Abstract
Heat shock proteins HSP27, HSP70 and HSP90 are molecular chaperones whose expression is increased after many different types of stress. They have a protective function helping the cell to cope with lethal conditions. The cytoprotective function of HSPs is largely explained by their anti-apoptotic function. HSPs have been shown to interact with different key apoptotic proteins. As a result, HSPs can block essentially all apoptotic pathways, most of them involving the activation of cystein proteases called caspases. Apoptosis and differentiation are physiological processes that share many common features, for instance, chromatin condensation and the activation of caspases are frequently observed. It is, therefore, not surprising that many recent reports imply HSPs in the differentiation process. This review will comment on the role of HSP90, HSP70 and HSP27 in apoptosis and cell differentiation. HSPs may determine de fate of the cells by orchestrating the decision of apoptosis versus differentiation.
Introduction
Stress or heat shock proteins (HSPs) were first discovered in 1962Citation1 as a set of highly conserved proteins whose expression was induced by different kinds of stress. It has subsequently been shown that most HSPs have strong cytoprotective effects and behave as molecular chaperones for other cellular proteins. HSPs are also induced at specific stages of development, differentiation and during oncogenesis.Citation2 Mammalian HSPs have been classified into five families according to their molecular size: HSP100, HSP90, HSP70, HSP60 and the small HSPs. Each family of HSPs is composed of members expressed either constitutively or regulated inducibly, and/or targeted to different sub-cellular compartments. The most studied HSPs are HSP90, the inducible HSP70 (also called HSP72) and the small heat shock protein HSP27.
HSP90 is a constitutively abundant chaperone that makes up 1–2% of cytosolic proteins. It is an ATP-dependent chaperone that accounts for the maturation and functional stability of a plethora of proteins termed HSP90 client proteins. In mammals, HSP90 comprises 2 homologue proteins (HSP90α and HSP90β) encoded by separated but highly conserved genes that arose through duplication during evolution.Citation3 Most studies do not differentiate between the two isoforms because for a long time they have been considered as having the same function in the cells. However, recent data and notably out-of-function experiments indicate that at least some functions of the beta isoform are not overlapped by HSP90α's functions.Citation4 HSP70, like HSP90, binds ATP and undergoes a conformational change upon ATP binding, needed to facilitate the refolding of denatured proteins. The chaperone function of HSP70 is to assist the folding of newly synthesized polypeptides or misfolded proteins, the assembly of multi-protein complexes and the transport of proteins across cellular membranes.Citation5,Citation6 HSP90 and HSP70 chaperone activity is regulated by co-chaperones like Hip, CHIP or Bag-1 that increase or decrease their affinity for substrates through the stabilization of the ADP or ATP bound state. In contrast to HSP90 and HSP70, HSP27 is an ATP-independent chaperone, its main chaperone function being protection against protein aggregation.Citation7 HSP27 can form oligomers of more than 1000 Kda. The chaperone role of HSP27 seems modulated by its state of oligomerization, the multimer being the chaperone competent state.Citation8 This oligomerization is a very dynamic process modulated by the phosphorylation of the protein that favors the formation of small oligomers. Cell-cell contact and methylglyoxal can also modulate the oligomerization of the protein.Citation9
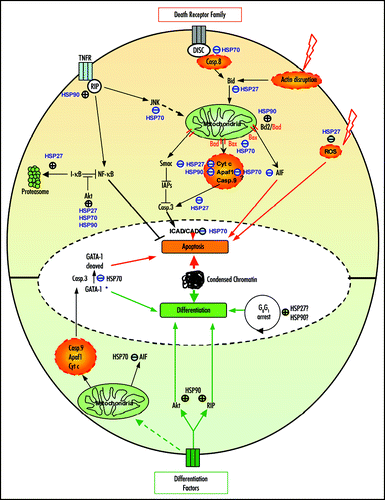
It is now well accepted that HSPs are important modulators of the apoptotic pathway. Apoptosis, or programmed cell death, is a type of death essential during embryogenesis and, latter on in the organism, to assure cell homeostasis. Apoptosis is also a very frequent type of cell death observed after treatment with cytotoxic drugs.Citation10 Mainly, two pathways of apoptosis can be distinguished, although cross-talk between the two signal transducing cascades exists (). The extrinsic pathway is triggered through plasma membrane proteins of the tumor necrosis factor (TNF) receptor family known as death receptors, and leads to the direct activation of the proteases called caspases, starting with the receptor-proximal caspase-8. The intrinsic pathway involves intracellular stress signals that provoke the permeabilization of the outer mitochondrial membrane, resulting in the release of pro-apoptotic molecules normally confined to the inter-membrane space. Such proteins translocate from mitochondria to the cytosol in a reaction that is controlled by Bcl-2 and Bcl-2-related proteins.Citation11 One of them is the cytochrome c, which interacts with cytosolic apoptosis protease-activating factor-1 (Apaf-1) and pro-caspase-9 to form the apoptosome, the caspase-3 activation complex.Citation12 Apoptosis inducing factor (AIF) and the Dnase, EndoG, are other mitochondria intermembrane proteins released upon an apoptotic stimulus. They translocate to the nucleus and trigger caspase-independent nuclear changes.Citation13,Citation14 Two additional released mitochondrial proteins, Smac/Diablo and Htra2/Omi, activate apoptosis by neutralizing the inhibitory activity of the inhibitory apoptotic proteins (IAPs) that associate with and inhibit caspasesCitation15 ().
Apoptosis and differentiation are two physiological processes that share different features like chromatin condensation or the need of caspase activity.Citation16 It has been demonstrated in many differentiation models that the activation of caspases is preceded by a mitochondrial membrane depolarization and release of mitochondria apoptogenic molecules.Citation17,Citation18 This suggests that the mitochondrial-caspase dependent apoptotic pathway is a common intermediate for conveying apoptosis and differentiation. Timing, intensity and cellular compartmentalization might determine whether a cell is to die or differentiate. HSPs might be essential to orchestrate this decision. This review will describe the role of HSP90, HSP70 and HSP27 in apoptosis and cell differentiation.
