 60 mL.Citation70 The AbioCor replacement heart received FDA approval under a Humanitarian Device Exemption in September 2006. It is approved for use in severe, biventricular end stage heart disease patients who are not eligible for heart transplant and have no other viable treatment options.
Indications for implantation of a total artificial heart include irreversible biventricular heart failure, acute decompensation after cardiotomy, cardiogenic shock after acute myocardial infarction, stone heart, irreversible cardiac rejection, failed LVAD or BiVAD, decompensating heart failure with left ventricular thrombus, acquired ventricular septal defect, prosthetic or incompetent native aortic valve in cardiogenic shock or unresponsive ventricular arrhythmias.Citation71 The AbioCor is only indicated in non-transplant-eligible patients and uses a scoring system to determine eligibility.Citation72 However, the respective devices generally share the same guidelines for TAH insertion.
TAH complications.
Impediments to the widespread use and application of the TAH are related to several serious complications. The most frequent complications are infection, severe post-op bleeding and thromboembolism.Citation73–Citation75 Potentially serious but less frequent complications are renal, hepatic, pulmonary and neurologic dysfunction and complications due to technical problems.Citation75 Other complicating factors for its use include patient selection, device size, implantation timing and location, the need for extensive surgery at implantation and the reliability of support equipment.
Life-threatening infections have been the most important complication. In the Jarvik-7 experience, all patients supported for many months developed serious infections that eventually contributed to their deaths.Citation76 Patients supported by a TAH for shorter periods while awaiting heart transplantation had infection rates of 30 to 40%.Citation74,Citation75 In the more recent bridge-to-transplant experience with the CardioWest TAH, the infection rate was no more than 20%.Citation76,Citation77
Post-op bleeding is a frequent and serious complication, occurring in 40 to 50% of TAH or VAD recipients.Citation73 Contributing factors include severe CHF and associated hepatic dysfunction, the extensive surgery and lengthy CPB time required for implantation and the necessity for postoperative anticoagulation therapy.
Thrombosis within the TAH is of particular concern. Five of the first six Jarvik-7 recipients suffered thromboembolic events. Frequency has decreased significantly since that initial experience and is now an estimated 10 to 15%.Citation73,Citation74,Citation78 Generally, heparin and warfarin are used as antithrombotic therapy, and aspirin or dipyridamole or both are also used.
Sizing and portability issues have also been significant problems. The AbioCor device is quite large, and in its current form it will only fit in approximately 50% of men, 20% of women and no children.
Pediatric VADs
Acute heart failure can occur in children as a result of hemodynamic insults imposed on the heart by structural defects or in a structurally normal heart with myocarditis. When present, acute heart failure in children justifies aggressive therapy because of the high potential for complete recovery. Mechanical circulatory support devices have been used successfully as a bridge to recovery in children, especially in the management of acute fulminant myocarditis or postcardiotomy heart failure.Citation57,Citation80,Citation81 The use of these devices as a bridge to transplantation has also been shown to decrease waiting list mortality and improve the efficiency of organ utilization in children.Citation82 However, current mechanical circulatory support options for infants and children are quite limited, especially with regard to size and duration of support. The devices currently available include the Berlin Heart EXCOR® (Berlin Heart GmbH, Berlin, Germany) the Medos HIA (MEDOS Medizintechnik GmbH, Stollberg, Germany), and the MicroMed Debakey VAD Child (MicroMed Technology Inc., Houston, TX).
The Berlin Heart EXCOR pediatric VAD was the first commercially available ventricular assist system designed specifically for the pediatric population, with miniaturized pumps and special cannula. It is a paracorporeally placed, air-driven pump in a polyurethane, translucent, semi-rigid chamber that houses the blood-contacting membrane. The blood pumps come in 10, 12, 15, 25 or 30 mL stroke volumes (and adult sizes up to 80 ml) and offer support options for pediatric patients as small as 2.5 kg (). The pumps are driven by a pulsatile electropneumonic system and can be used as a right, left or biventricular device. The EXCOR has been widely implanted in European centers, and experience in the US is growing rapidly.
