Abstract
Removal of toxic substances from the blood depends on patent connections between the kidneys, ureters and bladder that are established when the ureter is transposed from its original insertion site in the Wolffian duct, to the bladder trigone, its final insertion site. According to the Ureteric Bud Theory of Mackie and Stephens, this repositioning of the ureter orifice occurs as the trigone forms from the common nephric duct, the caudal-most Wolffian duct segment. The availability of mouse models has enabled us to re-examine this hypothesis in the context of normal and abnormal development. We find than in contrast to what has been previously thought, the common nephric duct does not differentiate into the bladder trigone but instead undergoes apoptosis, a crucial step in ureter transposition. Interestingly, apoptosis only occurs in close proximity with the sinus ridge, a raised epithelial structure located at the dorsal aspect of the urogenital sinus, suggesting that signals from this site may be important for normal ureter insertion. These studies provide new insights into the normal process of ureter maturation, and shed light on possible causes of obstruction and reflux, ureteral abnormalities that affect 1–2% of the human population.
Introduction
A crucial feature in embryonic development is the assembly of independently formed organs into complex systems that conduct substances such as food, air and waste into and out of the embryo. The organs that comprise the upper (kidney and ureter) and lower (bladder and urethra) urinary tract form independently, connecting at mid-gestation to generate an outflow tract that conducts urine from the kidneys to the bladder for storage and excretion.
The kidneys, ureters and Wolffian ducts, paired epithelial tubes that form most of the male genital tract, are largely derived from intermediate mesoderm, a strip of tissue lying between the lateral plate and the paraxial mesoderm. During early stages of development, the Wolffian ducts open into the cloaca, an endodermal sac that becomes partitioned into the hindgut and the urogenital sinus, the primordium of the bladder and urethra. The upper urinary tract derives from the ureteric bud, an epithelial tube that sprouts from the caudal aspect of the Wolffian duct between E10.5 and E11.5; the distal ureteric bud invades adjacent kidney mesenchyme and forms the renal collecting duct system, while the proximal ureteric bud differentiates into the ureter, a muscular tube that conducts urine from the kidney to the bladder. Initially the ureters join the bladder indirectly, via the CND (). However, to establish direct connections with the bladder, the ureters disassociate from the Wolffian ducts and merge with the bladder epithelium in the trigone, the muscular region located at the base of the bladder (,).
Precise positioning of the ureter orifice in the trigone depends on the ureteric bud sprouting at a stereotypical position on the Wolffian duct; abnormalities in this process can result in reflux and obstruction. The Ureteral Bud Theory formulated by Mackie and Stephens in the 1970s,Citation1 proposed that the final integration site of the ureter could be predicted from the sprouting site of the primary ureteric bud (). According to this model, a high ureteric bud tends to remain attached to the Wolffian duct or joins the lower urinary tract at a more posterior position than normal, for example in the urethra, an abnormality that would result in obstruction. On the other hand, a ureter associated with a ureteric bud that forms too low on the Wolffian duct tends to join the bladder at a position lateral or anterior to the proper insertion site in the trigone, causing reflux. In this case, reflux is thought to be due to shortening of the intra-vesicular ureteral segment disrupting the valve mechanism that prevents backflow of urine to the kidney.
Mackie and Stephens further proposed a mechanism by which normal ureter insertion occurs to explain how abnormalities during ureteric bud formation could induce changes in the final position of the ureter orifice (). They hypothesized that ureter positioning depends on remodeling of the CND which inserts into the bladder neck, expands and differentiates into the trigone. Expansion of the CND would enable the ureter to move away from the Wolffian duct, while insertion of the CND into the bladder and its differentiation into the trigone would reposition the ureter orifice at the proper site.Citation1 Since it is not possible to distinguish the CND from surrounding bladder tissue by histology or morphology, this theory has never been tested experimentally. However, the availability of mouse models in which the CND and ureter are selectively labeled with fluorescent markers together with genetic marking techniques for lineage analysis has enabled us to re-examine this hypothesis.
