Abstract
Out of the several signaling pathways controlling craniofacial development, the role of planar cell polarity (PCP) signaling is relatively poorly understood. This pathway, originally identified as a mechanism to maintain cell polarity within the epithelial cells of the Drosophila wing, has been linked to the proper development of a wide variety of tissues in vertebrates and invertebrates. While many of the pathway members are conserved, it appears that some of the members of the pathway act in a tissue-specific manner. Here, we discuss the role of this pathway in vertebrate craniofacial development, highlighting cranial neural crest migration, skull and palate formation and the role of non-traditional modulators of PCP signaling within this developmental process.
Introduction
Despite a variety of different facial features, the basic process of craniofacial formation, a key step in vertebrates' evolution, is remarkably similar.Citation1,Citation2 Craniofacial development begins with the delamination and migration of the cranial neural crest cells (NCC). These cells migrate out of the dorsal neural tube and condense to form the pharyngeal skeleton and neurocranium.Citation2 The skull forms as a result of the replacement of the initial cartilaginous skeleton by bone and direct membranous ossification. In mammals, formation of the secondary palate requires a sequence of complex morphogenetic tissue movements. Numerous developmental signals have been linked to these processes, one of which is the Wnt/planar cell polarity (PCP) pathway. The role of this pathway was first identified in establishing polarity within the epithelial cells of the Drosophila wing. In vertebrates, the Wnt/PCP pathway has been linked to a variety of developmental processes, including the orientation of the sensory hair cells in the mammalian ear, and controls the narrowing of the mediolateral axis and the elongation of the anterior posterior axis during gastrulation (referred to as convergent extension in Xenopus, or convergence and extension in zebrafish).Citation3 In this review, we will discuss the role of Wnt/PCP pathway proteins involved in the critical stages of craniofacial development.
Cranial Neural Crest Migration
Many of the core Wnt/PCP signaling proteins are expressed in the cranial neural crest cellsCitation4–Citation7 and are required for its proper migration.Citation8,Citation9 Here, we will briefly discuss this role; for a more detailed review, see Clay and Halloran.Citation10
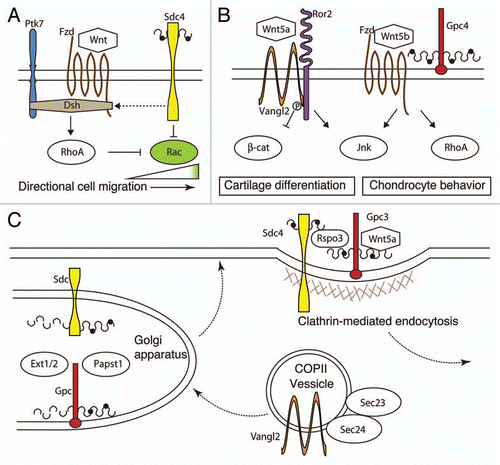
Studies in zebrafish and Xenopus suggest that Wnt/PCP restricts lamellipodial protrusions to the leading edge of the cells during NCC migration. This signaling pathway interacts with fibronectin-stimulated syndecan 4 (sdc4), a transmembrane proteoglycan expressed in neural crest cells, which acts to inhibit rac, a small GTPase at the trailing edge of the cells.Citation11 As rhoA, a downstream effector of the PCP pathway, also inhibits rac, sdc4 acts in a parallel pathway to regulate neural crest migration. The localization of PCP signaling elements, such as wnt11, frizzled 7 (fzd7) and dishevelled (dsh), with cell-cell contact is important for inhibition of NCC locomotion. When NCCs come in contact with each other, they change direction and retract their protrusions, a process that requires proper PCP signaling.Citation8 Transmembrane molecules such as protein tyrosine kinase 7 (ptk7) are thought to mediate the migration of NCCs through the activation of the PCP pathway. In Xenopus, ptk7 is thought to form a complex with fzd7 that recruits dsh to the membrane.Citation12
Role of Wnt/PCP Signaling in Skull Formation
Wnt signaling plays an important role in skeleton formation, and multiple groups have demonstrated that Wnt/PCP signaling is required for proper craniofacial development.Citation13–Citation16 Of the many Wnt molecules currently studied, Wnt5 ligands seem to be critical for craniofacial development. There are two known wnt5 genes in vertebrates, wnt5a and wnt5b.Citation17–Citation19 Interestingly, in mammals, Wnt5a is associated with craniofacial development, whereas in teleost, the wnt5b homolog is critical.Citation20,Citation21 Overexpression or a lack of Wnt5a results in a developmental delay of chondrocytes transitioning from proliferative to hypertrophic chondrocytes. Wnt5a signaling has been shown to regulate expression of type II collagen, upregulate c-Jun expression and activate the JNK-pathway, depending on the cartilage element assayed.Citation22,Citation23