HSP27, HSP70 and HSP90 are Anti-Apoptotic Proteins
Overexpression of HSP27, HSP70 or HSP90 prevents apoptosis triggered by various stimuli, including hyperthermia, oxidative stress, staurosporine, ligation of the Fas/Apo-1/CD95 death receptor or anticancer drugs.Citation2,Citation19–Citation21 Downregulation or inhibition of HSP27, HSP70 or HSP90 have been shown to be enough to sensitize a cell to apoptosis, proving that endogenous levels of those chaperones seem to be sufficiently high to control apoptosis.Citation22–Citation24 It is now known that these chaperones can interact with key proteins of the apoptotic signaling pathways ().
HSP90: A survival protein through its client proteins.
HSP90 client proteins include a number of signaling proteins like ligand-dependent transcription factors and signal transducing kinases that play a role in the apoptotic process. Upon binding and hydrolysis of ATP, the conformation of HSP90 changes and the client protein, which is no longer chaperoned, is ubiquitinated and degraded by the proteasome.Citation25
A function for HSP90 in the serine/threonine protein kinase Akt pathway was first suggested by studies using an HSP90 inhibitor that promoted apoptosis in HEK293T and resulted in suppressed Akt activity.Citation26 A direct interaction between Akt and HSP90 was reported later.Citation27 Binding of HSP90 protects Akt from protein phosphatase 2A (PP2A)-mediated dephosphorylation.Citation26 Phosphorylated Akt can then phosphorylate the Bcl-2 family protein Bad and caspase-9 leading to their inactivation and to cell survival.Citation28,Citation29 But Akt has been also shown to phosphorylate IkB kinase, which results in promotion of NFkB-mediated inhibition of apoptosis.Citation30 When the interaction HSP90/Akt was prevented by HSP90 inhibitors, Akt was dephosphorylated and destabilized and the likelihood of apoptosis increased.Citation27 Additional studies showed that another chaperone participates in the Akt-HSP90 complex, namely Cdc37.Citation26 Together this complex protects Akt from proteasome degradation. In human endothelial cells during high glucose exposure, apoptosis can be prevented by HSP90 through augmentation of the protein interaction between eNOS and HSP90 and recruitment of the activated Akt.Citation31 HSP90 has also been shown to interact with and stabilize the receptor interacting protein (RIP). Upon ligation of TNFR-1, RIP-1 is recruited to the receptor and promotes the activation of NFκB and JNK. Degradation of RIP-1 in the absence of HSP90 precludes activation of NFκB mediated by TNFα and sensitizes cells to apoptosis.Citation32 Another route by which HSP90 can affect NFκB survival activity is via the IKK complex.Citation33 The HSP90 inhibitor geldanamycin prevents TNF-induced activation of IKK, highlighting the role of HSP90 in NFκB activation. Some other HSP90 client proteins through which this chaperone could participate in cell survival are p53Citation34 and the transcription factors Her2 and Hif1α.Citation35,Citation36
But the anti-apoptotic role of HSP90 can also be explained by its effect and interaction with proteins not defined as HSP90 client proteins (i.e., whose stability is not regulated by HSP90). HSP90 overexpression in human leukemic U937 cells can prevent the activation of caspases in cytosolic extracts treated with cytochrome c probably because HSP90 can bind to Apaf-1 and inhibit its oligomerization and further recruitment of procaspase-9.Citation37
Unfortunately, most studies do not differentiate between HSP90α and HSP90β. It has recently been demonstrated in multiple myeloma, in which an over expression of HSP90 is necessary for cell survival, that depletion of HSP90β by siRNA is sufficient to induce apoptosis. This effect is strongly increased when also HSP90α is also depleted,Citation23 suggesting different and cooperating anti-apoptotic properties for HSP90α and HSP90β. Confirming this assumption, in mast cells, HSP90β has been shown to associate with the anti-apoptotic protein Bcl-2. Depletion of HSP90β with a siRNA or inhibion of HSP90 with geldanamycin inhibits HSP90β interaction with Bcl-2 and results in cytochrome c release, caspase activation and apoptosis.Citation38
In conclusion, HSP90 anti-apoptotic functions can largely be explained by its chaperone role assuring the stability of different proteins. Recent studies suggest that the two homologue proteins, HSP90α and HSP90β, might have different survival properties. It would be interesting to determine whether HSP90α and HSP90β bind to different client proteins or bind with different affinity.
HSP70: A quintessential inhibitor of apoptosis.
HSP70 loss-of-function studies demonstrated the important role of HSP70 in apoptosis. Cells lacking hsp70.1 and hsp70.3, the two genes that code for inductive HSP70, are very sensitive to apoptosis induced by a wide range of lethal stimuli.Citation39 Further, the testis specific isoform of HSP70 (hsp70.2) when ablated, results in germ cell apoptosis.Citation40 In cancer cells, depletion of HSP70 results in spontaneous apoptosis.Citation41
HSP70 has been shown to inhibit the apoptotic pathways at different levels (). At the pre-mitochondrial level, HSP70 binds to and blocks c-Jun N-terminal Kinase (JNK1) activity.Citation42,Citation43 Confirming this result, HSP70 deficiency induces JNK activation and caspase-3 activationCitation44 in apoptosis induced by hyperosmolarity. HSP70 also has been shown to bind to non-phosphorylated protein kinase C (PKC) and Akt, stabilizing both proteins.Citation45
At the mitochondrial level, HSP70 inhibits Bax translocation and insertion into the outer mitochondrial membrane. As a consequence, HSP70 prevents mitochondrial membrane permeabilization and release of cytochrome c and AIF.Citation46
At the post-mitochondrial level HSP70 has been demonstrated to bind directly to Apaf-1, thereby preventing the recruitment of procaspase-9 to the apoptosome.Citation47 However, these results have been contradicted by a study in which the authors demonstrated that HSP70 do not have any direct effect on caspase activation. They explain these contradictory results by showing that it is a high salt concentration and not HSP70 that inhibits caspase activation.Citation48
HSP70 also prevents cell death in conditions in which caspase activation does not occur.Citation49 Indeed, HSP70 binds to AIF, inhibits AIF nuclear translocation and chromatin condensation.Citation39,Citation50,Citation51 The interaction involves a domain of AIF between aminoacids 150 and 228.Citation52 AIF sequestration by HSP70 has been shown to reduce neonatal hypoxic/ischemic brain injury.Citation53 HSP70 has also been shown to associate with EndoG and to prevent DNA fragmentationCitation54 but since EndoG can form complexes with AIF, its association with HSP70 could involve AIF as a molecular bridge.