Expansion of the CND is a First Step in Repositioning the Ureter Orifice
Hoxb7-Gfp transgenic miceCitation2 express Green Fluorescent Protein (Gfp) in the ureter, Wolffian duct and CND, but not in the bladder enabling us for the first time to visualize CND remodeling in the context of ureter maturation (). Analysis of whole mount urogenital tracts from Hoxb7-Gfp embryos between E11 and E13 dpc revealed that the CND expanded to form a wedge-shaped multi-layered epithelium whose morphology was distinct from the upper ureter or Wolffian duct segments which are simple tubes (). Analysis of Hoxb7-Gfp embryos at later stages, revealed persistent expression of the Hoxb7-Gfp reporter in the Wolffian ducts and in the ureters, but surprisingly, by E14 the CND could no longer be distinguished (). These observations suggest that either the CND undergoes regression, or alternately, CND cells may differentiate into the trigone but down-regulate Hoxb7 promoter activity making Gfp expression undetectable. To distinguish between these possibilities, we performed lineage analysis, using a Cre-Lox recombination system to permanently label the CND and its daughter cells.Citation3 Hoxb7-Cre mice, a line expressing Cre recombinase in the CND, ureter and Wolffian ductCitation4 were crossed with R26RlacZ reporter miceCitation5 to yield Hoxb7-Cre;R26RlacZ mice. In cells expressing both R26RlacZ and Hoxb7-Cre, lacZ expression is permanently activated, enabling us to follow the fate of the CND and ureter orifice during maturation. These studies revealed that lacZ expression followed a similar pattern as Hoxb7-Gfp expression; lacZ labeled cells were visible in the CND up to about E13, after which, expression diminished and became undetectable by birth (). These studies suggest that the CND is likely to regress during ureter maturation rather than differentiate into the trigone and that ureter maturation occurs differently than previously thought.
Apoptosis is Important at Distinct Stages for the Regression of the CND and for Opening of Ureteral Lumen
The disappearance of the CND and its daughter cells suggests that the CND cells may undergo programmed cell death as a normal part of remodeling. To address this question, we analyzed Hoxb7-Gfp embryos with TUNEL and with antibodies directed against activated Caspase-3, both of which label apoptotic cells. Consistent with the results of the lineage studies, analysis at E11 and at subsequent stages revealed numerous TUNEL and Caspase-3 positive cells in the CND, but few if any apoptotic cells in the upper Wolffian duct and ureter ().
Interestingly, programmed cell death was also observed at later stages as the ureteral orifice, which was initially occluded, became patent. Analysis of Hoxb7-Gfp urogenital tracts at E15 revealed numerous apoptotic cells in the caudal ureter, which was blocked at this stage (). Analysis 24h later revealed an open ureteral orifice devoid of cells, suggesting that apoptosis in the distal ureter may be important for generating a patent outflow tract (). This localized cell death may represent the degeneration of Schwalla's membrane which is thought to occur late in gestation as renal function begins to increase, which is crucial for generating patent connections between the kidney and bladder.Citation6,Citation7 It has been suggested that Schwalla's membrane is part of the bladder epithelium, however, our studies suggest that this membrane is actually the ureteral lumen since the cells undergoing apoptosis are labeled with Hoxb7-Gfp, which is not expressed in the bladder.
Apoptosis has been shown to be crucial for many aspects of embryonic development, including palatal morphogenesis when the shelves fuse,Citation8 during limb bud formation when PCD of the inter-digital mesenchyme individuates and shapes the digits,Citation9 for opening of the vagina,Citation10 for sexual differentiationCitation11 and for pruning in the CNS.Citation12 Our studies reveal a wave of apoptosis in the CND that is likely to be important for separating the ureters from the Wolffian ducts (E11–E13)Citation3 and also appears to be important for opening of the ureteral lumen at E15. That apoptosis occurs close to and within the bladder epithelium raises the interesting possibility that this site could be a source of signals that induce programmed cell death.
Repositioning of the Ureter Orifice Depends on Fusion with the Urogenital Sinus Epithelium and Expansion of the Bladder
Expansion of the CND and its subsequent apoptosis are likely to be important for separation of the ureter from the Wolffian duct, however it is unclear how once separated, the ureter attains its final position in the bladder which is at a distance from the Wolffian duct. A number of studies suggest that the ureter orifice which is attached to the CND/trigone, reaches its final position by migrating along the urogenital sinus, while other studies suggest that the ureter is repositioned as it engulfed by the expanding urogenital sinus.Citation13–Citation15 To directly visualize the changing relationship between the ureter and bladder, we stained vibratome sections of urogenital tracts from Hoxb7-Gfp embryos with pan-cytokeratin, a marker that labels epithelial cells. At E12, the Wolffian duct and ureter formed a loop that aligned the CND with the urogenital sinus, positioning the ureter just above the site where 24h later it would fuse with the urothelium (). Staining of Hoxb7-Gfp E13 urogenital tracts for expression of Laminin, a component of the basement membrane, enabled us to visualize the ureter orifice which was now fused with the bladder epithelium. At this stage the ureter orifice was still close to the Wolffian duct (). By E14 however, the position of the ureter orifice was shifted dramatically, and was now at the bladder neck, while the Wolffian duct remained in its original insertion site in the urethra (). These observations suggest that ureter insertion depends on distinct events, including apoptosis between E11 and E12 that enables separation from the Wolffian duct, fusion with the bladder epithelium that occurs on E13 and finally growth of the bladder, which expands enormously between E12 and E14.