While Wnt5 ligands have been classified as “non-canonical,” the role they play in PCP signaling is controversial, as they can affect multiple Wnt receptor pathways, such as Frizzleds and Ror2.Citation16 The Ror protein family is highly evolutionarily conserved from Caenorhabditis elegans to humans and consists of an extracellular Frizzled-like, cysteine-rich domain, which can bind directly to Wnt5a, and a cytoplasmic tyrosine kinase domain that can be hyperphosphorylated.Citation24–Citation26 Expression of Ror2 and Wnt5a have been detected in the developing mouse craniofacial cartilage and teeth.Citation19,Citation27 While the Ror2 mouse mutant has a shortened snout and a cleft palateCitation28–Citation31 and Wnt5a mutant mice display hypertelorism, micrognathia and a triangular mouth,Citation15 a direct tie of these two genes with PCP signaling has yet to be identified in craniofacial cartilage. However, recent reports on limb formation have genetically and physically linked Wnt5a/Ror2 to van Gogh-like 2 (Vangl2) and PCP signaling.Citation24,Citation32 Previous research has shown that the Vangl2−/− mouse has digit and claw defects very similar to those observed in humans with mutations in WNT5A and ROR2, which lead to a rare form of short-limbed dwarfism called Robinow syndrome and brachydactyly type B.Citation15,Citation31,Citation33–Citation35 To identify whether Wnt5a and Ror2 mutations modulate the Vangl2 phenotype, double heterozygous and homozygous Wnt5a; Vangl2 and Ror2; Vangl2 mice were generated.Citation24,Citation32 While the Vangl2−/−; Wnt5a+/− mouse had shorter and wider digits and a stronger long-bone phenotype than the Vangl2 mutant alone, the Vangl2−/−; Ror2−/− was even more severe and was similar to that of the Wnt5a mutant. The loss of both Vangl2 and Ror2 lead to an increase in Wnt/β-catenin signaling in the limb, a result of the loss of Wnt5a repression on the canonical pathway. Co-immunoprecipitation and FRET analysis revealed that VANGL2 and ROR2 directly interact in the cytoplasm, where WNT5A-activated ROR2 receptor mediates VANGL2's phosphorylation. These results from the Vangl2−/−; Ror2−/− mouse leads to a model where the WNT5A gradient sets up distinct levels of Vangl2 phosphorylation in the cytoplasm, which is required for proper limb formation. As all three of these molecules are expressed during vertebrate craniofacial development, it suggests a possible role for them during craniofacial development.Citation24
Non-Traditional Modulators of Wnt/PCP Signaling in Cartilage and Bone Formation
Several studies have found mutations in glycoproteins, and the genes associated with their processing and targeting to the plasma membrane produce congenital defects reminiscent of Wnt/PCP mutants. Glypicans, extracellular proteins that are found throughout the animal kingdom, are composed of a cysteine-rich globular protein core, GPI anchor and heparan sulfate (HS) side chains located close to the plasma membrane. The HS side chains allow for glypicans to interact with a multitude of signaling molecules.Citation36,Citation37 A zebrafish mutant in the glypican 4 (gpc4) gene was first identified due to its compressed anterior-posterior body axis caused by a reduction in Wnt/PCP signaling that is required for convergence and extension movement of cells during gastrulation and that results in late embryonic lethality.Citation13,Citation38 A closer examination of the gpc4 mutant revealed shortened cartilages of the pharyngeal and neurocranium skeleton due to an inability of the chondrocytes to elongate and intercalate into a stacked cartilage element.Citation13,Citation14 To better understand the role of Wnt/PCP signaling in cartilage and bone formation in gpc4-deficient zebrafish embryos, the lethal gastrulation defect was suppressed with the addition of gpc4 mRNA.Citation14 This allowed observations of the larval and adult role of gpc4 in skull formation and identified a persistent loss of stacked chondrocyte organization in both juvenile and adult mutants. Interestingly, the early larva disorganization of cartilage elements resulted in the loss of particular facial bones, such as the symplectic, as a consequence of an expansion of neighboring ossification centers. These studies demonstrate a clear role for gpc4 and likely Wnt/PCP in the formation of craniofacial cartilages and subsequent skull ossification.
