Abstract
A simple diagnostic test is described for the detection of TSE in bovine, ovine and human brain and lymphoid tissue that obviates the use of proteinase K as a discriminating reagent. The immunoassay utilises high affinity anti-peptide antibodies that appear blind to the normal isoform of prion protein (PrPC). These reagents have been produced with novel N-terminal chimeric peptides and we hypothesise that the retention and stability of the extreme N-terminus of PrP in the disease-associated aggregate makes it an operationally specific marker for TSE. Accordingly, the assay involves homogenisation of the tissue directly in 8M guanidine hydrochloride, a simple one-step capture of PrPSc followed by detection with a europium-labelled anti-PrPC antibody. This rapid assay clearly differentiates between levels of disease-associated PrP extracted from brain and lymphoid tissues taken from confirmed TSE positive and negative cattle and sheep. The assay can also be used to detect PrPSc in cases of vCJD.
Introduction
The conversion of a normal membrane glycoprotein, the cellular prion protein (PrPC) to an insoluble aggregated isoform (PrPSc) is thought to be the key process in the pathogenesis of the transmissible spongiform encephalopathies (TSEs).Citation1 Consequently, the specific detection of PrPSc has formed the basis for the biochemical diagnosis of TSEs which include bovine spongiform encephalopathy (BSE), scrapie in sheep, hamsters and mice, Creutzfeld-Jacob Disease (CJD) in humans and chronic wasting disease (CWD) in white-tailed deer.Citation2,Citation3
The specificity of disease diagnosis in TSE diseases is usually achieved by the differential proteolysis of PrPC using enzymes such as proteinase K (PK) or trypsin, prior to the detection of a protease-resistant core of PrP (designated PrPres) by Western blotting or other techniques.Citation4–Citation9
The detection of PrPSc independent of the requirement to use PK requires the development of antibodies or ligands that recognise the abnormal form of PrP and which fail to react with PrPC.
In this paper, we describe the development and use of a sensitive immunoassay for the measurement of disease-associated ovine, bovine and human PrP without the requirement to use PK as a discriminating reagent. This has been made possible by the development of antibodies (both monoclonal and mini-antibodies produced by phage display) recognising linear epitopes at the very N-terminus of PrP, which are only exposed and detected after the solubilization of disease-associated aggregated PrP using 8M-GdHCl. These N-terminal epitopes are not detected to any appreciable extent in endogenous PrPC. Extraction with 8M-GdHCl facilitates the direct quantitative measurement of PrPSc in a rapid two-site DELFIA® using the novel antibodies as capture reagents together with a commercially available high affinity Mab to PrPC (SAF32) as the europium-labelled detecting reagent.
The potential utility of this approach as a pre-clinical diagnostic for TSE is discussed. In addition, we consider the significance of the reactivity of these unique reagents and speculate what the implications might be for a better understanding of the disease process.
Materials and Methods
Peptide synthesis.
The following peptides derived from the bovine and human PrP (Swiss Proteins P10279 and P04156, respectively) were synthesized by solid phase peptide synthesis on an automated multiple peptide synthesizer using Fmoc/tBU chemistry (Quartett Gmbh, Berlin, Germany):
A. Bovine
KKRPKPGGGWNTQPHGGGWG (PrP chimeric peptide 25–36/62–69); KKRPKPGGGWNT (PrP 25–36); QPHGGGWG (PrP 62–69)
B. Human
KKRPKPGGWNTQPHGGGWG (PrP chimeric peptide 23–33/59–66); KKRPKPGGWNT (PrP 23–33); QPHGGGWG (PrP 59–66)
The peptides were coupled using EDC chemistry to carrier proteins concholepas concholepas hemocyanin (CCH) and human transferrin (Trf ) (Biogenesis Ltd, Poole, England).
Production of Mab YWH1.
The structure of the bovine chimeric peptide immunogen is shown in . The reason for this approach was simply convenience. It was our intention to raise a panel of monoclonal antibodies (Mabs) recognising epitopes across the protein. Utilising two sequences in the chimera allowed for the isolation of Mabs recognising at least two different epitopes following a single immunisation. Hybridomas to this and other similar i mmunogens were prepared in BALB/c mice according to procedures previously described.Citation10 Many Mab-secreting hybridomas were indeed produced using this approach. Only one Mab (designated YWH1), however, possessed the necessary affinity and specificity that allowed for the development of the sensitive immunoassay described in this report.
Production of phage display antibody YWH2.