CND Apoptosis Depends on Signals from the Urogenital Sinus
Analysis of duplicated collecting duct systems from humans and animals led to the formulation of the Weigert-Meyer law.Citation1,Citation14,Citation16 This model suggests that when two ureteric bud branches form on the Wolffian duct, the upper branch fails to join the bladder normally, causing obstruction, while the lower branch, that forms closer to the bladder, tends to insert at the normal position in the trigone. An interesting explanation for these observations could be that CND apoptosis depends on signals from the bladder, and only occurs in close proximity to these signals. Hence, the CND associated with the lower branch closest to the bladder undergoes apoptosis enabling the ureter detach from the Wolffian duct and re-insert in the trigone, but the CND associated with the upper branch persists, as a consequence, this ureter is unable to separate properly from the Wolffian duct and fails to join the bladder normally. To address this possibility we examined ureter maturation in a model of supernumerary ureteric bud formation induced by maternal exposure to high levels of retinoic acid during ureteric bud formation, a gain-of-function phenotype resulting from inappropriate activation of retinoid signaling at the stage when ureteric buds sprout from the Wolffian duct.Citation3
RA-treated Hoxb7-Gfp embryos displayed massive obstruction at E18 (compare ). Analysis at the stage of ureteric bud formation revealed 2 ureteric bud branches had formed on the Wolffian duct; the lower ureteric bud branch (ub1) sprouted close to the bladder at the normal position, the second (ub2) at a more anterior position (). To test whether failure in CND apoptosis could underlie obstruction seen at E18, ages, we used lineage analysis to follow the fate of CND cells associated with ub1 and ub2 after RA-exposure. Caspase-3 staining of E14 urogenital tracts from controls revealed that the CND had regressed, and numerous apoptotic cells were visible (, white arrows). On the other hand, cnd2 which was associated with the upper ureteric bud branch, persisted, and few if any apoptotic cells were visible (, white arrows).
We next performed lineage studies in Hoxb7-Cre;R26RlacZ fetuses to follow the fate of CNDs associated with the low and high ureter branches in RA-induced duplicated systems. Analysis of control E18 Hoxb7-Cre:R26RlacZ embryos revealed lacZ expression in the Wolffian ducts and ureters, but not in the region in between suggesting that cnd1 underwent apoptosis (). In the embryo shown in , duplicated ureters were present on one side. One of these joined the bladder normally (ureter 1), the other, ureter,Citation2 joined the bladder at an abnormally posterior site closer to the urethra (; ureter2). These studies suggest that formation of the ureteric bud at a position close to the urogenital sinus may be crucial for receiving signals that induce programmed cell death of the CND, while CNDs associated with ureteric bud branches formed at higher positions on the Wolffian duct may persist since they are out of range of the bladder-derived signals that normally induce apoptosis, resulting in obstruction.
Molecular Pathways Important for Urinary Tract Formation
A number of genetic pathways have been identified that when mutated in humans or mice result in urogenital tract malformations, including Six1 and Eya1,Citation17 Pax2,Citation18,Citation19 Gata3,Citation20 Slit2,Citation21 Robo2,Citation22 HoxA13,Citation23 Sall1,Citation24 Sprouty1,Citation25 Foxc1/c2,Citation26 Ret27 and Rara; Rarb2Citation27 and Bmp4 [reviewed in: 28 29]. Among these, mutations in Sprouty 25, Slit/Robo21, Foxc1/c2 26, Ret and Rara/Rarb2 27 result in ectopic ureteric bud formation and obstruction due to mis-regulation of Ret/Gdnf signaling which is normally required both for positioning the ureteric bud at the proper position, and for suppressing ectopic ureteric bud formation. Wolffian ducts are capable of forming epithelial sprouts along most of their rostrocaudal axes that are maintained in some species including fish and amphibians as functional pronephric or mesonephric kidneys. In mammals however, pronephric and mesonephric kidneys regress and only a single duct persists, the ureteric bud, which drains the metanephric kidney. A number of genetic pathways have been identified that are crucial for restricting Ret signaling to a localized domain in the caudal Wolffian duct, including Sprouty, Slit/Robo or Foxc1/c2Citation21,Citation25,Citation26,Citation30 which when inactivated, result in increased Ret signaling, supernumerary ureteric bud formation and obstruction.