Two zebrafish mutants in exostosin genes (ext1 and 2) encoding glycosyltransferases and papst1, a 3′-phosphoadenosine 5′-phosphosulfate (PAPS) transporter, also exhibit severe craniofacial cartilage defects.Citation39 This provides further evidence that glypicans and their HS side chains play a critical role in PCP signaling, as both of these genes allow for the proper posttranslational modification of proteoglycans in the Golgi. Loss of these genes results in shorter cartilage elements composed of rounded disorganized cells instead of the thin elongated stacked chondrocytes, defects very similar to the phenotype seen in wnt5b and gpc4 mutants.Citation13,Citation21 Interestingly, mutations in human EXT1 and EXT2 genes were found to be the cause of hereditary multiple exostoses, a disease in which patients develop benign long-bone tumors during childhood.Citation40–Citation42 In addition, the Ext1 mice mutants display smaller craniofacial structures,Citation43 suggesting a role of EXTs in mammalian craniofacial development.
Another possible modulator of Wnt/PCP signaling via proteoglycans is r-spondin 3 (rspo3), a member of the secreted protein family, which was once thought to only interact with Wnt/β-catenin signalingCitation44,Citation45 but now has been shown to be important in the Wnt/PCP pathway. In a search for r-spondin receptors, rspo3 was found to bind specifically to cells expressing gpc3 and sdc4, both known proteoglycans that interact with Wnt signaling, but was unable to bind to cells expressing lrp6, kermen1 or frizzled5, all well-established elements of Wnt/β-catenin signaling.Citation46 To prove that rspo3 and sdc4 are part of the Wnt/PCP pathway, morpholinos (MO) against rspo3 were targeted to different germ layers of Xenopus embryos. Not only did rspo3 MO disrupt the morphogenetic process of convergent extension movements during gastrulation without disrupting the specification of mesoderm, it also disrupted the intercalation and stacking process of craniofacial cartilage, both phenotypes very reminiscent of the zebrafish Wnt/PCP mutant gpc4.Citation13,Citation46 Interestingly, rspo3 and wnt5a are required for sdc4 PCP signaling via a clathrin-mediated endocytosis process, suggesting an important role for endocytosis in the control of Wnt/PCP signaling ().Citation46
Mutations in genes associated with the secretion of signaling proteins result in disorganized craniofacial phenotypes similar to those discussed above, suggesting a potential role in PCP signaling. Two genes that encode proteins involved in this process in humans and zebrafish are sec23a and sec24d.Citation47–Citation49 Both of these genes are part of the COPII complex, which transports newly translated proteins, such as Col2a1 and proteoglycans, from the endoplasmic reticulum to the Golgi, where posttranslational modification take place. The Sec23 and Sec24 group of proteins are known to heterodimerize, where Sec24 proteins selectively bind cargo, and Sec23 proteins help to create the structural part of the COPII coat.Citation50,Citation51 There are four known sec24 genes in mammals, a through d. While there is some functional redundancy between sec24a and b and between sec24c and d, each has been shown to bind preferentially to specific protein cargo.Citation52 Interestingly, sec24b and sec24d are crucial for different aspects of PCP signaling. Two groups independently identified mouse lines with mutations in Sec24b with classical Wnt/PCP defects, such as neural tube closure defects known as craniorachischisis, abnormal organ of Corti hair cell arrangement and cardiac defects.Citation53,Citation54 Disruption of Sec24b results in abnormal trafficking of VANGL2, a key modulator of PCP cellular morphogenesis, but not other membrane-bound proteins. In zebrafish, sec23a and sec24d mutants have smaller disorganized craniofacial cartilage elements composed of rounded cells that are unable to secrete Col2a1a and other extracellular matrix (ECM) proteins that are stuck in the rough ER.Citation47,Citation49 The malformations of craniofacial cartilage elements of the sec24d mutant are not a result of a reduction of cell number but are due to abnormal cell shape, resembling phenotypes of the wnt5b and gpc4 mutants.Citation13,Citation21,Citation49 While the authors of these papers suggest that the cell shape defect is due to the loss of ECM, they do not preclude the possibility that Wnts and other signaling molecules could be affected by the loss of sec24d. Not surprisingly, studies in humans have found that cranio-lenticula-sutural dysplasia (CLSD), a disorder in which the anterior fontanels are developmentally delayed, results from a mutation in the SEC23A gene.Citation55 Patients with CLSD also present with prominent foreheads, hypertelorism, prominent brow ridges and broad noses. Based on the studies reviewed here, proteoglycans, such as gpc4 and sdc4, and their required posttranslational modifications and transport play a critical role in the PCP of craniofacial skeletal formation.