HSP70 can also rescue cells from a later phase of apoptosis than any known survival protein, downstream caspase-3 activation.Citation55 During the final phases of apoptosis, chromosomal DNA is digested by the DNase CAD (caspase activated DNase), following activation by caspase-3. The enzymatic activity and proper folding of CAD has been reported to be regulated by HSP70.Citation56
At the death receptors level, HSP70 binds to DR4 and DR5, thereby inhibiting TRAIL-induced assembly and activity of death inducing signaling complex (DISC).Citation57 Finally, HSP70 has been shown to inhibit lysosomal membrane permeabilization thereby preventing cathepsines release, proteases also implicated in apoptosis.Citation58,Citation59
In conclusion, HSP70 is a quintessential regulator of apoptosis that can interfere with all main apoptotic pathways. Interestingly, the ATP binding domain of HSP70 is not always required. For instance, while the ATPase function is needed for the Apaf-1Citation50 and AIF binding,Citation51 it is dispensable for JNKCitation60 or GATA-1Citation61 binding/protection. In this way, in erythroblasts, in which HSP70 blocks apoptosis by protecting GATA-1 from caspase-3 cleavage, a HSP70 mutant that lacks the ATP binding domain of HSP70 is as efficient as wild type HSP70 in assuring the protection of erythroblasts.Citation61
HSP27: An inhibitor of caspase activation.
HSP27 depletion reports demonstrate that HSP27 essentially blocks caspase-dependent apoptotic pathways. Small interefence targeting HSP27 induces apoptosis through caspase-3 activation.Citation62,Citation63 This may be consequence of the association of HSP27 with cytochrome c in the cytosol, thereby inhibiting the formation of the caspase-3 activation complex as demonstrated in leukemia and colon cancer cells treated with different apoptotic stimuli.Citation64–Citation66 This interaction involves amino-acids 51 and 141 of HSP27 and do not need the phosphorylation of the protein.Citation65 In multiple myeloma cells treated with dexamethasone, HSP27 has also been shown to interact with Smac.Citation67
HSP27 can also interfere with caspase activation upstream of the mitochondria.Citation66 This effect seems related to the ability of HSP27 to interact and regulate actin microfilaments dynamics. In L929 murine fibrosarcoma cells exposed to cytochalasin D or staurosporine, overexpressed HSP27 binds to F-actinCitation68 preventing the cytoskeletal disruption, Bid intracellular redistribution and cytochrome c releaseCitation66 (). HSP27 has also important anti-oxidant properties. This is related to its ability to uphold glutathione in its reduced form,Citation69 to decrease reactive oxygen species cell content,Citation19 and to neutralize the toxic effects of oxidized proteins.Citation70 These anti-oxidant properties of HSP27 seem particularly relevant in HSP27 protective effect in neuronal cells.Citation71
HSP27 has been shown to bind to the kinase Akt, an interaction that is necessary for Akt activation in stressed cells. In turn, Akt could phosphorylate HSP27, thus leading to the disruption of HSP27-Akt complexes.Citation72 HSP27 also affects one downstream event elicited by Fas/CD95. The phosphorylated form of HSP27 directly interacts with Daxx.Citation73 In LNCaP tumor cells, HSP27 has been shown to induce cell protection through its interaction with the activators of transcription 3 (Stat3).Citation74 Finally, HSP27 protective effect can also be consequence of its effect favouring the proteasomal degradation of certain proteins under stress conditions. Two of the proteins that HSP27 targets for their ubiquitination/proteasomal degradation are the transcription factor nuclear factor κB (NFκB) inhibitor IκBα and p27kip1. The pronounced degradation of IkBα induced by HSP27 overexpression increases NFκB dependent cell survivalCitation75 while that of p27kip1 facilitates the passage of cells to the proliferate phases of the cellular cycle. As a consequence HSP27 allows the cells to rapidly resume proliferation after a stress.Citation76
Therefore, HSP27 is able to block apoptosis at different stages because of its interaction with different partners. The capacity of HSP27 to interact with one or another partner seems to be determined by the oligomerization/phosphorylation status of the protein, which, at its turn, might depend on the cellular model/experimental conditions. We have demonstrated in vitro and in vivo that for HSP27 caspase-dependent anti-apoptotic effect, large non-phosphorylated oligomers of HSP27 were the active form of the protein.Citation77 Confirming these results, it has recently been demonstrated that methylglyoxal modification of HSP27 induces large oligomers formation and increases the anti-apoptotic caspase-inhibitory properties of HSP27.Citation78 In contrast, for HSP27 interaction with the F-actin and with Daxx, phosphorylated and small oligomers of HSP27 were necessaryCitation73,Citation79 and it is its phosphorylated form that protects against neurotoxicity.Citation80
HSP27, HSP70 and HSP90 and Cell Differentiation
Under the prescribed context of HSPs as powerful inhibitors of apoptosis, it is reasonable to assume that an increase or decrease in their expression might modulate the differentiation program. The first evidence of the role of HSPs in cell differentiation comes from their tightly regulated expression at different stages of development and cell differentiation. For instance during the process of endochondrial bone formation, they are differentially expressed in a stage-specific manner.Citation81 In addition, during post-natal development, time at which extensive differentiation takes place, HSPs expression is regulated in neuronal and non-neuronal tissues.Citation82 In hemin-induced differentiation of human K562 erythroleukemic cells, genes coding for HSPs are induced.Citation83
In leukemic cells HSP27 has been described as a pre-differentiation markerCitation84 because its induction occurs early during differentiation.Citation85–Citation88 HSP27 expression has also been suggested as a differentiation marker for skin keratinocytesCitation89 and for C2C12 muscle cells.Citation90 This role for HSP27 in cell differentiation might be related to the fact that HSP27 expression increases as cells reach the non proliferative/quiescent phases of the cellular cycle (G0/G1).Citation19,Citation76
Subcellular localization is another mechanism whereby HSPs can determine whether a cell is to die or to differentiate. We, and others, have recently demonstrated the essential function of nuclear HSP70 for erythroid differentiation. During red blood cells' formation, HSP70 and activated caspase-3 accumulate in the nucleus of the erythroblast.Citation91 HSP70 directly associates with GATA-1 protecting this transcription factor required for erythropoiesis from caspase-3 cleavage. As a result, erythroblats continue their differentiation process instead of dying by apoptosis.Citation61 HSP70, during erythropoiesis in TF-1 cells, have been shown to bind to AIF and thereby to block AIF-induced apoptosis, thus allowing the differentiation of erythroblasts to proceed.Citation18