The structure of the human chimeric peptide antigen is shown in . Recombinant human antibodies were generated from the HuCAL GOLD collection of human antibody genesCitation11 using proprietary methods (AbD Serotec, a Division of MorphoSys, Munich, Germany). The YWH Bivalent mini-antibodies contain a self-dimerizing helix-turn-helix motif,Citation12 a myc-tag (EQKLISEEDL) and a His6 tag (used for antibody purification and detection). The antibodies obtained as crude extracts were selected in ELISA with immobilized Trf conjugates for the presence of antibody fragments binding to the conjugates used in the panning, as well as to the other conjugated peptides. Only colonies displaying strong (at least 5-fold over background) binding in the primary screening ELISA with KKRPKPGGWNTQPHGGGWG-Trf and KKRPKPGGWNT-Trf but not with QPHGGGWG-Trf were selected for sequencing of the antibody VH CDR regions and for future production and purification. E. coli TG1F-cultures (250 ml) containing the YWH2 antibody genes were grown, harvested and chemically lysed. The soluble crude extract was subjected to one-step NiNTA chromatography and the purified eluted antibodies were tested by Coomassie blue-stained SDS-PAGE and ELISA.
Assay reagents.
The basic reagents for tissue homogenization and DELFIA® were obtained from Sigma-Aldrich Co Ltd., Fancy Road, Poole, Dorset, UK. These have been described in detail previously.Citation13 DELFIA® enhancement solution (Cat. No. 1244-105) and labelling reagent (Cat. No. 1244-302) were obtained from PerkinElmer UK Ltd, Chalfont Road, Seer Green, Beaconsfield, UK.
DELFIA® equipment.
Equipment necessary to perform DELFIA® may be obtained from PerkinElmer UK Ltd. This has been described in detail previously.Citation13 NUNC Maxisorp® microtitre plates (Cat. No. DIS-971-070U) and plate sealer (Cat. No. DIS-984-505J) may be obtained from Fisher Scientific UK Ltd., Bishop Meadow Road, Loughborough, UK.
Immunoassay standard.
Recombinant ovine PrP (ARR genotype) was obtained from the TSE Resource Centre, Institute for Animal Health, Compton, Newbury, Berkshire, UK. A stock solution was prepared in 8M-GdHCl at a concentration of 20 µg/ml and stored at 4°C. Immediately prior to assay, the stock solution was diluted in assay buffer to give six working standards, namely, 0, 0.32, 1.6, 8, 40 and 200 ng/ml recombinant ovine PrP.
Detecting antibody.
Purified BSA-free Mab SAF32 recognising the whole octapeptide repeat region of PrPC spanning residues 51 to 98Citation14 was obtained from IDS Ltd, Boldon Business Park, Boldon, Tyne & Wear, UK. The antibody was labelled with europium according to a method described previously.Citation13
Preparation of the YWH-coated plates.
Purified YWH antibodies were diluted in 0.1M PBS coating buffer (pH 7.4) to a concentration of 5 µg/ml. Two hundred (200) µl of coating solution (1 µg IgG per well) was added to each well of NUNC Maxisorp® microtitre plates using a 12-channel digital pipette. The plates were sealed and stored overnight at 4°C. Subsequently, the plates were washed once with wash solution and tapped dry on absorbent paper. Two hundred (200) µl of blocking buffer (0.1M PBS containing 2% BSA) was added to each well, the plates resealed and stored at 4°C until use.
Tissues.
Samples of ovine medulla (caudal and rostral to the obex), palatine tonsil and retropharyngeal lymph node were obtained from the Veterinary Laboratories Agency (VLA), New Haw, Addlestone, Surrey KT15 3NB, UK. These tissues included validated material from animals that had been diagnosed with classical scrapie by the VLA using a panel of diagnostic tests including histology, immunohistochemistry, Western blot analysis and other accredited immunoassays. Equivalent genotype-matched tissues were also received from a New Zealand (NZ) derived sheep flock that had never been exposed to the disease. In addition, homogenates of bovine caudal medulla from confirmed BSE positives and negatives were generously made available by the VLA, Whitley Road, Longbenton, Newcastle upon Tyne NE12 9SE, UK. A limited number of human brain homogenates are also available from the NIBSC CJD Resource Centre, Blanche Lane, South Mimms, Potters Bar EN6 3QG, UK.Citation15
Homogenisation of tissue.