Palate Formation
Several developmentally important signaling pathways control the complex formation of the secondary palate.Citation56 Both the β-catenin-dependent and -independent Wnt signaling pathways have been implicated in this process. Deficiency of two Wnt ligands associated with the β-catenin-dependent pathway, the Wnt9b−/− mouse knockout and humans with mutations in WNT3, result in clefting of the palate.Citation57,Citation58 The β-catenin-independent signaling molecule Wnt11 was also proposed to be important in the final steps of secondary palate formation.Citation59 In addition, WNT5A deficiency leads to a complete cleft formation in mice.Citation60 WNT5A controls directional cell migration and proliferation in this process and is mediated by Ror2. Frizzleds, the typical Wnt receptors, also play a role in palate closure; in particular, mouse embryos deficient in Fzd2 frequently develop cleft palate. This defect is fully penetrant in double Fzd1−/−; Fzd2−/− mutants.Citation61 In addition, Fzd1−/−; Fzd2−/− mice exhibit shortened lower jaws (hypognathia). Interestingly, WNT9A and WNT3 were shown to induce robust induction of β-catenin signaling by reporter expression when co-expressed with FZD1 or FZD2 in contrast to WNT5A or WNT11, which did not elucidate such a response.Citation61 While a strong interaction was observed between the core PCP genes Vangl2 and Fzd1 and Fzd2 in neural tube closure, no enhancement of palate formation defects has been demonstrated.Citation61 In addition, Vangl2−/− mouse mutants do not have defects in palate closure,Citation62–Citation64 leaving the question of the role of PCP signaling in palategenesis open.
Conclusion
Much of what is known about the role of the Wnt/PCP signaling pathway in craniofacial development is projected from its role in other tissues. While manipulation of the individual members of the pathway clearly leads to a disruption of normal craniofacial development, in most cases, the targets of the pathway have yet to be identified. Therefore, in order to gain a clear understanding of the pathway's role within craniofacial development, it needs to be determined whether the downstream targets of PCP signaling within other tissues also play a role within craniofacial development.
Figures and Tables
Figure 1 The role of PCP signaling in craniofacial development. The core planar cell polarity (PCP) signaling molecules, Frizzled (Fzd), Disheveled (Dsh) and Van Gogh-like 2 (Vangl2), interact with multiple proteins to transmit planar cell polarity information to and from cells. (A) In migrating neural crest cells, Wnt/PCP signaling activates RhoA to inhibit Rac activity in the trailing edge of the cell. Both Syndecan 4 (Sdc4) and protein tyrosine kinase 7 (Ptk7) can interact with Dsh, while Sdc4 can directly inhibit Rac. (B) During craniofacial cartilage formation, core PCP proteins' interaction with Ror2 and proteoglycans, such as Glypican 4 (Gpc4), inhibit Wnt/β-catenin signaling and activate RhoA and Jun signaling. (C) Transport of the proteins involved in PCP processes by a Sec23/24-dependent mechanism or their modification in the Golgi is essential for their function. A clathrin-mediated endocytosis process requiring R-Spondin 3 (Rspo3) and Wnt5a leads to the removal of Sdc4 and Gpc3 from the membrane. Perturbation of any of these processes affect Wnt/PCP signaling and craniofacial development.
Acknowledgments
This work was supported by the National Institutes of Health—NIDCR Grants R01DE016678 (J.T.), F32DE019058 (B.E.S.), and F32DE019986 (R.M.D.).