HSP90 has been required for erythroid differentiation of leukemia K562 cells induced by sodium butyrateCitation92 and for DMSO-differentiated HL-60 cells. Regulation of HSP90 isoforms may be a critical event in the differentiation of human embryonic carcinoma cells and may be involved in differentiation into specific cell lineages.Citation93 This effect of HSP90 in cell differentiation is probably because multiple transduction proteins essential for differentiation are client proteins of HSP90 such as Akt,Citation94 RIPCitation32 or Rb.Citation95 Loss of function studies confirm that HSP90 plays a role in cell differentiation and development. In Drosophila melanogaster, point mutations of HSP83 (the drosophila HSP90 gene) are lethal as homozygotes. Heteterozygous mutant combinations produce viable adults with the same developmental defect: sterility.Citation96 In Caenorhabditis elegans, DAF-21, the homologue of HSP90, is necessary for oocyte development.Citation97 In zebrafish, HSP90 is expressed during normal differentiation of triated muscle fibres. Disruption of the activity of the proteins or the genes give rise to failure in proper somatic muscle development.Citation98 In mice, loss-of-function studies demonstrate that while HSP90α loss-of-function phenotype appears to be normal, HSP90β is lethal. HSP90β is essential for trophoblasts differentiation and thereby for placenta development and this function can not be performed by HSP90α.Citation4
HSP90 inhibitors have also been used to study the role of HSP90 in cell differentiation. These inhibitors such as the benzoquinone ansamycin geldanamycin or its derivative the 17-allylamino-17-demethoxygeldanamycin (17-AAG), bind to the ATP-binding “pocket” of HSP90 with higher affinity than natural nucleotides and thereby HSP90 chaperone activity is impaired and its client proteins are degraded. As could be expected by the reported role of HSP90 in cell differentiation, inhibitors of HSP90 block C2C12 myoblasts differentiation.Citation99 In cancer cells and human leukemic blasts, 17-AAG induces a retinoblastoma-dependent G1 block. These G1 arrested cells do not differentiate but instead die by apoptosis.Citation100
However, some reports describe that inhibitors of HSP90 can induce the differentiation process. In acute myeloid leukemia cells, 17-AAG induced apoptosis or differentiation depending on the dose and time of the treatment.Citation101 Opposite effects on cell differentiation and apoptosis are also obtained with the HSP90 inhibitor geldanamycin: in PC12 cells it induced apoptosis while in murin neuroblastoma N2A cells it induced differentiation.Citation102 In breast cancer cells, 17-AAG-induced G1 block is accompanied by differentiation followed by apoptosis.Citation103 The HSP90 inhibitor PU3, a synthetic purine that like 17-AAG binds with high affinity to the ATP “pocket” of HSP90, caused breast cancer cells arrest in G1 phase and differentiation.Citation104
These contradictory reports concerning the inhibitors of HSP90 and cell differentiation could be explained if we consider that these drugs, depending on the experimental conditions, can have some side effects more or less independent of HSP90. Another possibility is that these studies do not differentiate between the amount of HSP90α and HSP90β inhibited. It is presently unknown whether HSP90 inhibitors equally block both isoforms, HSP90α and HSP90β. It not known neither whether post-translational modifications of HSP90 (acetylation, phosphorylation.) can affect their affinity for the inhibitors. HSP90α has been reported to be induced by lethal stimuli while the HSP90β can be induced by growth factors or cell differentiating signals.Citation105 Mouse embryos out-of-function studies clearly show the role of HSP90β in the differentiation process and, at least for HSP90β role in embryo cell differentiation, there is not an overlap with HSP90α functions. Therefore, we can hypothesized that it can be the degree of inhibition of HSP90β by the HSP90 inhibitors that would determine whether or not there is a blockade of the differentiation process. This degree of inhibition of the different HSP90 isoforms might be conditioned by their cellular localization and their post-translational modifications. It should be noted, however, that the relative relevance of HSP90β in the differentiation process might depend on the differentiation model studied.
To summarize, we can hypothesize that the role in the differentiation process of a chaperone will be determined by its transient expression, subcellular redistribution and/or post-translational modifications induced at a given stage by a differ- entiation factor. How can HSPs affect the differentiation process? First by their anti-apoptotic role interfering with caspase activity, we and other authors have shown that caspase activity was generally required for cell differentiation.Citation16,Citation17 Therefore, HSPs by interfering with caspase activity at a given moment, in a specific cellular compartment, may orchestrate the decision differentiation versus apoptosis. In this way, we have recently shown that HSP70 was a key protein to orchestrate this decision in erythroblasts.Citation61 Second, HSPs may affect the differentiation process by regulating the nuclear/cytosolic shuttling of proteins that take place during differentiation. For instance, c-IAP1 is translocated from the nucleus to the cytosol during differentiation of hematopoietic and epithelial cells, and we have demonstrated that HSP90 is needed for this c-IAP1 nuclear export.Citation106 It has also been shown that, during erythroblast differentiation, HSP70 is needed to inhibit AIF nuclear translocation.Citation18 Third, in the case of HSP90, the role in the differentiation process could be through certain of its client proteins, like RIP or Akt, whose stability is assured by the chaperone.
Repercussions and Concluding Remarks
The ability of HSPs to modulate the fate of the cells might have important repercussions in pathological situations such as cancer. Apoptosis, differentiation and oncogenesis are very related processes. Defaults in differentiation and/or apoptosis are involved in many cancer cells' aetiology. HSPs are abnormally constitutively high in most cancer cells and, in clinical tumors, they are associated with poor prognosis. In experimental models, HSP27 and HSP70 have been shown to increase cancer cells' tumorigenicty and their depletion can induce a spontaneous regression of the tumors.Citation24,Citation107 Several components of tumor cell-associated growth and survival pathways are HSP90 client proteins. These qualities have made HSPs targets for anticancer drug development. Today, although many research groups and pharmaceutical companies look for soluble specific inhibitors of HSP70 and HSP27, only specific soluble inhibitors of HSP90 are available for clinical trials. For some of them (17-AAG) phase II clinical trials are almost finished.Citation108 However, considering the new role of HSP90β in cell differentiation, it seems essential to re-evaluate the functional consequences of HSP90 blockade.