The Micromed Debakey VAD Child is a miniaturized electromagnetically actuated axial flow pump that is fully implantable (). It provides nonpulsatile assistance for the heart through continuous unloading of the left ventricle. This device is currently the only VAD that is FDA approved for children 5–16 years of age with a BSA between 0.7 and 1.5 m2. It is approved as a bridge to transplantation for patients with end-stage heart failure who are refractory to medical therapy.
The Medos HIA VAD is similar to the Berlin Heart VAD. It is a pulsatile VAD, pneumatically driven with a pump chamber made of polyurethane and is available in many sizes from 10 to 80 mL. It can also be used in a right, left or biventricular configuration.
It is estimated that the number of children that might benefit from support with ventricular assist devices ranges from 100–500 per year in the US.Citation83 Designing circulatory support devices for the pediatric patient is challenged by the wide range of patient size, the small size and fragility of the vasculature along with significant anatomic variations that may affect cannulation. Recognizing these limitations, the National Heart, Lung and Blood Institute solicited proposals to develop novel circulatory support systems for infants and children. The types of devices intended to be developed included left and right VADs, extracorporeal membrane oxygenation (ECMO) systems and other novel bioengineered systems for children ranging in weight from 2 to 25 kg. The goals set for the ideal pediatric device included: (1) routine deployment and functioning in less than one hour after the decision to initiate support, (2) low priming volume, (3) flexible cannulation suitable for abnormal anatomy, (4) minimal blood product exposure, (5) minimal risk of infection, bleeding, hemolysis and thrombosis and (6) suitability for long-term support (6 months).Citation84 The following five projects were awarded contracts and are currently in development.
The PediaFlow™ VAD (LaunchPoint Technologies Inc., Goleta, CA) is an implantable, magnetically suspended mixed-flow turbodynamic blood pump that is being designed and developed to provide chronic (6 months) circulatory support to patients from birth to 2 years of age (3 to 15 kg body weight) with congenital or acquired heart disease. The device is based on rotary blood pump technology, has a single percutaneous lead crossing the skin for energy transmission and may be used as a right, left or biventricular device.
The PediPump™ (Foster-Miller Technologies, Albany, NY) is a magnetic, bearing-supported, rotary dynamic circulatory support pump designed specifically for children. There will be two configurations for deployment based on patient size, with intravascular implantation in larger children and extravascular, intracorporeal implantation for smaller children.
The Pediatric Cardiopulmonary Assist System (pCAS) (Ension Inc., Pittsburgh, PA) can deliver both continuous and pulsatile flow. The system is based around a compact, paracorporeal rotary flow device capable of simultaneously pumping blood and providing oxygenation. The device rotor is fabricated from layers of microporous hollow fibers that include a custom coating to both increase fiber life and minimize requirements for systemic anticoagulation.
The Pediatric Jarvik 2000 (Jarvik Heart Inc., New York, NY) is an axial-flow blood pump designed to support patients in all size ranges (3 to 25 kg) (). These devices will be implanted in the LV apex with the pump totally in the LV chamber as with the adult Jarvik 2000.
The Penn State Pediatric Ventricular Assist Device (PVAD) is a pulsatile, pneumatically actuated blood pump. It is intended primarily for paracorporeal placement but will also be implantable for bridge to transplantation applications. It will be available in two sizes with stroke volumes of 12 and 25 ml. It can be used for left, right or biventricular support for up to 6 months.
Conclusion
The advancements in mechanical circulatory support have been remarkable over the last fifty years. This initial progress laid the cornerstone of open heart surgery through use and refinement of the heart-lung machine and paved the way for the development of ventricular assist devices and the total artificial heart. The quality and quantity of life for heart failure patients worldwide are now being improved by the use of cardiac assist devices as a bridge to heart transplant or myocardial recovery, and more recently, as a destination therapy. Miniaturization of these devices now promises similar support for treatment of heart failure in the pediatric population. Future trends in the development of newer devices will predictably include: further miniaturization of pumps for increased implantability and biventricular support, use of less thrombogenic materials to reduce the need for anticoagulation, improved pump durability by refinements in design, materials and the use of newer technologies, increased use of transcutaneous energy systems to avoid the need for drive lines exiting the body, use of improved and miniaturized energy sources to allow heart failure patients to live more “untethered” and unrestricted lives and, ultimately, the further development of a durable, reliable and totally implantantable device for effective long-term replacement of the human heart.