Tissue was cut, accurately weighed and transferred to an ETDS VIII homogeniser tube (The Design Village, Lowlands Estate, Braye Road, Vale, Guernsey, Channel Islands). An equivalent volume of 8M GdHCl was added. The tube was assembled and the tissue homogenised for 20 seconds (brain) or 40 seconds (lymphoid tissue) using the ETDS VIII homogeniser. Subsequently, the homogenates were diluted (1 in 20 v/v) by the addition of assay buffer (through the central tube of the disposable device). The diluted homogenates were mixed using the EDTS VIII homogeniser for a further 10 seconds and used without additional treatment.
In the case of the BSE and CJD homogenates that had already been prepared, a modified protocol was adopted. Briefly, 50 µL of homogenate was carefully pipetted into a 2 mL Eppendorf Safe-Lock® tube and 50 µL 8MGdHCl added. The tubes were stoppered and vortexed. Subsequently, 900 µL of assay buffer were added, the tubes stoppered, vortexed and then spun in a microfuge at 13,000 rpm for 5 minutes. The supernatants were used without further treatment.
Immunoassay.
YWH-coated microtiter plates were washed twice with wash solution and tapped dry on absorbent paper. Two hundred (200) µl of each standard or sample were added in duplicate to appropriate wells of the coated plate. The plates were sealed and incubated on the shaker for 2 hours at 18°C. Subsequently, the plates were washed three times with wash solution, tapped dry on absorbent paper, and 200 ml of europium-labelled anti-PrP detecting antibody (SAF32; diluted at 1:2000 v/v in assay buffer) added to each well. The plates were incubated on the shaker at 18°C for a further 60 minutes. The plates were then washed six times, tapped dry, turned around 180°, and rewashed a further six times. The plates were again tapped dry on absorbent paper. Two hundred (200) µl of enhancement solution were added, the plates shaken for 5 minutes at 18°C and the fluorescence measured in the time-resolved fluorometer. The concentrations of PrP were determined using the proprietary PerkinElmer data reduction package Multicalc®.
Western blot analysis.
Three samples of ovine caudal medulla (two confirmed scrapie positives and one negative) were homogenised in BioRad TeSeE buffer using BioRad Precess 24 (Bio-Rad Laboratories Ltd. Bio-Rad House, Maylands Avenue, Hemel Hempstead, Herts HP2 7TD, U.K.) to yield 10% brain tissue homogenates. For the samples to be digested, 8 µL PK (200 µg/ml) were added to 24 µl 10% brain homogenate to give a final concentration of 50 µg/mL. The mixtures were incubated at 37°C for 45 minutes. Subsequently, the digestion was terminated by the addition of 8 µl 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF; Sigma-Aldrich A8456; 1 mM final) followed by the addition of 40 µl sample loading buffer (2x) and boiling for 10 minutes. The samples were cooled on ice and spun at 13,000 rpm for 1 minute. The SDS extracts of digested and undigested samples were run on a 12% Tris-glycine gel with pre-stained molecular markers (Broad Range 6–175 kDa; New England Biolabs Cat. No. P7708L). The proteins were transferred to PVDF membrane (Biorad Immun blot PVDF 0.2 µm; Cat. No. 162-0177), blocked with 5% Marvel in TBS. The membranes were probed with Mab P4 (R-Biopharm Rhone Ltd, West of Scotland Science Park, Unit 3.06 Kelvin Campus Maryhill Road, Glasgow G20 0SP, U.K.; 2 µg/ml) followed by HRP-linked goat anti-mouse IgG (γ chain specific; 1:5000 v/v; Sigma, Fancy Road, Poole, Dorset UK; Cat. No. A3673).
Results
Specificity of YWH1 and YWH2.
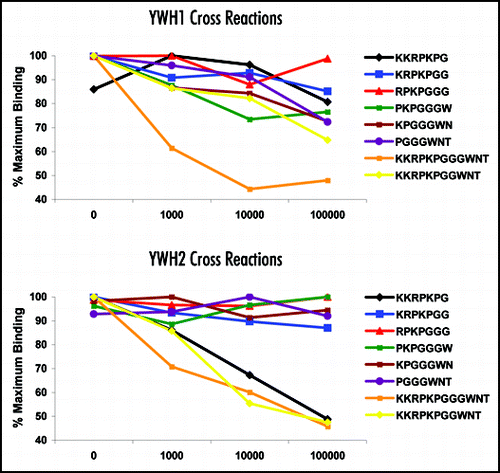
A series of peptides were prepared to ascertain the epitope specificity of the YWH series of antibodies by competitive inhibition using DELFIA. Each of these peptides was diluted in assay buffer containing 2 ng/mL recombinant ovine PrP to give a range of peptide concentrations (i.e., 0, 100, 1000 and 10,000 ng/mL). Two hundred (200) µL of each dilution was added in duplicate to appropriate wells of the coated plate and the inhibition of YWH reactivity for recombinant PrP immunoassay was completed as described previously. The results () show that for YWH1 the full-length peptide with three glycines is the best inhibitor. With the YWH2 antibody, peptides based on the extreme N terminus sequence are good inhibitors. Neither YWH1 nor YWH2 cross-reacted with the octapeptide repeat peptide (QPHGGGWG).