Figures and Tables
Figure 1 Modulation of apoptosis and differentiation by HSP90, HSP70 and HSP27. In apoptosis (upper part), HSP90 can inhibit caspase (casp.) activation by its interaction with Apaf1. HSP90 stabilizes proteins from the survival signaling including RIP, Akt and Bcl-2. HSP70 can block apoptosis by inhibiting JNK, Bax mitochondrial translocation, by interacting with AIF and/or Apaf-1 or by protecting essential nuclear proteins from caspase-3 cleavage. HSP27 can block caspase activation through its action on F-actin, Bid, ROS or cytochrome c. HSP27 also favors the proteasomal degradation of proteins like I-kBa. During differentiation (lower part), HSP90 stabilizes proteins involved in signaling induced by differentiating factors like Akt, RIP as well as Rb. HSP70 prevents the cleavage of GATA-1 by caspase-3 allowing erythoblast to differentiate instead of dying by apoptosis. HSP70 neutralization of AIF contributes also to the survival of the differentiating cells. HSP27 might be involved in the cell cycle arrest (→, stimulation; ⊥, inhibition; *, in erythroblasts).
Acknowledgements
We thank the Ligue contre le Cancer (Nièvre) for financial support. Celine Didelot has fellowship from la Fondation France contre la Leucemie, David Lanneau from the Ministère de l'Education, and Aurelie de Thonel from l'Association pour la Recherche contre le Cancer (ARC).
References
- Ritossa F. A new puffing pattern induced by heat shock and DNP in Drosophila. Experimentia 1962; 18:571 - 573
- Parcellier A, Gurbuxani S, Schmitt E, Solary E, Garrido C. Heat shock proteins, cellular chaperones that modulate mitochondrial cell death pathways. Biochem Biophys Res Commun 2003; 304:505 - 512
- Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer 2005; 5:761 - 772
- Voss AK, Thomas T, Gruss P. Mice lacking HSP90beta fail to develop a placental labyrinth. Development 2000; 127:1 - 11
- Beckmann RP, Mizzen LE, Welch WJ. Interaction of Hsp 70 with newly synthesized proteins: Implications for protein folding and assembly. Science 1990; 248:850 - 854
- Shi Y, Thomas JO. The transport of proteins into the nucleus requires the 70-kilodalton heat shock protein or its cytosolic cognate. Mol Cell Biol 1992; 12:2186 - 2192
- Ehrnsperger M, Graber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. Embo J 1997; 16:221 - 229
- Shashidharamurthy R, Koteiche HA, Dong J, McHaourab HS. Mechanism of chaperone function in small heat shock proteins: Dissociation of the HSP27 oligomer is required for recognition and binding of destabilized T4 lysozyme. J Biol Chem 2005; 280:5281 - 5289
- Garrido C. Size matters: Of the small HSP27 and its large oligomers. Cell Death Differ 2002; 9:483 - 4835
- Solary E, Droin N, Bettaieb A, Corcos L, Dimanche-Boitrel MT, Garrido C. Positive and negative regulation of apoptotic pathways by cytotoxic agents in hematological malignancies. Leukemia 2000; 14:1833 - 1849
- Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ 2006; 13:1423 - 1433
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997; 91:479 - 489
- Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, Ferri KF, Zamzami N, Wakeham A, Hakem R, Yoshida H, Kong YY, Mak TW, Zuniga-Pflucker JC, Kroemer G, Penninger JM. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 2001; 410:549 - 554
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999; 397:441 - 446
- Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 2000; 102:33 - 42
- Launay S, Hermine O, Fontenay M, Kroemer G, Solary E, Garrido C. Vital functions for lethal caspases. Oncogene 2005; 24:5137 - 5348
- Garrido C, Kroemer G. Life's smile, death's grin: Vital functions of apoptosis-executing proteins. Curr Opin Cell Biol 2004; 16:639 - 646
- Lui JC, Kong SK. Heat shock protein 70 inhibits the nuclear import of apoptosis-inducing factor to avoid DNA fragmentation in TF-1 cells during erythropoiesis. FEBS Lett 2007; 581:109 - 117
- Garrido C, Ottavi P, Fromentin A, Hammann A, Arrigo AP, Chauffert B, Mehlen P. HSP27 as a mediator of confluence-dependent resistance to cell death induced by anticancer drugs. Cancer Res 1997; 57:2661 - 2667
- Garrido C, Mehlen P, Fromentin A, Hammann A, Assem M, Arrigo AP, Chauffert B. Inconstant association between 27-kDa heat-shock protein (Hsp27) content and doxorubicin resistance in human colon cancer cells: The doxorubicin-protecting effect of Hsp27. Eur J Biochem 1996; 237:653 - 659
- Mehlen P, Kretz-Remy C, Preville X, Arrigo AP. Human hsp27, Drosophila hsp27 and human alphaB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFalpha-induced cell death. Embo J 1996; 15:2695 - 2706
- Nylandsted J, Wick W, Hirt UA, Brand K, Rohde M, Leist M, Weller M, Jaattela M. Eradication of glioblastoma, and breast and colon carcinoma xenografts by Hsp70 depletion. Cancer Res 2002; 62:7139 - 7142
- Chatterjee M, Jain S, Stuhmer T, Andrulis M, Ungethum U, Kuban RJ, Lorentz H, Bommert K, Topp M, Kramer D, Muller-Hermelink HK, Einsele H, Greiner A, Bargou RC. STAT3 and MAPK signaling maintain overexpression of heat shock proteins 90alpha and beta in multiple myeloma cells, which critically contribute to tumor-cell survival. Blood 2007; 109:720 - 728
- Gurbuxani S, Bruey JM, Fromentin A, Larmonier N, Parcellier A, Jaattela M, Martin F, Solary E, Garrido C. Selective depletion of inducible HSP70 enhances immunogenicity of rat colon cancer cells. Oncogene 2001; 20:7478 - 7485