Abbreviations
| CHF | = | congestive heart failure |
| CPB | = | cardiopulmonary bypass |
| IABP | = | intra-aortic balloon pump |
| TAH | = | total artificial heart |
| VAD | = | ventricular assist device |
| LVAD | = | left ventricular assist device |
| CVP | = | central venous pressure |
| ECG | = | electrocardiogram |
| VSD | = | ventricular septal defect |
| BiVAD | = | biventricular assist device |
| DT | = | destination therapy |
Figures and Tables
Figure 1 Three commonly used first generation centrifugal pumps (Ann Thorac Surg 1999; 68:666–71; , and ).
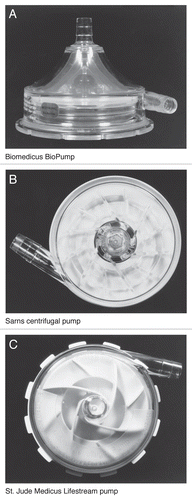
Figure 3 Bicaval implant technique. For RVAD support, the body of the right atrium is cannulated away from the superior and inferior vena caval anastomoses. Outflow from the RV AD is to a graft cannula sewn to the main pulmonary artery (PA). The graft is placed just distal to the pulmonary valve but proximal to the anastomosis between the donor and recipient PA. For LVAD support, the recipient left atrial cuff is cannulated adjacent to the right pulmonary vein, providing drainage to the pump. Outflow from the LVAD is to a graft cannula sewn to the recipient aorta just above the aortic valve (J Thorac Cardiovasc Surg 2003; 126:442-447; ).
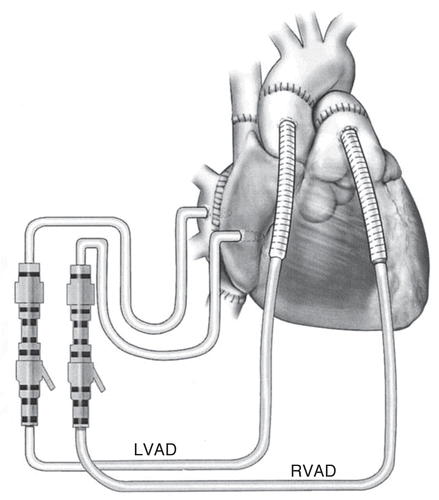
Figure 5 First-generation pulsatile devices. HeartMate LVAD (left), Thoratec pVAD (center) and Novacor VAD (right) (Curr Opin Org Transp 2009; 14:554–9; ).
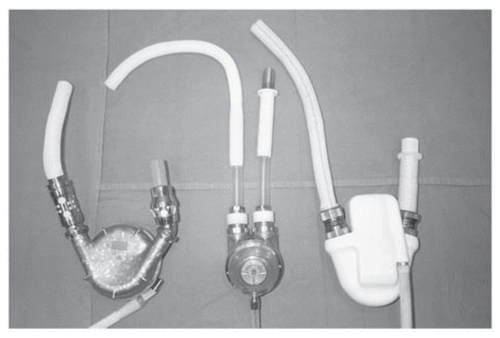
Figure 6 Thoratec pVAD implant configurations allow for LVAD, RVAD and BiVAD support, reprinted with permission from Thoratec Corporation.
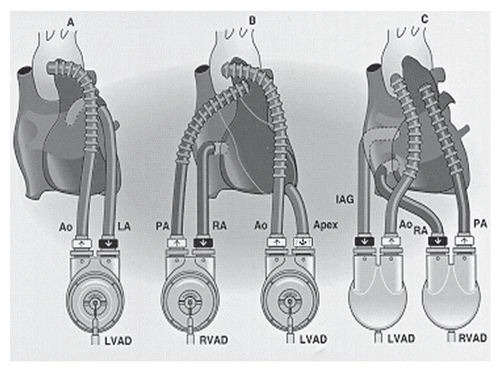
Figure 9 (A) CardioWest total artificial heart. (B) Implanted with Console (Ann Thorac Surg 1999; 68:698–704; and ).