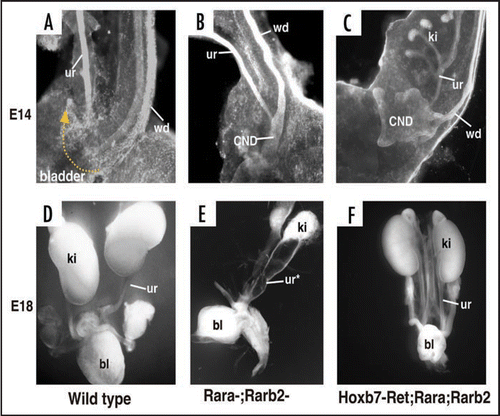
Loss-of-function mutations that result in down-regulation of the Ret/Gdnf signaling also cause obstruction, but in this case, the underlying defect is likely to be due to failure in CND remodeling. Retinoic acid, the active form of vitamin A, is required for kidney formation and ureter insertion by maintaining Ret expression in the ureteric bud and CND, respectively.Citation27,Citation31 Retinoic acid signals by binding to and activating RA-receptors, transcription factors belonging to the nuclear receptor superfamily.Citation32 Inactivation of two RA-receptor family members, Rara and Rarb2, results in renal hypoplasia due to impaired branching, and obstruction due to mal-positioned distal ureters that failed to join the bladder.Citation33 Cytokeratin staining at E14 revealed that in wild type embryos ureters had separated from the Wolffian ducts and joined the bladder. In contrast, Rara−/−;Rarb2−/− mutant ureters remained joined to the Wolffian ducts. Interestingly CND remodeling in these mutants was arrested at two distinct stages. In the Rara−/−;Rarb2−/− mutant urogenital tract shown, the CND on the left persisted and appeared not to have remodeled (), while on the contralateral side, the CND had expanded but was not inserted in the bladder and had not undergone apoptosis.
The observations that: (1) Ret is normally localized in CND cells and is down-regulated in Rara−/−;Rarb2−/− mutants, (2) Forced expression of Ret in the mutant CND rescued remodeling, suggest that retinoic acid regulates CND remodeling via Ret. Ret signaling may be important for the initial expansion of the CND, acting in a similar manner as in ureteric bud formation and also for apoptosis of the CND.Citation3,Citation34 At present it is unclear whether RA regulates Ret expression directly, or whether RA-regulated transcription in adjacent cells, for example in the bladder, may induce secreted signals that normally control Ret expression and CND apoptosis. Ret mutations in humans are associated with Hirschsprung's disease and renal defects.35 It will be interesting to determine whether ureteral abnormalities in humans may also be linked to loss of Ret function.
Questions and Answers
That was a beautiful talk, thank you. You had mentioned there are valves between the ureter and the bladder. It did not appear that any “positive cells” in your Hoxb7-GFP embryos or Hoxb7Cre; ROSAlacZ mice contribute to the valve formation.
I didn't think so either.
What do you think is the origin of the valves? You have the hedgehog-CreROSAlacZ model in which bladder epithelium is labeled. Did you look at that model and see if those epithelial cells contribute to the valves.
When I speak of a valve, what I'm talking about is a segment of ureter passing through the bladder sub mucosa. Therefore the ‘valve’ is not the epithelium so much as the intersection of the ureter and bladder muscle.
So you are not speaking of a structure like a heart valve.
Correct.
What is known about the enzyme that converts vitamin A to retinoic acid?
The enzyme activity is distributed widely and acts locally in embryonic cells. There are other enzymes that degrade retinoic acid.
In several of your animal models the ureter is dilated (hydroureter). You focused on the bladder and the trigone. However, you didn't speak about the kidney. Could you summarize what is the importance of the ureter in kidney development? Is there a ‘point of no return’ for ureter malformation when it comes to signaling kidney development?
I think what we are learning from knocking out genes that impact on ureter development is that their expression is important not only for ureter development, but also for kidney development per se. In other words, renal hypoplasia and mal-positioned ureter orifices could both be caused by loss of a single gene, whose function is important both in the kidney and during ureter maturation. There is no evidence to support the idea that the abnormal position of the ureteric bud results in renal hypoplasia, in fact, you can make lots of kidneys when you have supernumerary ureteric buds for example in sprouty mutants.
Do you think it is possible that death of the common nephric duct cells you show is due to loss of attachment, what some would designate anoikis?