- Sreedhar AS, Kalmar E, Csermely P, Shen YF. Hsp90 isoforms: Functions, expression and clinical importance. FEBS Lett 2004; 562:11 - 15
- Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem 2002; 277:39858 - 39866
- Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci USA 2000; 97:10832 - 10837
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997; 91:231 - 241
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science 1998; 282:1318 - 1321
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 1999; 401:82 - 85
- Lin LY, Lin CY, Ho FM, Liau CS. Up-regulation of the association between heat shock protein 90 and endothelial nitric oxide synthase prevents high glucose-induced apoptosis in human endothelial cells. J Cell Biochem 2005; 94:194 - 201
- Lewis J, Devin A, Miller A, Lin Y, Rodriguez Y, Neckers L, Liu ZG. Disruption of hsp90 function results in degradation of the death domain kinase, receptor-interacting protein (RIP), and blockage of tumor necrosis factor-induced nuclear factor-kappaB activation. J Biol Chem 2000; 275:10519 - 10526
- Chen G, Cao P, Goeddel DV. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol Cell 2002; 9:401 - 410
- Zhang Y, Wang JS, Chen LL, Cheng XK, Heng FY, Wu NH, Shen YF. Repression of hsp-90beta gene by p53 in UV irradiation-induced apoptosis of Jurkat cells. J Biol Chem 2004; 279:42545 - 42551
- Munster PN, Marchion DC, Basso AD, Rosen N. Degradation of HER2 by ansamycins induces growth arrest and apoptosis in cells with HER2 overexpression via a HER3, phosphatidylinositol 3′-kinase-AKT-dependent pathway. Cancer Res 2002; 62:3132 - 3137
- Hur E, Kim HH, Choi SM, Kim JH, Yim S, Kwon HJ, Choi Y, Kim DK, Lee MO, Park H. Reduction of hypoxia-induced transcription through the repression of hypoxia-inducible factor-1alpha/aryl hydrocarbon receptor nuclear translocator DNA binding by the 90-kDa heat-shock protein inhibitor radicicol. Mol Pharmacol 2002; 62:975 - 982
- Pandey P, Saleh A, Nakazawa A, Kumar S, Srinivasula SM, Kumar V, Weichselbaum R, Nalin C, Alnemri ES, Kufe D, Kharbanda S. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. Embo J 2000; 19:4310 - 4322
- Cohen-Saidon C, Carmi I, Keren A, Razin E. Antiapoptotic function of Bcl-2 in mast cells is dependent on its association with heat shock protein 90beta. Blood 2006; 107:1413 - 1420
- Schmitt E, Parcellier A, Gurbuxani S, Cande C, Hammann A, Morales MC, Hunt CR, Dix DJ, Kroemer RT, Giordanetto F, Jaattela M, Penninger JM, Pance A, Kroemer G, Garrido C. Chemosensitization by a non-apoptogenic heat shock protein 70-binding apoptosis-inducing factor mutant. Cancer Res 2003; 63:8233 - 8240
- Dix DJ, Allen JW, Collins BW, Mori C, Nakamura N, Poorman-Allen P, Goulding EH, Eddy EM. Targeted gene disruption of Hsp70–2 results in failed meiosis, germ cell apoptosis, and male infertility. Proc Natl Acad Sci USA 1996; 93:3264 - 3268
- Nylandsted J, Rohde M, Brand K, Bastholm L, Elling F, Jaattela M. Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2. Proc Natl Acad Sci USA 2000; 97:7871 - 7876
- Park HS, Lee JS, Huh SH, Seo JS, Choi EJ. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. Embo J 2001; 20:446 - 456
- Gabai VL, Yaglom JA, Volloch V, Meriin AB, Force T, Koutroumanis M, Massie B, Mosser DD, Sherman MY. Hsp72-mediated suppression of c-Jun N-terminal kinase is implicated in development of tolerance to caspase-independent cell death. Mol Cell Biol 2000; 20:6826 - 6836
- Lee JS, Lee JJ, Seo JS. HSP70 deficiency results in activation of c-Jun N-terminal Kinase, extracellular signal-regulated kinase, and caspase-3 in hyperosmolarity-induced apoptosis. J Biol Chem 2005; 280:6634 - 6641
- Gao T, Newton AC. The turn motif is a phosphorylation switch that regulates the binding of Hsp70 to protein kinase C. J Biol Chem 2002; 277:31585 - 31592
- Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem 2005; 280:38729 - 38739
- Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol 2000; 2:469 - 475
- Steel R, Doherty JP, Buzzard K, Clemons N, Hawkins CJ, Anderson RL. Hsp72 inhibits apoptosis upstream of the mitochondria and not through interactions with Apaf-1. J Biol Chem 2004; 279:51490 - 51499
- Creagh EM, Carmody RJ, Cotter TG. Heat shock protein 70 inhibits caspase-dependent and -independent apoptosis in Jurkat T cells. Exp Cell Res 2000; 257:58 - 66
- Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jaattela M, Penninger JM, Garrido C, Kroemer G. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol 2001; 3:839 - 843
- Ruchalski K, Mao H, Li Z, Wang Z, Gillers S, Wang Y, Mosser DD, Gabai V, Schwartz JH, Borkan SC. Distinct hsp70 domains mediate apoptosis-inducing factor release and nuclear accumulation. J Biol Chem 2006; 281:7873 - 7880
- Gurbuxani S, Schmitt E, Cande C, Parcellier A, Hammann A, Daugas E, Kouranti I, Spahr C, Pance A, Kroemer G, Garrido C. Heat shock protein 70 binding inhibits the nuclear import of apoptosis-inducing factor. Oncogene 2003; 22:6669 - 6678
- Matsumori Y, Hong SM, Aoyama K, Fan Y, Kayama T, Sheldon RA, Vexler ZS, Ferriero DM, Weinstein PR, Liu J. Hsp70 overexpression sequesters AIF and reduces neonatal hypoxic/ischemic brain injury. J Cereb Blood Flow Metab 2005; 25:899 - 910
- Kalinowska M, Garncarz W, Pietrowska M, Garrard WT, Widlak P. Regulation of the human apoptotic DNase/RNase Endonuclease G: Involvement of Hsp70 and ATP. Apoptosis 2005; 10:821 - 830
- Jaattela M, Wissing D, Kokholm K, Kallunki T, Egeblad M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. Embo J 1998; 17:6124 - 6134