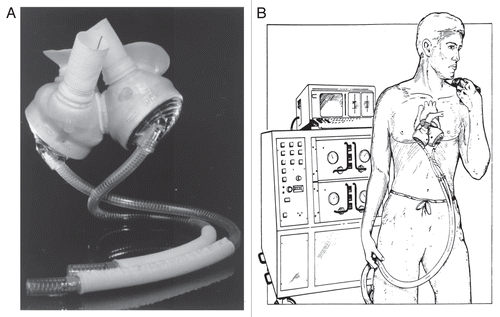
Figure 10 (A) The AbioCor total artificial heart (TAH) implanted, reprinted with permission from Abiomed, Inc. (B) AbioCor TAH components (J Thorac Cardiovasc Surg 2004; 127:131–41; ).
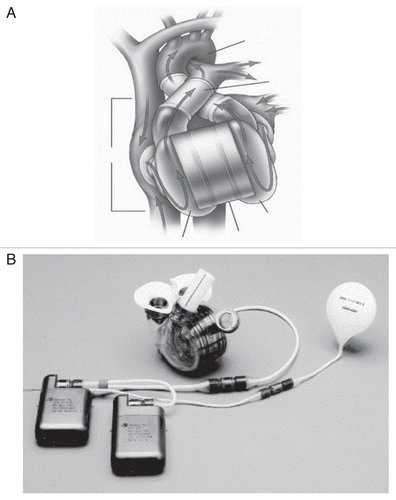
Figure 11 Berlin Heart EXCOR VAD for pediatric and adult use, reprinted with permission from Berlin Heart, Inc.
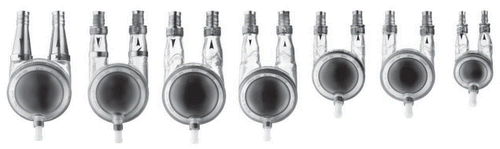
Figure 12 MicroMed Debakey CHILD LVAD, courtesy of www.micromedcv.com.
References
- Lillehei CW, Dewall RA, Read RC, Warden HE, Varco RL. Direct vision intracardiac surgery in man using a simple, disposable artificial oxygenator. Dis Chest 1956; 29:1 - 8
- DeBakey ME. Development of mechanical heart devices. Ann Thorac Surg 2005; 79:2228 - 2231
- Gemmato CJ, Forrester MD, Myers TJ, Frazier OH, Cooley DA. Thirty-Five years of mechanical circulatory support at the Texas Heart Institute. Tex Heart Inst J 2005; 32:168 - 177
- Mudge GH Jr, Fang JC, Smith C, Couper G. The physiologic basis for the management of ventricular assist devices. Clin Cardiol 2006; 29:285 - 289
- Frazier OH, Rose EA, McCarthy P, Burton NA, Tector A, Levin H, et al. Improved mortality and rehabilitation of transplant candidates treated with a long-term implantable left ventricular assist system. Ann Surg 1995; 222:337 - 338
- Dang NC, Topkara VK, Kim BT, Mercando ML, Kay J, Naka Y. Clinical outcomes in patients with chronic congestive heart failure who undergo left ventricular assist device implantation. J Thorac Cardiovasc Surg 2005; 130:1302 - 1309
- Bank AJ, Mir SH, Nguyen DQ, Bolman RM, Shumway SJ, Miller LW, et al. Effects of left ventricular assist devices on outcomes in patients undergoing heart transplantation. Ann Thorac Surg 2000; 69:1369 - 1374
- Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term mechanical left ventricular assist device for end-stage heart failure. N Engl J Med 2001; 345:1435 - 1443
- Rao V, Oz MC, Flannery MA, Catanese KA, Argenziano M, Naka Y. Revised screening scale to predict survival after insertion of a left ventricular assist device. J Thorac Cardiovasc Surg 2003; 125:855 - 862
- Kantrowitz A, Tjonneland S, Freed PS, Phillips SJ, Butner AN, Sherman JL Jr. Initial clinical experience with intraaortic balloon pumping in cardiogenic shock. JAMA 1968; 203:113 - 118
- Papaioannou TG, Stefanadis C. Basic principles of the intraaortic balloon pump and mechanisms affecting its performance. ASAIO 2005; 51:296 - 300
- Baughman KL, Jarcho JA. Bridge to life—cardiac mechanical support. N Engl J Med 2007; 357:846 - 849
- Kar B, Adkins LE, Civitello AB, Loyalka P, Palanichamy N, Gemmato CJ, et al. Clinical experience with the TandemHeart® percutaneous ventricular assist device. Tex Heart Inst J 2006; 33:111 - 115
- Wheeldon DR. Mechanical circulatory support: state of the art and future perspectives. Perfusion 2003; 18:159 - 169
- Samoukovic G, Rosu C, Giannetti N, Cecere R. The Impella® LP 5.0 as a bridge to long-term circulatory support. Interact CardioVasc Thorac Surg 2009; 8:682 - 683
- Litwak KN, Kihara S, Kameneva MV, Litwak P, Uryash A, Wu Z, et al. Effects of continuous flowlLeft ventricular assist device support on skin tissue microcirculation and aortic hemodynamics. ASAIO Journal 2003; 49:103 - 107