Immunoassay for BSE in brain tissue.
The concentrations of disease-associated PrP were determined in 400 samples of bovine brain tissue homogenate previously prepared at the VLA, Newcastle. These homogenates comprised 200 confirmed BSE-positives and 200 confirmed BSE-negatives. The immunoassay was performed using Mab YWH1 with SAF32 detection and the results are shown in . These results indicate that BSE positive brain samples have an extremely wide variation in PrP concentrations (0.928–80.35 ng/ml) compared to negative controls.
Immunoassay for scrapie in brain tissue.
The concentrations of disease-associated PrP were determined in equivalent samples of ovine brain tissue taken caudal or rostral to the obex using YWH1 with SAF32 detection and the results are shown in (Caudal Medulla) and (Rostral Medulla). Again these results show that in scrapie brain material, individual samples can vary widely in terms of PrP concentration when compared to negative controls.
Immunoassay for scrapie in lymphoid tissue.
The concentrations of disease-associated PrP were determined in samples of ovine palatine tonsil and retropharyngeal lymph node using YWH1 with SAF32 detection and the results are shown in and , respectively. It is important to note that the concentration of disease-associated PrP in lymphoid tissue may be 10- to 20-fold lower than in equivalent brain tissue.
Statistical analysis.
An analysis of our data has shown that the concentration of PrP in TSE positive tissue is not normally distributed. There are also differences between the variances across the two groups (positive and negative). To test for differences in the concentration of PrP between the groups, we used the Mann-Whitney (or two-sample Wilcoxon) test. This non-parametric test is useful in situations where the assumption of normality of the data is questionable. A potential problem is that this test statistic can give erroneous significant results when the variances of the comparison groups differ greatly. A possible solution to this was to rank the data in ascending order, and then perform a two-sample t-test on the ranks. In both cases (Mann-Whitney and t-test), analysis showed that the difference between the concentrations of PrP in positive and negative brain and lymphoid tissue is highly significant (p < 0.01).
Comparison between DELFIA® and Western blot analysis.
To establish whether the YWH1 assay can discriminate between the disease-associated and normal forms of PrP, we compared the YWH1 DELFIA® assay with a standard Western blot assay (using MabP4 detection) on the same tissues. Results from the two independent yet complimentary assays are shown in . The results indicate that in the absence of PK digestion, MabP4 detects normal PrPC in the Western Blot analysis of brain homogenate from a confirmed scrapie-negative sheep. Nevertheless, there is little measurable PrPC in this homogenate when using the YWH1 DELFIA® compared to scrapie positive animals. The two confirmed scrapie positive tissue chosen contain differing amounts of disease-associated PrP. This finding is reflected in the correlation between the quantitative data generated by the YWH1 DELFIA® and the differing intensities of the Western Blot analysis following PK digestion.
Measurement of disease-associated PrP in normal and vCJD human tissue.
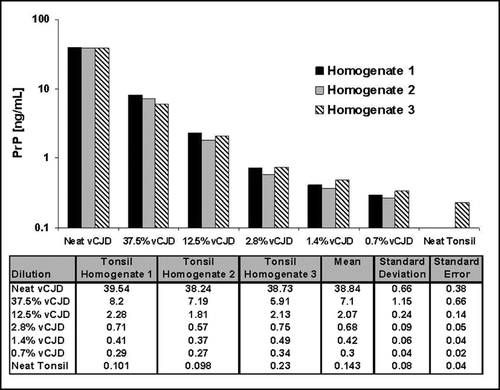
The concentrations of disease-associated PrP were determined using YWH2 with SAF32 detection in duplicate samples of homogenate prepared from three different normal human tonsil homogenates spiked with vCJD brain homogenate to produce mixtures containing 100%, 37.5%, 12.5%, 2.8%, 1.4%, 0.07% and 0% vCJD brain tissue. The results are shown in . The data indicates that the methodology is sufficiently sensitive to detect PrPSc in human vCJD tonsil should the tissues ever become available.