References
- Northcutt RG, Gans C. The genesis of neural crest and epidermal placodes: a reinterpretation of vertebrate origins. Q Rev Biol 1983; 58:1 - 28; PMID: 6346380; http://dx.doi.org/10.1086/413055
- Schilling TF. Genetic analysis of craniofacial development in the vertebrate embryo. Bioessays 1997; 19:459 - 468; PMID: 9204763; http://dx.doi.org/10.1002/bies.950190605
- Wansleeben C, Meijlink F. The planar cell polarity pathway in vertebrate development. Dev Dyn 2011; 240:616 - 626; PMID: 21305650; http://dx.doi.org/10.1002/dvdy.22564
- Bekman E, Henrique D. Embryonic expression of three mouse genes with homology to the Drosophila melanogaster prickle gene. Mech Dev 2002; 119:77 - 81; PMID: 14516664; http://dx.doi.org/10.1016/S09254773(03)00095-9
- Darken RS, Scola AM, Rakeman AS, Das G, Mlodzik M, Wilson PA. The planar polarity gene strabismus regulates convergent extension movements in Xenopus. EMBO J 2002; 21:976 - 985; PMID: 11867525; http://dx.doi.org/10.1093/emboj/21.5.976
- Goto T, Keller R. The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Dev Biol 2002; 247:165 - 181; PMID: 12074560; http://dx.doi.org/10.1006/dbio.2002.0673
- Nakaya MA, Habas R, Biris K, Dunty WC Jr, Kato Y, He X, et al. Identification and comparative expression analyses of Daam genes in mouse and Xenopus. Gene Expr Patterns 2004; 5:97 - 105; PMID: 15533824; http://dx.doi.org/10.1016/j.modgep.2004.06.001
- Carmona-Fontaine C, Matthews HK, Kuriyama S, Moreno M, Dunn GA, Parsons M, et al. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature 2008; 456:957 - 961; PMID: 19078960; http://dx.doi.org/10.1038/nature07441
- De Calisto J, Araya C, Marchant L, Riaz CF, Mayor R. Essential role of non-canonical Wnt signalling in neural crest migration. Development 2005; 132:2587 - 2597; PMID: 15857909; http://dx.doi.org/10.1242/dev.01857
- Clay MR, Halloran MC. Regulation of cell adhesions and motility during initiation of neural crest migration. Curr Opin Neurobiol 2011; 21:17 - 22; PMID: 20970990; http://dx.doi.org/10.1016/j.conb.2010.09.013
- Matthews HK, Marchant L, Carmona-Fontaine C, Kuriyama S, Larrain J, Holt MR, et al. Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development 2008; 135:1771 - 1780; PMID: 18403410; http://dx.doi.org/10.1242/dev.017350
- Shnitsar I, Borchers A. PTK7 recruits dsh to regulate neural crest migration. Development 2008; 135:4015 - 4024; PMID: 19004858; http://dx.doi.org/10.1242/dev.023556
- Topczewski J, Sepich DS, Myers DC, Walker C, Amores A, Lele Z, et al. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell 2001; 1:251 - 264; PMID: 11702784; http://dx.doi.org/10.1016/S1534-5807(01)00005-3
- LeClair EE, Mui SR, Huang A, Topczewska JM, Topczewski J. Craniofacial skeletal defects of adult zebrafish Glypican 4 (knypek) mutants. Dev Dyn 2009; 238:2550 - 2563; PMID: 19777561; http://dx.doi.org/10.1002/dvdy.22086
- Person AD, Beiraghi S, Sieben CM, Hermanson S, Neumann AN, Robu ME, et al. WNT5A mutations in patients with autosomal dominant Robinow syndrome. Dev Dyn 2010; 239:327 - 337; PMID: 19918918; http://dx.doi.org/10.1002/dvdy.22156
- Hartmann C. Skeletal development—Wnts are in control. Mol Cells 2007; 24:177 - 184; PMID: 17978569
- Geetha-Loganathan P, Nimmagadda S, Antoni L, Fu K, Whiting CJ, Francis-West P, et al. Expression of WNT signalling pathway genes during chicken craniofacial development. Dev Dyn 2009; 238:1150 - 1165; PMID: 19334275; http://dx.doi.org/10.1002/dvdy.21934