- Ootaki Y, Kamohara K, Akiyama M, Zahr F, Kopcak MW, Dessoffy R, et al. Phasic coronary blood flow pattern during a continuous flow left ventricular assist support. Eur J Cardiothorac Surg 2005; 28:711 - 716
- Letsou GV, Myers TJ, Gregoric ID, Delgado R, Shah N, Robertson K, et al. Continuous axial-flow left ventricular assist device (Jarvik 2000) maintains kidney and liver perfusion for up to 6 months. Ann Thorac Surg 2003; 76:1167 - 1170
- Saito S, Westaby S, Piggot D, Dudnikov S, Robson D, Catarino PA, et al. End-organ function during chronic nonpulsatile circulation. Ann Thorac Surg 2002; 74:1080 - 1085
- Thohan V, Stetson SJ, Nagueh SF, Rivas-Gotz C, Koerner MM, Lafuente JA, et al. Cellular and hemodynamics responses of failing myocardium to continuous flow mechanical circulatory support using the DeBakey-Noon left ventricular assist device: a comparative analysis with pulsatile-type devices. J Heart Lung Transplant 2005; 24:566 - 575
- Zimpfer D, Wieselthaler G, Czerny M, Fakin R, Haider D, Zrunek P, et al. Neurocognitive function in patients with ventricular assist devices: a comparison of pulsatile and continuous blood flow devices. ASAIO J 2006; 52:24 - 27
- Sezai A, Shiono M, Orime Y, Nakata K, Hata M, Iida M, et al. Major organ function under mechanical support: comparative studies of pulsatile and nonpulsatile circulation. Artificial Organs 1999; 23:280 - 285
- Reddy RC, Goldstein AH, Pacella JJ, Cattivera GR, Clark RE, Magovern GJ Sr. End organ function with prolonged nonpulsative circulatory support. ASAIO J 1995; 41:547 - 551
- Simon MA, Watson J, Baldwin JT, Wagner WR, Borovetz HS. Current and future considerations in the use of mechanical circulatory support devices. Annu Rev Biomed Eng 2008; 10:59 - 84
- Slater JP, Rose EA, Levin HR, Frazier OH, Roberts JK, Weinberg AD, et al. Low thromboembolic risk without anticoagulation using advanced-design left ventricular assist device. Ann Thorac Surg 1996; 62:1321 - 1328
- Mehta SM, Pae WE Jr, Rosenberg G, Snyder AJ, Weiss WJ, Lewis JP, et al. The LionHeart LVD-2000: a completely implanted left ventricular assist device for chronic circulatory support. Ann Thorac Surg 2001; 71:156 - 161
- Noon GP, Morley DL, Irwin S, Abdelsayed SV, Benkowski RJ, Lynch BE. Clinical experience with the MicroMed DeBakey ventricular assist device. Ann Thorac Surg 2001; 71:133 - 138
- Frazier OH, Myers TJ, Jarvik RK, Westaby S, Pigott DW, Gregoric ID, et al. Research and development of an implantable, axial-flow left ventricular assist device: the Jarvik 2000 Heart. Ann Thorac Surg 2001; 71:125 - 132
- Loforte A, Montallo A, Ranocchi F, Casali G, Luzi G, Della Monica PL, et al. Long-term mechanical support with the HeartMate® II LVAS. Transplant Proc 2009; 41:1357 - 1359
- Frazier OH, Gemmato C, Myers TJ, Gregoric ID, Radovancevic P, Loyalka P, et al. Initial clinical experience with the HeartMate® II Axial-Flow left ventricular assist device. Tex Heart Inst J 2007; 34:275 - 281
- Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 2007; 357:885 - 896
- Schmid C, Tjan TD, Etz C, Scmidt C, Wenzelburger F, Wilhelm M, et al. First clinical experience with the Incor left ventricular assist device. J Heart Lung Transplant 2005; 24:1188 - 1194