There could be a signal coming from the epithelium into the bladder that induces apoptosis.
Abbreviations
| CND | = | common nephric duct |
| Wd | = | wolffian duct |
| RA | = | retinoic acid |
Figures and Tables
Figure 1 Ureter maturation. (A and C) Whole mount and schematic of a mouse E11 urinary tract prior to ureter insertion. (B and D) Whole mount and schematic of an E14 urinary tract after ureter insertion. Note the ureter has now detached from the Wolffian duct and fused with the bladder epithelium.
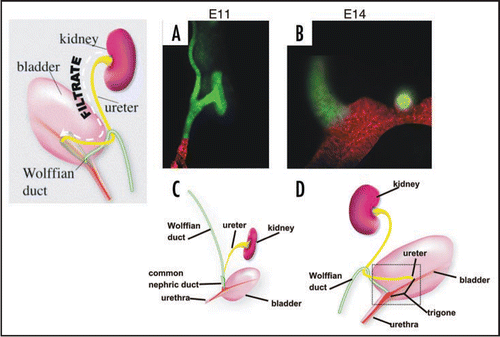
Figure 2 The Bladder Trigone. Top: Schematic of the ureteral valve mechanism and its physical relationship to the trigone. The trigone is a muscular structure located at the base of the bladder. When the bladder fills with urine, the trigonal muscle helps compress the ureteral orifice preventing back-flow of urine to the ureters and kidneys, which can cause severe damage. Bottom: The mouse ureteral valve. A vibratome section of a P0 urogenital tract stained with smooth muscle alpha actin (green) and uroplakin (red).
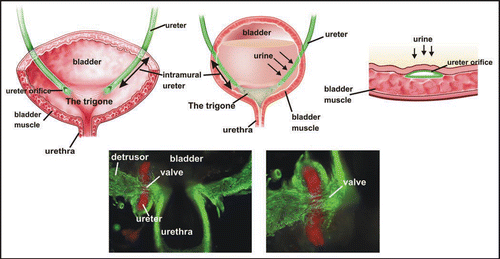
Figure 3 The Mackie Stephens hypothesis. Top: A ureteric bud emerging at the proper site on the Wolffian duct will join the trigone normally. Middle: A ureteric bud that forms too low on the Wolffian duct tends to join the bladder lateral or anterior to the normal insertion site causing reflux. Bottom: A ureteric bud forming too high on the Wolffian duct tends to remain attached to the sex ducts or joins the urogenital sinus posterior to its normal insertion site, causing obstruction. Green, Wolffian ducts and ureters; Red, cloacal endoderm and bladder trigone. Abbreviations: ub, ureteric bud; Wd, Wolffian duct.
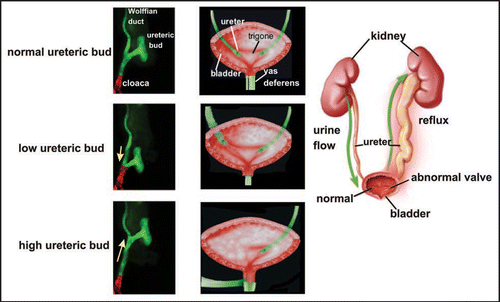
Figure 4 The original model of ureter insertion. Left: The ureter joins the bladder indirectly via the common nephric cut. Middle: The common nephric duct inserts into the bladder and expands, moving the ureter orifice anterior with respect to the Wolffian duct. Right: Further expansion of the trigone positions the ureter orifice in its final insertion site.
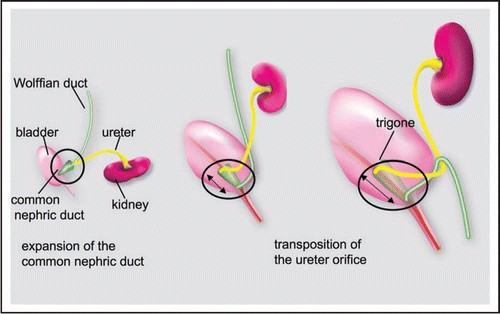
Figure 5 CND remodeling. (A) Left Top: Whole mount E12 Hoxb7-Gfp urogenital tract. Note that Gfp is present in Wolffian duct, ureter and CND but not in the bladder which is invisible in this image Bottom left: A sample of CND epithelium counterstained with E-cadherin showing the multilayered structure, as opposed to the simple tubular structure of the upper segments of Wolffian duct and ureter. Right: Images of Hoxb7-Gfp whole mount urogenital tracts between E11 and E14. Note the expansion of the CND (white dashed arrows at E11, E12 and E13) and its subsequent disappearance (E14, white dashed arrow). (B) Urogenital tracts from E11, E12 and E13 Hoxb7-Gfp embryos counterstained with cytokeratin (red) to illustrate expansion of the CND as the ureter moves into contact with the urogenital sinus. Abbreviations: CND, common nephric duct; ugs, urogenital sinus; Wd, Wolffian duct; ur, ureter.