- Sakahira H, Nagata S. Co-translational folding of caspase-activated DNase with Hsp70, Hsp40, and inhibitor of caspase-activated DNase. J Biol Chem 2002; 277:3364 - 3370
- Guo F, Sigua C, Bali P, George P, Fiskus W, Scuto A, Annavarapu S, Mouttaki A, Sondarva G, Wei S, Wu J, Djeu J, Bhalla K. Mechanistic role of heat shock protein 70 in Bcr-Abl-mediated resistance to apoptosis in human acute leukemia cells. Blood 2005; 105:1246 - 1255
- Gyrd-Hansen M, Nylandsted J, Jaattela M. Heat shock protein 70 promotes cancer cell viability by safeguarding lysosomal integrity. Cell Cycle 2004; 3
- Bivik C, Rosdahl I, Ollinger K. Hsp70 protects against UVB induced apoptosis by preventing release of cathepsins and cytochrome c in human melanocytes. Carcinogenesis 2006;
- Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, Massie B. The chaperone function of hsp70 is required for protection against stress induced apoptosis.. Mol Cell Biol 2000; 20:7146 - 7159
- Ribeil JA, Zermati Y, Vandekerckhove J, Cathelin S, Kersual J, Dussiot M, Coulon S, Moura IC, Zeuner A, Kirkegaard-Sorensen T, Varet B, Solary E, Garrido C, Hermine O. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature 2007; 445:102 - 105
- Rocchi P, Jugpal P, So A, Sinneman S, Ettinger S, Fazli L, Nelson C, Gleave M. Small interference RNA targeting heat-shock protein 27 inhibits the growth of prostatic cell lines and induces apoptosis via caspase-3 activation in vitro. BJU Int 2006; 98:1082 - 1089
- Garrido C, Bruey JM, Fromentin A, Hammann A, Arrigo AP, Solary E. HSP27 inhibits cytochrome c-dependent activation of procaspase-9. Faseb J 1999; 13:2061 - 2070
- Concannon CG, Orrenius S, Samali A. Hsp27 inhibits cytochrome c-mediated caspase activation by sequestering both pro-caspase-3 and cytochrome c. Gene Expr 2001; 9:195 - 201
- Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol 2000; 2:645 - 652
- Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP. Hsp27 as a negative regulator of cytochrome C release. Mol Cell Biol 2002; 22:816 - 834
- Chauhan D, Li G, Hideshima T, Podar K, Mitsiades C, Mitsiades N, Catley L, Tai YT, Hayashi T, Shringarpure R, Burger R, Munshi N, Ohtake Y, Saxena S, Anderson KC. Hsp27 inhibits release of mitochondrial protein Smac in multiple myeloma cells and confers dexamethasone resistance. Blood 2003; 102:3379 - 3386
- Guay J, Lambert H, Gingras-Breton G, Lavoie JN, Huot J, Landry J. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci 1997; 110(Pt 3):357 - 368
- Arrigo AP, Virot S, Chaufour S, Firdaus W, Kretz-Remy C, Diaz-Latoud C. Hsp27 consolidates intracellular redox homeostasis by upholding glutathione in its reduced form and by decreasing iron intracellular levels. Antioxid Redox Signal 2005; 7:414 - 422
- Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C, Paul C, Wieske M, Arrigo AP, Buchner J, Gaestel M. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem 1999; 274:18947 - 18956
- Wyttenbach A, Sauvageot O, Carmichael J, Diaz-Latoud C, Arrigo AP, Rubinsztein DC. Heat shock protein 27 prevents cellular polyglutamine toxicity and suppresses the increase of reactive oxygen species caused by huntingtin. Hum Mol Genet 2002; 11:1137 - 1151
- Rane MJ, Pan Y, Singh S, Powell DW, Wu R, Cummins T, Chen Q, McLeish KR, Klein JB. Heat shock protein 27 controls apoptosis by regulating Akt activation. J Biol Chem 2003; 278:27828 - 27835
- Charette SJ, Lavoie JN, Lambert H, Landry J. Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol 2000; 20:7602 - 7612
- Rocchi P, Beraldi E, Ettinger S, Fazli L, Vessella RL, Nelson C, Gleave M. Increased Hsp27 after androgen ablation facilitates androgen-independent progression in prostate cancer via signal transducers and activators of transcription 3-mediated suppression of apoptosis. Cancer Res 2005; 65:11083 - 11093
- Parcellier A, Schmitt E, Gurbuxani S, Seigneurin-Berny D, Pance A, Chantome A, Plenchette S, Khochbin S, Solary E, Garrido C. HSP27 is a ubiquitin-binding protein involved in I-kappaBalpha proteasomal degradation. Mol Cell Biol 2003; 23:5790 - 5802
- Parcellier A, Brunet M, Schmitt E, Col E, Didelot C, Hammann A, Nakayama K, Nakayama KI, Khochbin S, Solary E, Garrido C. HSP27 favors ubiquitination and proteasomal degradation of p27Kip1 and helps S-phase re-entry in stressed cells. Faseb J 2006; 20:1179 - 1181
- Bruey JM, Paul C, Fromentin A, Hilpert S, Arrigo AP, Solary E, Garrido C. Differential regulation of HSP27 oligomerization in tumor cells grown in vitro and in vivo. Oncogene 2000; 19:4855 - 4863
- Oya-Ito T, Liu BF, Nagaraj RH. Effect of methylglyoxal modification and phosphorylation on the chaperone and anti-apoptotic properties of heat shock protein 27. J Cell Biochem 2006; 99:279 - 291
- Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J Biol Chem 1994; 269:20780 - 20784
- Akbar MT, Lundberg AM, Liu K, Vidyadaran S, Wells KE, Dolatshad H, Wynn S, Wells DJ, Latchman DS, de Belleroche J. The neuroprotective effects of heat shock protein 27 overexpression in transgenic animals against kainate-induced seizures and hippocampal cell death. J Biol Chem 2003; 278:19956 - 19965
- Loones MT, Morange M. Hsp and chaperone distribution during endochondral bone development in mouse embryo. Cell Stress Chaperones 1998; 3:237 - 244
- Sarge KD, Cullen KE. Regulation of hsp expression during rodent spermatogenesis. Cell Mol Life Sci 1997; 53:191 - 197