- Esmore DS, Kaye D, Salamonsen R, Buckland M, Rowland M, Negri J, et al. First clinical implant of the VentrAssist Left Ventricular Assist System as destination therapy for end-stage heart failure. J Heart Lung Transplant 2005; 24:1150 - 1154
- Esmore D, Spratt P, Larbalestier R, Tsui S, Fiane A, Ruygrok P, et al. VentrAssist™ left ventricular assist device: clinical trial results and Clinical Development Plan update. Eur J Cardiothorac Surg 2007; 32:735 - 744
- Esmore D, Kaye D, Spratt P, Larbalesteir R, Ruygrok P, Tsui S, et al. A prospective, multicenter trial of the VentrAssist left ventricular assist device for bridge to transplant: safety and efficacy. J Heart Lung Transplant 2008; 27:579 - 588
- Farrar DJ, Bourque K, Dague CP, Cotter CJ, Poirier VL. Design features, development status and experimental results with the Heartmate III centrifugal left ventricular assist system with a magnetically levitated rotor. ASAIO Journal 2007; 53:310 - 315
- Loree HM II, Bourque K, Gernes DB, Richardson JS, Poirier VL, Barletta N, et al. The HeartMate III: Design and in vivo studies of a Maglev centrifugal left ventricular assist device. Artificial Organs 25:386 - 391
- Komoda T, Weng Y, Nojiri C, Hetzer R. Implantation technique for the DuraHeart left ventricular assist system. J Artif Organs 2007; 10:124 - 127
- Horvath DJ, Golding LAR, Massiello A, Medvedev AL, Gerhart RL, Ochiai T, et al. The CorAide blood pump. Ann Thorac Surg 2001; 71:191
- Gazzoli F, Alloni A, Pagani F, Pellegrini C, Longobardi A, Ricci D, et al. Arrow CorAide Left Ventricular Assist System: initial experience of the cardiothoracic surgery center in Pavia. Ann Thorac Surg 2007; 83:279 - 282
- Tuzun E, Roberts K, Cohn WE, Sargin M, Gemmato CJ, Radovancevic B, et al. In vivo evaluation of the HeartWare centrifugal ventricular assist device. Tex Heart Inst J 2007; 34:406 - 411
- Wood C, Maiorana A, Larbalestier R, Lovett M, Green G, O'Driscoll G. First successful bridge to myocardial recovery with a HeartWare HVAD. J Heart Lung Transplant 2008; 27:695 - 697
- Spanier T, Oz M, Levin H, Weinberg A, Stamatis K, Stern D. Activation of coagulation and fibrinolytic pathways in patients with left ventricular assist devices. J Thorac Cardiovasc Surg 1996; 112:1090 - 1097
- Holman WL, Park SJ, Long JW, Weinberg A, Gupta L, Tierney AR, et al. Infection in permanent circulatory support: experience from the REMATCH trial. J Heart Lung Transplant 2004; 23:1359 - 1365
- Holman WL, Skinner JL, Waites KB, Benza RL, McGiffin DC, Kirklin JK. Infection during circulatory support with ventricular assist devices. Ann Thorac Surg 1999; 68:711 - 716
- Holman WL, Rayburn BK, McGiffin DC, Foley BA, Benza RL, Bourge RC, et al. Infection in ventricular assist devices: prevention and treatment. Ann Thorac Surg 2003; 75:48 - 57
- Nurozler F, Argenziano M, Oz MC, Naka Y. Fungal left ventricular assist device endocarditis. Ann Thorac Surg 2001; 71:614 - 618
- Chinn R, Dembitsky W, Eaton L, Chillcott S, Stahovich M, Rasmusson B, et al. Multicenter experience: prevention and management of left ventricular assist device infections. ASAIO J 2005; 51:461 - 470
- Minami K, El-Banayosy A, Sezai A, Arusoglu L, Sarnowsky P, Fey O, et al. Morbidity and outcome after mechanical ventricular support using Thoratec, Novacar and HeartMate for bridging to heart transplantation. Artif Organs 24:421 - 426