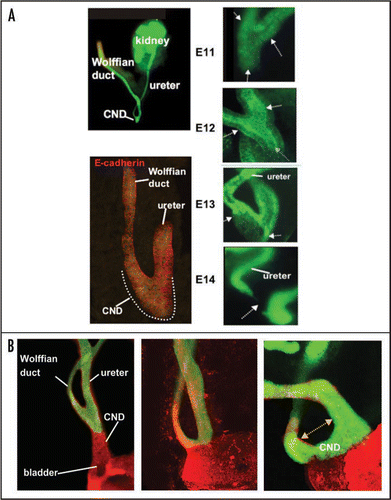
Figure 6 The CND does not form the trigone. Lineage analysis to determine the fate of CND cells. (A and D) A whole mount (A) and section (D) of a urogenital tract from an E13 Hoxb7-Cre;R26RlacZ embryo showing lacZ positive cells in the ureter, Wolffian duct and CND. (B and E) Whole mount (B) and section (E) of an E14 Hoxb7-Cre;R26RlacZ embryo showing lacZ expression at E14. Note the decline in lacZ expression in the CND but persistent expression in the ureters and Wolffian ducts. (C and F) Analysis of whole mount (C) and a section (F) of an urogenital tract from a E18 Hoxb7-Cre;R26RlacZ embryo. Note the absence of lacZ-labeled cells in the trigone, between the ureter and Wolffian duct where there is still strong lacZ expression. Bottom: A schematic showing a revised model of ureter maturation.
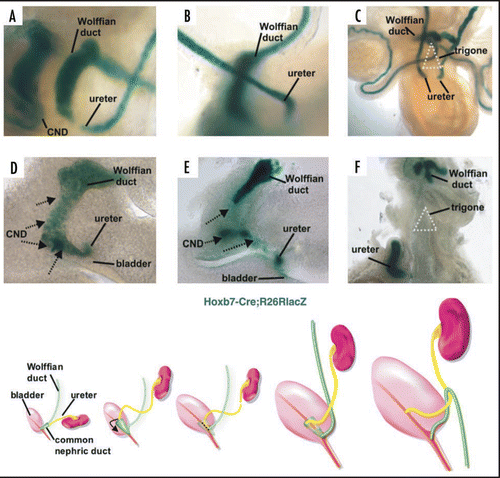
Figure 7 Apoptosis of the common nephric duct. Cryosection of am E11 Hoxb7-Gfp urogenital tract stained with activated caspase-3 antibody, (green) showing apoptosis of CND cells (red), but not cells in the Wolffian duct and ureter.
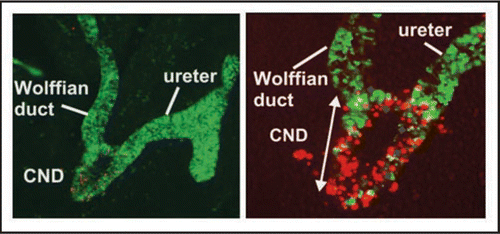
Figure 8 Fusion of the ureter orifice with the urogenital sinus and opening of the lumen. Left to right panels 1–6, Hoxb7-Gfp embryonic urogenital tracts stained with E-Cadherin (red) and activated caspase-3 (blue). Note apoptotic cells in the lumen of the ureter which is within the urogenital sinus epithelium at E15 (3,4) and the opening of the lumen on E16 (5,6).
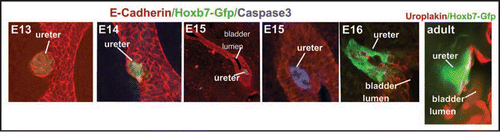
Figure 9 Ureter Insertion depends on alignment of the new ureter orifice with the bladder, regression of the CND, fusion of the ureter with the urogenital sinus epithelium and expansion of the bladder. Left: Vibratome sections of E12, E13 and E14 wild type urogenital tracts stained with antibody directed against cytokeratin (red).Note the enormous growth of the bladder relative to the urethra during this 3-day period. Right: Top, vibratome section of a Hoxb7-Gfp embryo (green) stained with E-cadherin (red). Note a loop has formed that helps align the ureter with the position in the urothelium (red) where it will fuse. Right middle and bottom: Laminin (red) stained Hoxb7-Gfp (green) urinary tracts showing the relative positions of the CND, ureter and Wolffian duct. At E13 the ureter orifice is fused with the urogenital sinus epithelium, but remains close to the Wolffian duct. Note the cell debris at this stage between the ureter and Wolffian duct where the CND was previously. Right Bottom: Between E13 and E14, the ureter orifice, which is tethered to the urogenital sinus epithelium, moves anteriorly as the bladder undergoes dramatic expansion.