- Witte F, Dokas J, Neuendorf F, Mundlos S, Stricker S. Comprehensive expression analysis of all Wnt genes and their major secreted antagonists during mouse limb development and cartilage differentiation. Gene Expr Patterns 2009; 9:215 - 223; PMID: 19185060; http://dx.doi.org/10.1016/j.gep.2008.12.009
- Yamaguchi TP, Bradley A, McMahon AP, Jones SA. Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 1999; 126:1211 - 1223; PMID: 10021340
- Dale RM, Sisson BE, Topczewski J. The emerging role of Wnt/PCP signaling in organ formation. Zebrafish 2009; 6:9 - 14; PMID: 19250029; http://dx.doi.org/10.1089/zeb.2008.0563
- Piotrowski T, Schilling TF, Brand M, Jiang YJ, Heisenberg CP, Beuchle D, et al. Jaw and branchial arch mutants in zebrafish II: anterior arches and cartilage differentiation. Development 1996; 123:345 - 356; PMID: 9007254
- Ryu JH, Chun JS. Opposing roles of WNT-5A and WNT-11 in interleukin-1beta regulation of type II collagen expression in articular chondrocytes. J Biol Chem 2006; 281:22039 - 22047; PMID: 16754689; http://dx.doi.org/10.1074/jbc.M601804200
- Yang Y, Topol L, Lee H, Wu J. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development 2003; 130:1003 - 1015; PMID: 12538525; http://dx.doi.org/10.1242/dev.00324
- Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, et al. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell 2011; 20:163 - 176; PMID: 21316585; http://dx.doi.org/10.1016/j.devcel.2011.01.001
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells 2003; 8:645 - 654; PMID: 12839624; http://dx.doi.org/10.1046/j.1365-2443.2003.00662.x
- Yoda A, Oishi I, Minami Y. Expression and function of the Ror-family receptor tyrosine kinases during development: lessons from genetic analyses of nematodes, mice and humans. J Recept Signal Transduct Res 2003; 23:1 - 15; PMID: 12680586; http://dx.doi.org/10.1081/RRS-120018757
- Lin M, Li L, Liu C, Liu H, He F, Yan F, et al. Wnt5a regulates growth, patterning, and odontoblast differentiation of developing mouse tooth. Dev Dyn 2011; 240:432 - 440; PMID: 21246660; http://dx.doi.org/10.1002/dvdy.22550
- DeChiara TM, Kimble RB, Poueymirou WT, Rojas J, Masiakowski P, Valenzuela DM, et al. Ror2, encoding a receptor-like tyrosine kinase, is required for cartilage and growth plate development. Nat Genet 2000; 24:271 - 274; PMID: 10700181; http://dx.doi.org/10.1038/73488
- Schwabe GC, Trepczik B, Suring K, Brieske N, Tucker AS, Sharpe PT, et al. Ror2 knockout mouse as a model for the developmental pathology of autosomal recessive Robinow syndrome. Dev Dyn 2004; 229:400 - 410; PMID: 14745966; http://dx.doi.org/10.1002/dvdy.10466
- Takeuchi S, Takeda K, Oishi I, Nomi M, Ikeya M, Itoh K, et al. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes Cells 2000; 5:71 - 78; PMID: 10651906; http://dx.doi.org/10.1046/j.1365-2443.2000.00300.x
- van Bokhoven H, Celli J, Kayserili H, van Beusekom E, Balci S, Brussel W, et al. Mutation of the gene encoding the ROR2 tyrosine kinase causes autosomal recessive Robinow syndrome. Nat Genet 2000; 25:423 - 426; PMID: 10932187; http://dx.doi.org/10.1038/78113
- Wang B, Sinha T, Jiao K, Serra R, Wang J. Disruption of PCP signaling causes limb morphogenesis and skeletal defects and may underlie Robinow syndrome and brachydactyly type B. Hum Mol Genet 2011; 20:271 - 285; PMID: 20962035; http://dx.doi.org/10.1093/hmg/ddq462
- Robinow M, Silverman FN, Smith HD. A newly recognized dwarfing syndrome. Am J Dis Child 1969; 117:645 - 651; PMID: 5771504