Discussion
This paper describes the characterization and use of antibodies to the extreme N terminal region of PrPC that can be used in an immunoassay to quantify levels of disease-associated PrP in brain and lymphoid tissue derived from ruminants with TSEs and humans with vCJD.
One of the significant characteristics of the antibodies is their relative high affinity for a linear epitope on PrPC. By competitive inhibition assays using synthetic peptides (); we have shown that the YWH1 Mab binds to an epitope at the N-terminus (i.e., PGGGWNT) of bovine and ovine PrP. Accordingly, YWH1 has a reduced cross-reactivity with human PrP comprising a slightly different sequence (i.e., PGGWNT) in this region. On the other hand, competitive inhibition assays show that the epitope for YWH2 is in the more basic region of the extreme N-terminus (i.e., KKRPKPG) which is highly conserved across species.
Although these antibodies were raised to synthetic peptides corresponding to the extreme N terminal regions of PrPC (see ), they appear to have operational specificity for disease-associated PrP. Unlike other Mabs to PrPC which cannot be used in a PK independent assay, the YWH antibodies react poorly with normal tissue PrPC and detect highly significant levels of disease-associated PrP in both BSE and scrapie brain tissue. In addition, the reagents can be used to detect disease-associated PrP in lymphoid tissue (tonsil and retropharyngeal lymph node) from scrapie infected animals. We have also shown, in a small number of experiments, that YWH2 is able to detect disease-associated PrP in vCJD brain and human tonsil spiked with vCJD brain. There was little reactivity with PrP in normal brain or tonsil tissue. Consequently, an assay for vCJD in tonsil tissue may be useful for the pre-clinical detection of this disease in the human population. Currently, studies are being undertaken on human tonsil tissue and any candidate assays must to be able to detect disease-associated PrP in this material.
Although these antibodies recognize linear epitopes at the very N-terminus of PrP, they are only exposed and detected after the solubilization of disease-associated aggregated PrP using 8M-GdHCl. The N-terminal epitopes are not detected to any appreciable extent in endogenous PrPC even in the presence of high molarity chaotrope. Somewhat paradoxically, the antibodies do react with recombinant PrPC used as the calibrator in the DELFIA®. This material, however, was produced in a bacterial expression system and is neither glycosylated nor subject to normal metabolism nor post-translational modification as PrP in mammalian cells. Using flow cytometry, we have been unable to show any reactivity of the YWH antibodies with the PrPC on the cell surface (paper in preparation).
To explain the fact that the antibodies can clearly discriminate between normal and disease-associated PrP derived from mammalian tissues, we hypothesize:
(1) The very N-terminal moiety of PrPC may be cleaved from the protein as a result of normal and rapid metabolic turnover or through the action of endogenous proteases that may be released during the homogenisation of the tissue.
(2) When PrPC undergoes conversion to PrPSc, normal metabolic turnover may be inhibited and the N-terminal regions of i ndividual PrP molecules are retained within intra-cellular aggregates of compartmentalized PrPSc in the cytosol.Citation16
(3) The N-terminal residues of PrPC may undergo post-translational modification whereas, in disease, this process is altered or inhibited in some way.
Thus in the pathogenesis of TSE, abnormal processing with the concomitant formation of PrPSc might lead to the retention of the intact N-terminus within the protein aggregate. Inevitably, this epitope would be exposed on the addition of 8M-GdHCl and, as a result, the antibodies would bind. The non-diagnostic use of YWH series antibodies may facilitate a better understanding of some of these processes.
The quantitation of disease-associated PrP with these antibodies is based on a simple extraction procedure that does not require the prior removal of PrPC. This extraction facilitates the direct quantitative measurement of PrPSc in a rapid two-site DELFIA® using the novel YWH antibodies as capture reagents together with a commercially available high affinity Mab to PrPC (SAF32) as the europium-labelled detecting reagent.
It is clear from the data that there is widespread variation in the levels of disease-associated PrP in all TSE positive tissues. This is not an artefact caused by assay variation. Once the tissue is homogenised, repeated analysis demonstrates high assay precision (). It is conceivable; however, that in the clinical situation, there is widespread variation in the amounts of disease-associated PrP. This is not as obvious when qualitative tests such as assays based on Western blotting or immunohistochemistry are used. Indeed, most of the accredited diagnostic tests for TSE remain essentially of the “yes-no” type. Nevertheless, closer inspection of the Western blot data shown in indicates that the intensity of Western blot detection following PK digestion can correlate with the quantitative data obtained using the YWH1 DELFIA® without the use of PK.