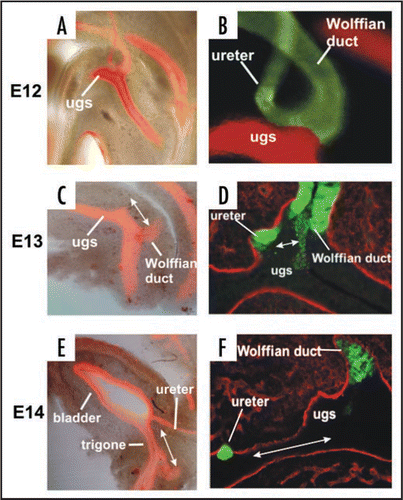
Figure 10 CND apoptosis depends on proximity to the bladder. (A and B) Control Hoxb7-Gfp urogenital tracts from E11 (A) and E18 (B) embryos. (C) Activated-Caspase-3 stained section of an E14 Hoxb7-Gfp embryo. (D) Ureter insertion in a P0 Hoxb7-Cre;R26RlacZ urogenital tract. (E and F) urogenital tracts from a Hoxb7-Gfp embryo exposed to retinoic acid; (E) E11, (F) E18. (G) Activated caspase-3 staining of the upper ureteric bud (ureteric bud 2) showing lack of apoptosis in the associated CND (CND2). (H) Ureter insertion in an E18 Hoxb7-Cre;R26RlacZ mouse with double ureters. Note the absence of CND1 and the persistence of the CND2.
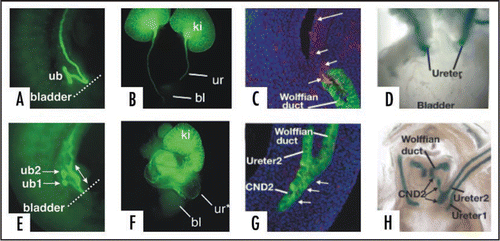
Figure 11 Defective CND remodeling in Rara-;Rarb2-mutants is rescued by Ret. (A) A cytokeratin stained E14 wild type urogenital tract. (B and C) Cytokeratin stained Rara-;Rarb2-mutant urogenital tract. (D) Whole mount E18 urogenital tract from a wild type embryo. (E) Whole mount E18 Rara-;Rarb2-urogenital tract. (F) Whole mount E18 Hoxb7-Cre;Rara-;Rarb2-urogenital tract.
Acknowledgements
We thank the members of the Mendelsohn lab for their hard work and dedication that made the studies on ureter maturation possible. We also thank Qais Al-awqati, Jonathan Barasch, Frank Costantini and Doris Herzlinger for discussions, advice and support.
Note
Edited transcripts of research conferences sponsored by Organogenesis and the Washington University George M. O'Brien Center for Kidney Disease Research (P30 DK079333) are published in Organogenesis. These conferences cover organogenesis in all multicellular organisms including research into tissue engineering, artificial organs and organ substitutes and are participated by faculty at Washington University School of Medicine, St. Louis Missouri.