- Afzal AR, Jeffery S. One gene, two phenotypes: ROR2 mutations in autosomal recessive Robinow syndrome and autosomal dominant brachydactyly type B. Hum Mutat 2003; 22:1 - 11; PMID: 12815588; http://dx.doi.org/10.1002/humu.10233
- Oldridge M, Fortuna AM, Maringa M, Propping P, Mansour S, Pollitt C, et al. Dominant mutations in ROR2, encoding an orphan receptor tyrosine kinase, cause brachydactyly type B. Nat Genet 2000; 24:275 - 278; PMID: 10700182; http://dx.doi.org/10.1038/73495
- Fico A, Maina F, Dono R. Fine-tuning of cell signaling by glypicans. Cell Mol Life Sci 2011; 68:923 - 929; PMID: 18087675; http://dx.doi.org/10.1007/s00018-007-7471-6
- Filmus J, Capurro M, Rast J. Glypicans. Genome Biol 2008; 9:224; PMID: 18505598; http://dx.doi.org/10.1186/gb-2008-9-5-224
- Solnica-Krezel L, Stemple DL, Mountcastle-Shah E, Rangini Z, Neuhauss SC, Malicki J, et al. Mutations affecting cell fates and cellular rearrangements during gastrulation in zebrafish. Development 1996; 123:67 - 80; PMID: 9007230
- Clément A, Wiweger M, von der Hardt S, Rusch MA, Selleck SB, Chien CB, et al. Regulation of zebrafish skeletogenesis by ext2/dackel and papst1/pinscher. PLoS Genet 2008; 4:1000136; PMID: 18654627; http://dx.doi.org/10.1371/journal.pgen.1000136
- Ahn J, Ludecke HJ, Lindow S, Horton WA, Lee B, Wagner MJ, et al. Cloning of the putative tumour suppressor gene for hereditary multiple exostoses (EXT1). Nat Genet 1995; 11:137 - 143; PMID: 7550340; http://dx.doi.org/10.1038/ng1095-137
- Wuyts W, Van Hul W, Wauters J, Nemtsova M, Reyniers E, Van Hul EV, et al. Positional cloning of a gene involved in hereditary multiple exostoses. Hum Mol Genet 1996; 5:1547 - 1557; PMID: 8894688; http://dx.doi.org/10.1093/hmg/5.10.1547
- Zak BM, Crawford BE, Esko JD. Hereditary multiple exostoses and heparan sulfate polymerization. Biochim Biophys Acta 2002; 1573:346 - 355; PMID: 12417417; http://dx.doi.org/10.1016/S0304-4165(02)00402-6
- Koziel L, Kunath M, Kelly OG, Vortkamp A. Ext1-dependent heparan sulfate regulates the range of Ihh signaling during endochondral ossification. Dev Cell 2004; 6:801 - 813; PMID: 15177029; http://dx.doi.org/10.1016/j.devcel.2004.05.009
- Kazanskaya O, Glinka A, del Barco Barrantes I, Stannek P, Niehrs C, Wu W. R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev Cell 2004; 7:525 - 534; PMID: 15469841; http://dx.doi.org/10.1016/j.devcel.2004.07.019
- Kim KA, Wagle M, Tran K, Zhan X, Dixon MA, Liu S, et al. R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol Biol Cell 2008; 19:2588 - 2596; PMID: 18400942; http://dx.doi.org/10.1091/mbc.E08-02-0187
- Ohkawara B, Glinka A, Niehrs C. Rspo3 binds syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis. Dev Cell 2011; 20:303 - 314; PMID: 21397842; http://dx.doi.org/10.1016/j.devcel.2011.01.006
- Lang MR, Lapierre LA, Frotscher M, Goldenring JR, Knapik EW. Secretory COPII coat component Sec23a is essential for craniofacial chondrocyte maturation. Nat Genet 2006; 38:1198 - 1203; PMID: 16980978; http://dx.doi.org/10.1038/ng1880
- Ohisa S, Inohaya K, Takano Y, Kudo A. sec24d encoding a component of COPII is essential for vertebra formation, revealed by the analysis of the medaka mutant, vbi. Dev Biol 2010; 342:85 - 95; PMID: 20346938; http://dx.doi.org/10.1016/j.ydbio.2010.03.016
- Sarmah S, Barrallo-Gimeno A, Melville DB, Topczewski J, Solnica-Krezel L, Knapik EW. Sec24D-dependent transport of extracellular matrix proteins is required for zebrafish skeletal morphogenesis. PLoS ONE 2010; 5:10367; PMID: 20442775; http://dx.doi.org/10.1371/journal.pone.0010367