- Goldstein DJ, Oz MC, Rose EA. Implantable left ventricular assist devices. N Engl J Med 1998; 339:1522 - 1533
- Dang NC, Topkara VK, Mercnado M, Kay J, Krugger KH, Aboodi MS, et al. Right heart failure after left ventricular assist device implantation in patients with chronic congestive heart failure. J Heart Lung Transplant 2006; 25:1 - 6
- Kavarana MN, Pessin-Minsley MS, Urtecho J, Catanese KA, Flannery M, Oz MC, et al. Right ventricular dysfunction and organ failure in left ventricular assist device recipients: a continuing problem. Ann Thorac Surg 2002; 73:745 - 750
- Morgan JA, John R, Lee BJ, Oz MC, Naka Y. Is severe right ventricular failure in left ventricular assist device recipients a risk factor for unsuccessful bridging to transplant and post-transplant mortality. Ann Thorac Surg 2004; 77:859 - 863
- Santamore WP, Gray LA. Left ventricular contributions to right ventricular systolic function during LVAD support. Ann Thorac Surg 1996; 61:350 - 356
- Van Meter CH Jr. Right heart failure: Best treated by avoidance. Ann Thorac Surg 2001; 71:220 - 222
- Argenziano M, Choudhri AF, Moazami N, Rose EA, Smith CR, Levin HR, et al. Randomized, double-blind trial of inhaled nitric oxide in LVAD recipients with pulmonary hypertension. Ann Thorac Surg 1998; 65:340 - 345
- Dembitsky WP, Tector AJ, Park S, Moskowitz AJ, Gelijins AC, Ronan NS, et al. Left ventricular assist device performance with long-term circulatory support: lessons from the REMATCH Trial. Ann Thorac Surg 2004; 78:2123 - 2130
- Cooley DA. The total artificial heart. Nat Med 2003; 9:108 - 111
- Gray NA Jr, Selzman CH. Current status of the total artificial heart. Am Heart J 2006; 152:4 - 10
- Frazier OH, Akutsu T, Cooley DA. Total artificial heart (TAH) utilization in man. Trans Am Soc Artif Intern Organs 1982; 28:534 - 538
- DeVries WC. The permanent artificial heart, four case reports. JAMA 1988; 259:849 - 859
- DeVries WC, Anderson JL, Joyce LD, Anderson FL, Hammond EH, Jarvik RK, et al. Clinical use of the total artificial heart. N Engl J Med 1984; 310:273 - 278
- Griffith BP, Hardesty RL, Kormos RL, Trento A, Borovetz HS, Thompson ME, et al. Temporary use of the Jarvik-7 total artificial heart before transplantation. N Engl J Med 1987; 316:130 - 134
- Copeland JG, Smith RG, Icenogle TB, Rhenman B, Williams R, Vasu MA. Early experience with the total artificial heart as a bridge to cardiac transplantation. Surg Clin North Am 1988; 68:621 - 635
- Johnson KE, Prieto M, Joyce LD, Pritzker M, Emery RW. Summary of the clinical use of the Symbion total artificial heart: a registry report. J Heart Lung Transplant 1992; 11:103 - 116
- Copeland JG, Smith RG, Arabia FA, Nolan PE, Sethi Gk, Tsau PH, et al. Cardiac replacement with a total artificial heart as a bridge to transplantation. N Engl J Med 2004; 351:859 - 867
- SoRelle R. Totally contained AbioCor artificial heart implanted July 3, 2001. Circulation 2001; 104:9005 - 9006
- Mussivand T, Holmes KS, Hum a, Keon WJ. Transcutaneous energy transfer with voltage regulation for rotary blood pumps. Artif Organs 1996; 20:621 - 624
- Ahn JM, Kang DW, Kim HC, Min BG. In Vivo performance evaluation of a transcutaneous energy and information transmission system for the total artificial heart. ASAIO Journal 1993; 39:208 - 212