Furthermore, the use of PK in conventional assays may destroy significant amounts of PK-sensitive disease-associated PrP leaving a consistent residue of PK-resistant PrP. There is increasing evidence that PK-sensitive PrP may be present in the early stages of TSE pathogenesis and in the derivation of TSE strains.Citation17–Citation19
Variation in brain or lymphoid tissue may also be critically dependent on the micro-anatomical distribution of disease-associated PrP. With lymphoid tissue, immunohistochemistry has shown that PrPSc is not uniformly distributed in germinal centres.Citation20 The detection of disease-associated PrP may be critically dependent on the micro-anatomical region taken in the biopsied tissue.
The search for a useful antibody that is specific for the disease-associated form of the protein has been an elusive quest although there have been several reports. For example, in 1997, Korth et al., described the Mab 15B3 that was able to specifically precipitate bovine, murine or human PrPSc but not PrPC.Citation21 This reagent was an IgM antibody. It is interesting to note that Paramithiotis et al., described another IgM—antibody raised against a synthetic peptide, containing a repeating YYR motif, that seemed to be specific for the disease-associated isoform of PrP.Citation22 However, the YYR motif is present in many proteins and this may compromise the specificity of this TSE diagnostic. In another report, Curin Serbec et al described the Mab V5B2 which was raised against a peptide from the C-terminal part of PrP and which recognized a conformational specific epitope in PrPSc aggregates.Citation23 By definition, any reagent recognizing a conformational epitope is likely to lose its ability to bind following any sample denaturation.
The YWH antibodies allow the direct detection of disease-associated PrP in brain and lymphoid tissue without the need for prior treatment of the tissue with PK to remove normal PrPC. To date, most accredited tests for the post-mortem detection of PrPSc in TSEs have involved the use of PK to discriminate between normal and disease-associated protein.Citation24 There are several problems associated with the use of this protease. Firstly, the conditions employed for the experimental proteolysis are somewhat arbitrary. Sufficient protease is required to digest all the PrPC while leaving the majority of the PrPSc to be detected. Some loss of PrPSc may be tolerated when there are sufficiently high levels of aggregated protein. Accordingly, the methods have been shown to work reliably when the target tissue is brain stem collected from animals in the terminal stages of the disease.Citation5,Citation7,Citation8
The application of this approach as a pre-clinical test is considerably more problematic.Citation25–Citation28 Firstly, in the early stages of disease, there is little detectable disease-associated PrP in the central nervous system. Consequently, it is necessary to target primary and secondary lymphoid tissue to identify disease-associated material. Furthermore, concentrations of aggregated material are relatively low, particularly in the early stages of the disease. Thus, the use of PK as a discriminating reagent in this situation is complicated because of the difficulty of knowing in advance exactly how much protease to use. Another problem with the continuing use of PK is the constraints arising with high throughput automation where inadequate control of proteolysis may give rise to false positive and/or false negative results.
Both YWH1 and YWH2 need to be evaluated as pre-clinical detectors of disease since there is still a compelling need for rapid diagnostic tests for TSEs that are sensitive, specific and quantitative. At the present time, work is continuing to evaluate and fully validate the disease-specific diagnostic for TSEs in cattle, sheep, hamsters, mice and humans. In particular, we are using the methodology to gain a better understanding of pathogenesis by monitoring the appearance of disease-associated aggregated PrP in primary and secondary lymphoid tissue obtained from scrapie-susceptible (VRQ/VRQ) and resistant (ARR/ARR) lambs exposed to the disease from birth.
Abbreviations
| PrPC | = | cellular prion protein |
| PrPSc | = | disease-associated prion protein |
| TSE | = | transmissible spongiform encephalopathy |
| BSE | = | bovine spongiform encephalopathy |
| CJD | = | Creutzfeld Jacob Disease |
| PK | = | proteinase K |
| DELFIA® | = | dissociation enhanced lanthanide fluoroimmunoassay |
| GdHCl | = | guanidine hydrochloride |
| Mab | = | monoclonal antibody |
Figures and Tables
Figure 1 N-terminal bovine (A) and human (B) chimeric peptide used as immunogens for the production of YWH antibodies.
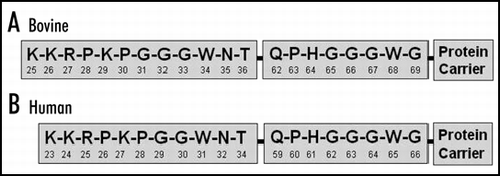
Figure 3 Measurement of disease-associated PrP in histology-confirmed scrapie positive and negative rostral medulla using Western blot and DELFIA®.