- Hicke L, Schekman R. Yeast Sec23p acts in the cytoplasm to promote protein transport from the endoplasmic reticulum to the Golgi complex in vivo and in vitro. EMBO J 1989; 8:1677 - 1684; PMID: 2670558
- Yoshihisa T, Barlowe C, Schekman R. Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Science 1993; 259:1466 - 1468; PMID: 8451644; http://dx.doi.org/10.1126/science.8451644
- Wendeler MW, Paccaud JP, Hauri HP. Role of Sec24 isoforms in selective export of membrane proteins from the endoplasmic reticulum. EMBO Rep 2007; 8:258 - 264; PMID: 17255961; http://dx.doi.org/10.1038/sj.embor.7400893
- Merte J, Jensen D, Wright K, Sarsfield S, Wang Y, Schekman R, et al. Sec24b selectively sorts Vangl2 to regulate planar cell polarity during neural tube closure. Nat Cell Biol 2010; 12:41 - 46; PMID: 19966784; http://dx.doi.org/10.1038/ncb2002
- Wansleeben C, Feitsma H, Montcouquiol M, Kroon C, Cuppen E, Meijlink F. Planar cell polarity defects and defective Vangl2 trafficking in mutants for the COPII gene Sec24b. Development 2010; 137:1067 - 1073; PMID: 20215345; http://dx.doi.org/10.1242/dev.041434
- Boyadjiev SA, Justice CM, Eyaid W, McKusick VA, Lachman RS, Chowdry AB, et al. A novel dysmorphic syndrome with open calvarial sutures and sutural cataracts maps to chromosome 14q13-q21. Hum Genet 2003; 113:1 - 9; PMID: 12677423; http://dx.doi.org/10.1007/s00439-003-0932-6
- Greene RM, Pisano MM. Palate morphogenesis: Current understanding and future directions. Birth Defects Res C Embryo Today 2010; 90:133 - 154; PMID: 20544696; http://dx.doi.org/10.1002/bdrc.20180
- Niemann S, Zhao C, Pascu F, Stahl U, Aulepp U, Niswander L, et al. Homozygous WNT3 mutation causes tetra-amelia in a large consanguineous family. Am J Hum Genet 2004; 74:558 - 563; PMID: 14872406; http://dx.doi.org/10.1086/382196
- Juriloff DM, Harris MJ, McMahon AP, Carroll TJ, Lidral AC. Wnt9b is the mutated gene involved in multifactorial nonsyndromic cleft lip with or without cleft palate in A/WySn mice, as confirmed by a genetic complementation test. Birth Defects Res A Clin Mol Teratol 2006; 76:574 - 579; PMID: 16998816; http://dx.doi.org/10.1002/bdra.20302
- Lee JM, Kim JY, Cho KW, Lee MJ, Cho SW, Kwak S, et al. Wnt11/Fgfr1b cross-talk modulates the fate of cells in palate development. Dev Biol 2008; 314:341 - 350; PMID: 18191119; http://dx.doi.org/10.1016/j.ydbio.2007.11.033
- He F, Xiong W, Yu X, Espinoza-Lewis R, Liu C, Gu S, et al. Wnt5a regulates directional cell migration and cell proliferation via Ror2-mediated noncanonical pathway in mammalian palate development. Development 2008; 135:3871 - 3879; PMID: 18948417; http://dx.doi.org/10.1242/dev.025767
- Yu H, Smallwood PM, Wang Y, Vidaltamayo R, Reed R, Nathans J. Frizzled 1 and frizzled 2 genes function in palate, ventricular septum and neural tube closure: general implications for tissue fusion processes. Development 2010; 137:3707 - 3717; PMID: 20940229; http://dx.doi.org/10.1242/dev.052001
- Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet 2001; 28:251 - 255; PMID: 11431695; http://dx.doi.org/10.1038/90081
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature 2003; 423:173 - 177; PMID: 12724779; http://dx.doi.org/10.1038/nature01618
- Murdoch JN, Doudney K, Paternotte C, Copp AJ, Stanier P. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum Mol Genet 2001; 10:2593 - 2601; PMID: 11709546; http://dx.doi.org/10.1093/hmg/10.22.2593