- Dowling RD, Etoch SW, Stevens KA, Johnson AC, Gray LA Jr. Current status of the AbioCor Implantable Replacement Heart. Ann Thorac Surg 2001; 71:147 - 149
- Copeland JG, Smith RG, Bose RK, Tsau PH, Nolan PE, Slepian MJ. Risk factor analysis for bridge to transplantation with the CardioWest Total Artificial Heart. Ann Thorac Surg 2008; 85:1639 - 1644
- Dowling RD, Gray LA Jr, Etoch SW, Laks H, Marelli D, Samuels L, et al. Initial experience with the AbioCor Implantable Replacement Heart System. J Thorac Cardiovasc Surg 2004; 127:131 - 141
- Quaini E, Pavie A, Chieco S, Mambrito B. The Concerted Action ‘Heart’ European registry on clinical application of mechanical circulatory support systems: bridge to transplant. The Registry Scientific Committee. Eur J Cardiothorac Surg 1997; 11:182 - 188
- Mehta SM, Aufiero TX, Pae WE Jr, Miller CA, Pierce WS. Combined registry for the clinical use of mechanical ventricular assist pumps and the total artificial heart in conjunction with heart transplantation: sixth official report. J Heart Lung Transplant 1995; 14:585 - 593
- Conger JL, Inman RW, Tamez D, Frazier OH, Branislav R. Infection and thrombosis in total artificial heart technology: past and future challenges-a historical review. ASAIO J 2000; 46:22 - 27
- Copeland JG, Pavie A, Duveau D, Keon WJ, Masters R, Pifarre R, et al. Bridge to transplantation with the CardioWest Total Artificial Heart: The international experience 1993 to 1995. J Heart Lung Transplant 1996; 15:94 - 99
- Copeland JG, Smith RG, Arabia FA, Nolan PE, Mehta VK, McCarthy MS, et al. Comparison of the CardioWest Total Artificial Heart, the Novacor Left Ventricular Assist System and the Thoractec Ventricular Assist System in bridge to transplantation. Ann Thorac Surg 2001; 71:92 - 97
- Didisheim P, Olsen DB, Farrar DJ, Portner PM, Griffith BP, Pennington DG, et al. Infections and thromboembolism with implantable cardiovascular devices. Trans Am Soc Artif Intern Organs 1989; 35:54 - 70
- Chen YS, Yu HY, Huang SC, Chiu KM, Lin TY, Lai LP, et al. Experience and result of extracorporeal membrane oxygenation in treating fulminant myocarditis with shock: what mechanical support should be considered first?. J Heart Lung Transplant 2005; 24:81 - 87
- Duncan BW, Bohn DJ, Atz AM, French JW, Laussen PC, Wessel DL. Mechanical circulatory support for the treatment of children with acute fulminant myocarditis. J Thorac Cardiovasc Surg 2001; 122:440 - 448
- Grinda JM, Chevalier P, D'Attellis N, Bricourt MO, Berrebi A, Guibourt P, et al. Fulminant myocarditis in adults and children: bi-ventricular assist device for recovery. Eur J Cardiothorac Surg 2004; 26:1169 - 1173
- Goldman AP, Cassidy J, de Leval M, Haynes S, Brown K, Whitmore P, et al. The waiting game: bridging to paediatric heart transplantation. Lancet 2003; 362:1948 - 1949
- Webber S. Clinical Trials Working Group. Pediatric circulatory support contractors' meeting: Report of the Clinical Trials Working Group. ASAIO J 2009; 55:10 - 12
- Baldwin JT, Borovetz HS, Duncan BW, Gartner MJ, Jarvik RK, Weiss WJ, Hoke TR. The National Heart, Lung and Blood Institute Pediatric Circulatory Support Program. Circulation 2006; 113:147 - 155
- Thoratec Corporation. Thoratec 2011; Available from: www.thoratec.com