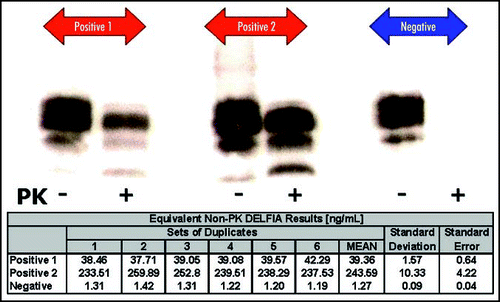
Table 1 Measurement of disease-associated PrP in histology-confirmed BSE positive and negative bovine caudal medulla.
Table 2 Measurement of disease-associated PrP in histology-confirmed Scrapie positive and negative ovine caudal medulla (A), rostral medulla (B), palatine tonsil (C) and retropharyngeal lymph node (D)
Acknowledgements
The authors would like to thank VLA for their support and encouragement, particularly in the provision of confirmed positive and negative tissues. Without their support, none of this work could have been accomplished. In particular, we would like to acknowledge our colleagues at the VLA in Newcastle for the opportunity to perform aspects of the work using their facilities. In addition, we are grateful to PerkinElmer Life Sciences for their support and for the provision of equipment and reagents. We thank Drs. Raymond Bujdoso and Alana Thackray for constructive discussions and TJ McKinley, Department of Veterinary Medicine, Madingley Road, Cambridge CB3 0ES, UK for expert advice regarding the statistical analysis of the data. We are grateful to the TSE Joint Funders for financial support (BBSRC Grants 8/BSD17730 and BB/D004500).
Conflict of Interest/Financial Disclosure Statement
The work described in this paper is subject to the UK Patent Application (PCT/GB2006/003494). This patent application, however, has not influenced our scientific judgment.
References
- Prusiner SB. The prion diseases. Brain Pathol 1998; 8:499 - 513
- Kascsak RJ, Fersko R, Pulgiano D, Rubenstein R, Carp RI. Immunodiagnosis of prion disease. Immunol Invest 1997; 26:259 - 268
- Kubler E, Oesch B, Raeber AJ. Diagnosis of prion diseases. Br Med Bull 2003; 66:267 - 279
- Madec JY, Groschup MH, Buschmann A, Belli P, Calavas D, Baron T. Sensitivity of the Western blot detection of prion protein PrPres in natural sheep scrapie. J Virol Methods 1998; 75:169 - 177
- Schaller O, Fatzer R, Stack M, Clark J, Cooley W, Biffiger K, Egli S, Doherr M, Vandevelde M, Heim D, Oesch B, Moser M. Validation of a western immunoblotting procedure for bovine PrP(Sc) detection and its use as a rapid surveillance method for the diagnosis of bovine spongiform encephalopathy (BSE). Acta Neuropathol (Berl) 1999; 98:437 - 443
- Oesch B, Doherr M, Heim D, Fischer K, Egli S, Bolliger S, Biffiger K, Schaller O, Vandevelde M, Moser M. Application of Prionics Western blotting procedure to screen for BSE in cattle regularly slaughtered at Swiss abattoirs. Arch Virol 2000; Suppl 189 - 195
- Madec JY, Belli P, Calavas D, Baron T. Efficiency of Western blotting for the specific immunodetection of proteinase K-resistant prion protein in BSE diagnosis in France. Vet Rec 2000; 146:74 - 76
- Cooley WA, Clark JK, Ryder SJ, Davis LA, Farrelly SS, Stack MJ. Evaluation of a rapid western immunoblotting procedure for the diagnosis of bovine spongiform encephalopathy (BSE) in the UK. J Comp Pathol 2001; 125:64 - 70
- Stack MJ, Chaplin MJ, Clark J. Differentiation of prion protein glycoforms from naturally occurring sheep scrapie, sheep-passaged scrapie strains (CH1641 and SSBP1), bovine spongiform encephalopathy (BSE) cases and Romney and Cheviot breed sheep experimentally inoculated with BSE using two monoclonal antibodies. Acta Neuropathol (Berl) 2002; 104:279 - 286
- Price KM, Cuthbertson AS, Varndell IM, Sheppard PW. The production and characterisation of monoclonal antibodies to myc, c-erbB-2 and EFG-receptor using a synthetic peptide approach. Dev Biol Stand 1990; 71:23 - 31
- Kretzschmar T, von Ruden T. Antibody discovery: Phage display. Curr Opin Biotechnol 2002; 13:598 - 602