References
- Mackie GG, Stephens FD. Duplex kidneys: a correlation of renal dysplasia with position of the ureteral orifice. J Urol 1975; 114:274 - 280
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 2001; 1:4
- Batourina E, Tsai S, Lambert S, Sprenkle P, Viana R, Dutta S, et al. Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Nat Genet 2005; 37:1082 - 1089
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 1999; 21:70 - 71
- Alcaraz A, Vinaixa F, Tejedo-Mateu A, Fores MM, Gotzens V, Mestres CA, et al. Obstruction and recanalization of the ureter during embryonic development. J Urol 1991; 145:410 - 416
- Ruano-Gil D, Coca-Payeras A, Tejedo-Mateu A. Obstruction and normal recanalization of the ureter in the human embryo. Its relation to congenital ureteric obstruction. Eur Urol 1975; 1:287 - 293
- Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell 1998; 94:727 - 737
- Zuzarte-Luis V, Hurle JM. Programmed cell death in the embryonic vertebrate limb. Semin Cell Dev Biol 2005; 16:261 - 269
- Rodriguez I, Araki K, Khatib K, Martinou JC, Vassalli P. Mouse vaginal opening is an apoptosis-dependent process which can be prevented by the overexpression of Bcl2. Dev Biol 1997; 184:115 - 121
- Roberts LM, Hirokawa Y, Nachtigal MW, Ingraham HA. Paracrine-mediated apoptosis in reproductive tract development. Dev Biol 1999; 208:110 - 122
- Buss RR, Sun W, Oppenheim RW. Adaptive roles of programmed cell death during nervous system development. Annual review of neuroscience 2006; 29:1 - 35
- Tanagho EA. Devlopment of the Ureter 1981; New York Springer-Verlag
- Meyer R. Normal and abnormal development of the ureter in the human emryo-a mechanistic consideration. Anatomical Records 1946; 68:355 - 371
- Hutch JA. Anatomy and physiology of the bladder, trigone and urethra 1972; London, New York Butterworths Appleton-Century-Crofts
- Weiss JP. Embryogenesis of ureteral anomalies: a unifying theory. Aust N Z J Surg 1988; 58:631 - 638
- Ruf RG, Xu PX, Silvius D, Otto EA, Beekmann F, Muerb UT, et al. SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes. Proc Natl Acad Sci USA 2004; 101:8090 - 8095
- Favor J, Sandulache R, Neuhauser-Klaus A, Pretsch W, Chatterjee B, Senft E, et al. The mouse Pax2(1Neu) mutation is identical to a human PAX2 mutation in a family with renal-coloboma syndrome and results in developmental defects of the brain, ear, eye and kidney. Proc Natl Acad Sci USA 1996; 93:13870 - 13875
- Sanyanusin P, Schimmenti LA, McNoe LA, Ward TA, Pierpont ME, Sullivan MJ, et al. Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies and vesicoureteral reflux. Nat Genet 1995; 9:358 - 364
- Muroya K, Hasegawa T, Ito Y, Nagai T, Isotani H, Iwata Y, et al. GATA3 abnormalities and the phenotypic spectrum of HDR syndrome. J Med Genet 2001; 38:374 - 380
- Lu W, van Eerde AM, Fan X, Quintero-Rivera F, Kulkarni S, Ferguson H, et al. Disruption of ROBO2 is associated with urinary tract anomalies and confers risk of vesicoureteral reflux. Am J Hum Genet 2007; 80:616 - 632
- Mortlock DP, Innis JW. Mutation of HOXA13 in hand-foot-genital syndrome. Nat Genet 1997; 15:179 - 180
- Engels S, Kohlhase J, McGaughran J. A SALL1 mutation causes a branchio-oto-renal syndrome-like phenotype. J Med Genet 2000; 37:458 - 460
- Murawski IJ, Gupta IR. Vesicoureteric reflux and renal malformations: a developmental problem. Clinical genetics 2006; 69:105 - 117
- Airik R, Kispert A. Down the tube of obstructive nephropathies: The importance of tissue interactions during ureter development. Kidney Int 2007;
- Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, et al. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell 2005; 8:229 - 239
- Grieshammer U, Le M, Plump AS, Wang F, Tessier-Lavigne M, Martin GR. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev Cell 2004; 6:709 - 717
- Shakya R, Jho EH, Kotka P, Wu Z, Kholodilov N, Burke R, et al. The role of GDNF in patterning the excretory system. Dev Biol 2005; 283:70 - 84
- Kume T, Deng K, Hogan BL. Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development 2000; 127:1387 - 1395
- Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter [In Process Citation]. J Clin Invest 2000; 105:863 - 873
- Batourina E, Choi C, Paragas N, Bello N, Hensle T, Costantini FD, et al. Distal ureter morphogenesis depends on epithelial cell remodeling mediated by vitamin A and Ret. Nat Genet 2002; 32:109 - 115
- Mendelsohn C, Lohnes D, Decimo D, Lufkin T, LeMeur M, Chambon P, et al. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development 1994; 120:2749 - 2771
- Wilson J, Ga WJ. Malformations in the genito-urinary tract induced by maternal vitamin A deficiency in the rat. Am J Anat 1948; 83:357 - 407
- McGrane MM. Vitamin A regulation of gene expression: molecular mechanism of a prototype gene. The Journal of nutritional biochemistry 2007; 18:497 - 508
- Runeberg-Roos P, Saarma M. Neurotrophic factor receptor RET: structure, cell biology and inherited diseases. Ann Med 2007; 39:572 - 580