- Trinklein ND, Chen WC, Kingston RE, Myers RM. Transcriptional regulation and binding of heat shock factor 1 and heat shock factor 2 to 32 human heat shock genes during thermal stress and differentiation. Cell Stress Chaperones 2004; 9:21 - 28
- Mehlen P, Mehlen A, Godet J, Arrigo AP. Hsp27 as a switch between differentiation and apoptosis in murine embryonic stem cells. J Biol Chem 1997; 272:31657 - 31665
- Shakoori AR, Oberdorf AM, Owen TA, Weber LA, Hickey E, Stein JL, Lian JB, Stein GS. Expression of heat shock genes during differentiation of mammalian osteoblasts and promyelocytic leukemia cells. J Cell Biochem 1992; 48:277 - 287
- Spector NL, Ryan C, Samson W, Levine H, Nadler LM, Arrigo AP. Heat shock protein is a unique marker of growth arrest during macrophage differentiation of HL-60 cells. J Cell Physiol 1993; 156:619 - 625
- Spector NL, Mehlen P, Ryan C, Hardy L, Samson W, Levine H, Nadler LM, Fabre N, Arrigo AP. Regulation of the 28 kDa heat shock protein by retinoic acid during differentiation of human leukemic HL-60 cells. FEBS Lett 1994; 337:184 - 188
- Chaufour S, Mehlen P, Arrigo AP. Transient accumulation, phosphorylation and changes in the oligomerization of Hsp27 during retinoic acid-induced differentiation of HL-60 cells: Possible role in the control of cellular growth and differentiation. Cell Stress Chaperones 1996; 1:225 - 235
- Ishiwatari S, Suzuki T, Hitomi T, Yoshino T, Matsukuma S, Tsuji T. Effects of methyl paraben on skin keratinocytes. J Appl Toxicol 2007; 27:1 - 9
- Ito H, Kamei K, Iwamoto I, Inaguma Y, Kato K. Regulation of the levels of small heat-shock proteins during differentiation of C2C12 cells. Exp Cell Res 2001; 266:213 - 221
- Zermati Y, Garrido C, Amsellem S, Fishelson S, Bouscary D, Valensi F, Varet B, Solary E, Hermine O. Caspase activation is required for terminal erythroid differentiation. J Exp Med 2001; 193:247 - 254
- Grebenova D, Kuzelova K, Pluskalova M, Peslova G, Halada P, Hrkal Z. The proteomic study of sodium butyrate antiproliferative/cytodifferentiation effects on K562 cells. Blood Cells Mol Dis 2006; 37:210 - 217
- Yamada T, Hashiguchi A, Fukushima S, Kakita Y, Umezawa A, Maruyama T, Hata J. Function of 90-kDa heat shock protein in cellular differentiation of human embryonal carcinoma cells. In Vitro Cell Dev Biol Anim 2000; 36:139 - 146
- Yun BG, Matts RL. Hsp90 functions to balance the phosphorylation state of Akt during C2C12 myoblast differentiation. Cell Signal 2005; 17:1477 - 1485
- Chen CF, Chen Y, Dai K, Chen PL, Riley DJ, Lee WH. A new member of the hsp90 family of molecular chaperones interacts with the retinoblastoma protein during mitosis and after heat shock. Mol Cell Biol 1996; 16:4691 - 4699
- Yue L, Karr TL, Nathan DF, Swift H, Srinivasan S, Lindquist S. Genetic analysis of viable Hsp90 alleles reveals a critical role in Drosophila spermatogenesis. Genetics 1999; 151:1065 - 1079
- Inoue T, Hirata K, Kuwana Y, Fujita M, Miwa J, Roy R, Yamaguchi Y. Cell cycle control by daf-21/Hsp90 at the first meiotic prophase/metaphase boundary during oogenesis in Caenorhabditis elegans. Dev Growth Differ 2006; 48:25 - 32
- Krone PH, Evans TG, Blechinger SR. Heat shock gene expression and function during zebrafish embryogenesis. Semin Cell Dev Biol 2003; 14:267 - 274
- Yun BG, Matts RL. Differential effects of Hsp90 inhibition on protein kinases regulating signal transduction pathways required for myoblast differentiation. Exp Cell Res 2005; 307:212 - 223
- Nimmanapalli R, O'Bryan E, Bhalla K. Geldanamycin and its analogue 17-allylamino-17-demethoxygeldanamycin lowers Bcr-Abl levels and induces apoptosis and differentiation of Bcr-Abl-positive human leukemic blasts. Cancer Res 2001; 61:1799 - 1804
- Yu W, Rao Q, Wang M, Tian Z, Lin D, Liu X, Wang J. The Hsp90 inhibitor 17-allylamide-17-demethoxygeldanamycin induces apoptosis and differentiation of Kasumi-1 harboring the Asn822Lys KIT mutation and downregulates KIT protein level. Leuk Res 2006; 30:575 - 582
- Lopez-Maderuelo MD, Fernandez-Renart M, Moratilla C, Renart J. Opposite effects of the Hsp90 inhibitor Geldanamycin: Induction of apoptosis in PC12, and differentiation in N2A cells. FEBS Lett 2001; 490:23 - 27
- Munster PN, Srethapakdi M, Moasser MM, Rosen N. Inhibition of heat shock protein 90 function by ansamycins causes the morphological and functional differentiation of breast cancer cells. Cancer Res 2001; 61:2945 - 2952
- Chiosis G, Timaul MN, Lucas B, Munster PN, Zheng FF, Sepp-Lorenzino L, Rosen N. A small molecule designed to bind to the adenine nucleotide pocket of Hsp90 causes Her2 degradation and the growth arrest and differentiation of breast cancer cells. Chem Biol 2001; 8:289 - 299
- Cullinan SB, Whitesell L. Heat shock protein 90: A unique chemotherapeutic target. Semin Oncol 2006; 33:457 - 465
- Plenchette S, Cathelin S, Rebe C, Launay S, Ladoire S, Sordet O, Ponnelle T, Debili N, Phan TH, Padua RA, Dubrez-Daloz L, Solary E. Translocation of the inhibitor of apoptosis protein c-IAP1 from the nucleus to the Golgi in hematopoietic cells undergoing differentiation: A nuclear export signal-mediated event. Blood 2004; 104:2035 - 2043
- Garrido C, Fromentin A, Bonnotte B, Favre N, Moutet M, Arrigo AP, Mehlen P, Solary E. Heat shock protein 27 enhances the tumorigenicity of immunogenic rat colon carcinoma cell clones. Cancer Res 1998; 58:5495 - 5499
- Dai C, Whitesell L. HSP90: A rising star on the horizon of anticancer targets. Future Oncol 2005; 1:529 - 540