- Pluckthun A, Pack P. New protein engineering approaches to multivalent and bispecific antibody fragments. Immunotechnology 1997; 3:83 - 105
- Barnard G, Sy MS. Krull I. The diagnosis of transmissible spongiform encephalopathies using differential extraction and DELFIA. Prions and Mad Cow Disease 2003; New York Marcel Dekker 277 - 315
- Monnet C, Marthiens V, Enslen H, Frobert Y, Sobel A, Marc Mége R. Heterogeneity and regulation of cellular prion protein glycoforms in neuronal cell lines. European Journal of Neuroscience 2003; 18:542 - 549
- Minor P, Newham J, Jones N, Bergeron C, Gregori L, Asher D, van Engelenburg F, Stroebel T, Vey M, Barnard G, Head M. Standards for the assay of Creutzfeldt-Jakob disease specimens. J Gen Virol 2004; 85:1777 - 1784
- Kristiansen M, Messenger MJ, Klohn PC, Brandner S, Wadsworth JD, Collinge J, Tabrizi SJ. Disease-related prion protein forms aggresomes in neuronal cells leading to caspase activation and apoptosis. J Biol Chem 2005; 280:38851 - 38861
- Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M, Cohen FE, Prusiner SB. Eight prion strains have PrP(Sc) molecules with different conformations. Nat Med 1998; 4:1157 - 1165
- Safar J, Cohen FE, Prusiner SB. Quantitative traits of prion strains are enciphered in the conformation of the prion protein. Arch Virol Suppl 2000; 227 - 235
- Thackray AM, Hopkins L, Bujdoso R. Proteinase K-sensitive disease-associated ovine prion protein revealed by conformation-dependent immunoassay. Biochem J 2007; 401:475 - 483
- Heggebo R, Press CM, Gunnes G, Gonzalez L, Jeffrey M. Distribution and accumulation of PrP in gut-associated and peripheral lymphoid tissue of scrapie-affected Suffolk sheep. J Gen Virol 2002; 83:479 - 489
- Korth C, Stierli B, Streit P, Moser M, Schaller O, Fischer R, Schulz-Schaeffer W, Kretzschmar H, Raeber A, Braun U, Ehrensperger F, Hornemann S, Glockshuber R, Riek R, Billeter M, Wuthrich K, Oesch B. Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature 1997; 390:74 - 77
- Paramithiotis E, Pinard M, Lawton T, LaBoissiere S, Leathers VL, Zou WQ, Estey LA, Lamontagne J, Lehto MT, Kondejewski LH, Francoeur GP, Papadopoulos M, Haghighat A, Spatz SJ, Head M, Will R, Ironside J, O'Rourke K, Tonelli Q, Ledebur HC, Chakrabartty A, Cashman NR. A prion protein epitope selective for the pathologically misfolded conformation. Nat Med 2003; 9:893 - 899
- Curin Serbec V, Bresjanac M, Popovic M, Pretnar Hartman K, Galvani V, Rupreht R, Cernilec M, Vranac T, Hafner I, Jerala R. Monoclonal antibody against a peptide of human prion protein discriminates between Creutzfeldt-Jacob's disease-affected and normal brain tissue. J Biol Chem 2004; 279:3694 - 3698
- Moynagh J, Schimmel H. Tests for BSE evaluated. Bovine spongiform encephalopathy. Nature 1999; 400:105
- Hamir AN, Miller JM, Schmerr MJ, Stack MJ, Chaplin MJ, Cutlip RC. Diagnosis of preclinical and subclinical scrapie in a naturally infected sheep flock utilizing currently available postmortem diagnostic techniques. J Vet Diagn Invest 2001; 13:152 - 154
- van Keulen LJ, Vromans ME, van Zijderveld FG. Early and late pathogenesis of natural scrapie infection in sheep. Apmis 2002; 110:23 - 32
- Heggebo R, Press CM, Gunnes G, Ulvund MJ, Tranulis MA, Lsverk T. Detection of PrPSc in lymphoid tissues of lambs experimentally exposed to the scrapie agent. J Comp Pathol 2003; 128:172 - 181
- Buschmann A, Biacabe AG, Ziegler U, Bencsik A, Madec JY, Erhardt G, Luhken G, Baron T, Groschup MH. Atypical scrapie cases in Germany and France are identified by discrepant reaction patterns in BSE rapid tests. J Virol Methods 2004; 117:27 - 36